Abstract
Background
Ulcerative colitis (UC) is the most common form of inflammatory bowel disease in the UK. Medical management aims to induce and maintain remission and to avoid complications and the necessity for surgical intervention. Colectomy removes the source of inflammation but is associated with morbidity and mortality. Newer anti-tumour necrosis factor (TNF)-α therapies may improve medical outcomes, albeit at an increased cost.
Objective
Our objective was to assess the incremental cost effectiveness of infliximab, adalimumab and golimumab versus conventional therapy and surgery from a National Health Service (NHS) and Personal Social Services (PSS) perspective over a lifetime horizon.
Methods
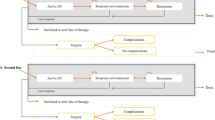
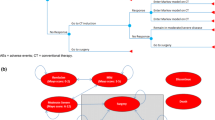
A Markov model was developed with health states defined according to whether the patient is alive or dead, current treatments received, history of colectomy and level of disease control. Transition probabilities were derived from network meta-analyses (NMAs) of trials of anti-TNF-α agents in the moderate-to-severe UC population. Health utilities, colectomy rates, surgical complications and resource use estimates were derived from literature. Unit costs were drawn from standard costing sources and literature and were valued at year 2013/2014 values.
Results
For patients in whom surgery is an option, colectomy is expected to dominate all medical treatment options. For patients in whom colectomy is not an option, infliximab and golimumab are expected to be ruled out due to dominance, whilst the incremental cost-effectiveness ratio (ICER) for adalimumab versus conventional treatment is expected to be approximately £50,278 per quality-adjusted life-year (QALY) gained.
Conclusions
Based on the NMAs, the ICERs for anti-TNF-α therapy versus conventional treatment or surgery are expected to be at best, in excess of £50,000 per QALY gained. The cost effectiveness of withdrawing biologic therapy upon remission and re-treating relapse is unknown.


Similar content being viewed by others
References
National Institute for Health and Care Excellence. Ulcerative colitis: Management in adults, children and young people. NICE Clinical Guideline Number 166. London: NICE; 2013, pp 1–37.
Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. Br Med J 2013; 346. http://www.bmj.com/content/346/bmj.f432.long. Accessed 01 May 2014.
Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53(10):1471–8.
Bayless TM, Hanauer SB. Advanced therapy in inflammatory bowel disease: volume 1—IBD and ulcerative colitis. 3rd ed. Shelton: Connecticut: People’s Medical Publishing House—USA; 2011.
Royal College of Physicians. Report of the results for the national clinical audit of adult inflammatory bowel disease inpatient care in the UK. 1-67. London: RCP; 2012, p. 1–68.
European Medicines Agency. Summary of product characteristics—infliximab. London: EMA; 2009. p. 1–56.
European Medicines Agency. Summary of product characteristics—adalimumab. London: EMA; 2009. p. 1–292.
European Medicines Agency. Summary of product characteristics—golimumab. London: EMA; 2009. p. 1–199.
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olsen A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76.
Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer SB, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7.
Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257–65.
Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85–95.
Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):96–109.
Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson AM, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49(2):283–94.
Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392–400.
Probert CS, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, Arnott ID, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52(7):998–1002.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. London: NICE; 2013. p. 1–102.
Archer R, Tappenden P, Ren S, Martyn St-James M, Harvey R, Basarir H et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy: Clinical effectiveness systematic review and economic model. Final report to the National Institute for Health and Care Excellence. Sheffield: University of Sheffield 2014, p. 1–438.
Cooney RM, Warren BF, Altman DG, Abreu MT, Travis SP. Outcome measurement in clinical trials for ulcerative colitis: towards standardisation. Trials 2007;8(17). http://www.trialsjournal.com/content/8/1/17. Accessed 01 June 2014).
Woehl A, Hawthorne AB, McEwan P. The relation between disease activity, quality of life and health utility in patients with ulcerative colitis. Gut. 2008;57(Suppl1):A153.
Arseneau KO, Sultan S, Provenzale DT, Onken J, Bickston SJ, Foley E, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol. 2006;4(9):1135–42.
Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44(4):431–40.
Arai K, Koganei K, Kimura H, Akatani M, Kitoh F, Sugita A, et al. Incidence and outcome of complications following restorative proctocolectomy. Am J Surg. 2005;190(1):39–42.
BMJ Group and the Royal Pharmaceutical Society of Great Britain. Br Natl Formul. 2014. https://www.medicinescomplete.com/mc/bnf/current/. Accessed 01 May 2014.
Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Therapeut. 2008;28(10):1230–9.
Merck, Sharp, Dohme. Manufacturer’s submission of evidence to the National Institute for Health and Care Excellence: Infliximab. Hertfordshire: MSD; 2014.
Department of Health. NHS Reference Costs 2012/13. London: DH. https://www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013. Accessed 04 May 2014.
Buchanan J, Wordsworth S, Ahmad T, Perrin A, Vermeire S, Sans M, et al. Managing the long term care of inflammatory bowel disease patients: The cost to European health care providers. J Crohn Colitis. 2011;5(4):301–16.
Dias S, Welton N, Sutton A, Ades A. NICE Decision Support Unit Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Sheffield: University of Sheffield; 2011. p. 1–98.
Thomas A, O’Hara B, Ligges U, Sturtz S. Making BUGS open. R News. 2015;6:12–7.
Office for National Statistics. Interim life tables 2009–2011. 2013. http://www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2009-2011/stb-2009-2011.html. Accessed 05 May 2014.
Swinburn P, Elwick H, Bean K, Curry A, Patel S, Bodger K, et al. The impact of surgery on health related quality of life in ulcerative colitis. Gut. 2012;61(Suppl2):A237.
Van der Valk ME, Mangen MJ, Dijkstra G, Bodegraven AA, Fiddler H, De Jong DJ et al. Is there a difference in quality of life and costs between ulcerative colitis patients with a pouch or an ileostomy? Dig Dis Wk; 2012.
Richards DM, Hughes SA, Irving MH, Scott NA. Patient quality of life after successful restorative proctocolectomy is normal. Colorectal Dis. 2001;3(4):223–6.
Kuruvilla K, Osler T, Hyman NH. A comparison of the quality of life of ulcerative colitis patients after IPAA vs ileostomy. Dis Colon Rectum. 2012;55(11):1131–7.
Curtis L. Unit costs of health and social care 2014. 1–294. Personal Social Services Research Unit: Kent; 2014.
Xie F, Blackhouse G, Assasi N, Gaebel K, Robertson D, Goeree R. Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis. Cost Eff Resour Alloc. 2009;7:20.
Acknowledgments
The authors would like to thank MSD and AbbVie for providing additional data over the course of the National Institute for Health and Care Excellence (NICE) appraisal for which the model was developed.
Author Contributions
Paul Tappenden and Hasan Basarir developed the health economic model. Rachel Archer and Marrissa Martyn-St James undertook the systematic review of clinical effectiveness evidence. Shijie Ren, Rebecca Harvey and John Stevens undertook the network meta-analyses. Sami Hoque and Alan Lobo provided ongoing clinical advice during the model development. All authors contributed to the preparation of this manuscript. Paul Tappenden will act as the overall guarantor for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme to inform the NICE Technology Appraisal Programme (Project Number 12/51/01). Alan Lobo has received money for attending an advisory board for Takeda Pharmaceuticals. Paul Tappenden, Shijie Ren, Rachel Archer, Rebecca Harvey, Marrissa Martyn-St James, Hasan Basarir, John Stevens and Sami Hoque do not have any conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tappenden, P., Ren, S., Archer, R. et al. A Model-Based Economic Evaluation of Biologic and Non-Biologic Options for the Treatment of Adults with Moderately-to-Severely Active Ulcerative Colitis after the Failure of Conventional Therapy. PharmacoEconomics 34, 1023–1038 (2016). https://doi.org/10.1007/s40273-016-0409-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0409-9