Abstract
Objective
The AntiRetroviral Therapy with TMC114 ExaMined In naive Subjects (ARTEMIS) clinical trial examined the efficacy and safety of two ritonavir-boosted protease inhibitors (PI/r), darunavir/r 800/100 mg once daily (QD) and lopinavir/r 800/200 mg daily, both used in combination with tenofovir disoproxil fumarate/emtricitabine. This study aimed to assess the cost effectiveness of the darunavir/r regimen compared with the lopinavir/r regimen in treatment-naive adults with HIV-1 infection in Canada.
Methods
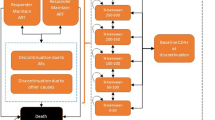
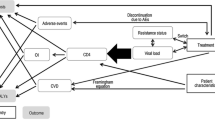
A Markov model with a 3-month cycle time and six CD4 cell-count-based health states (>500, 351–500, 201–500, 101–200, 51–100, and 0–50 cells/mm3) followed a cohort of treatment-naive adults with HIV-1 infection through initial darunavir/r or lopinavir/r combination therapy and a common set of subsequent regimens over the course of their remaining lifetimes. Population characteristics and transition probabilities were estimated from the ARTEMIS clinical trial and other trials. Costs (in 2014 Canadian dollars), utilities, and mortality were estimated from Canadian sources and published literature. Costs and health outcomes were discounted at 5 % per year. One-way and probabilistic sensitivity analyses were performed, including a simple indirect comparison of the darunavir/r initial regimen with an atazanavir/r-based regimen.
Results
In the base-case lifetime analysis, individuals receiving initial therapy with the darunavir/r regimen experienced 0.25 more quality-adjusted life-years (QALYs) with lower antiretroviral drug costs (−$14,246) and total costs (−$18,402) than individuals receiving the lopinavir/r regimen, indicating that darunavir/r dominated lopinavir/r. In an indirect comparison with an atazanavir/r-based regimen, the darunavir/r regimen remained the dominant choice, but with lower cost savings (−$2,303) and QALY gains (0.02). Results were robust to a wide range of other changes in input parameter values, population characteristics, and modeling assumptions. The probabilistic sensitivity analysis demonstrated that the darunavir/r regimen was cost effective compared with the lopinavir/r regimen in over 86 % of simulations for willingness-to-pay thresholds between $0 and $100,000 per QALY gained.
Conclusions
Darunavir/r 800/100 mg QD may be a cost-effective PI/r component of initial antiretroviral therapy for treatment-naive adults with HIV-1 infection in Canada.


Similar content being viewed by others
References
Pomerantz RJ, Horn DL. Twenty years of therapy for HIV-1 infection. Nat Med. 2003;9:867–73.
Joint United Nations Programme on HIV/AIDS (UNAIDS). World Health Organization (WHO). AIDS epidemic update; 2009. http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2009/default.asp. Accessed 6 Sep 2010.
Braithwaite RS, Justice AC, Chang CC, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118:890–8.
Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–9.
Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9.
Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–7.
Hutchinson AB, Farnham PG, Dean HD, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences. J Acquir Immune Defic Syndr. 2006;43:451–7.
Hellinger FJ. The lifetime cost of treating a person with HIV. JAMA. 1993;270:474–8.
Levy AR, James D, Johnston KM, et al. The direct costs of HIV/AIDS care. Lancet Infect Dis. 2006;6:171–7.
Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:54–62.
Krentz HB, Gill MJ. Cost of medical care for HIV-infected patients within a regional population from 1997 to 2006. HIV Med. 2008;9:721–30.
Yamashita TE, Phair JP, Munoz A, et al. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. AIDS. 2001;15:735–46.
Powderly WG, Saag MS, Chapman S, et al. Predictors of optimal virological response to potent antiretroviral therapy. AIDS. 1999;13:1873–80.
Gross R, Yip B, Lo Re V 3rd, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–14.
Legorreta A, Yu A, Chernicoff H, et al. Adherence to combined lamivudine + zidovudine versus individual components: a community-based retrospective medicaid claims analysis. AIDS Care. 2005;17:938–48.
Maitland D, Jackson A, Osorio J, et al. Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Med. 2008;9:667–72.
Rosenblum M, Deeks SG, van der Laan M, Bangsberg DR. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One. 2009;4:e7196.
Hill A, Sawyer W. Effects of nucleoside reverse transcriptase inhibitor backbone on the efficacy of first-line boosted highly active antiretroviral therapy based on protease inhibitors: meta-regression analysis of 12 clinical trials in 5168 patients. HIV Med. 2009;10:527–35.
Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–33.
European AIDS Clinical Society. Guidelines on the clinical management and treatment of HIV infected adults in Europe (Version 5-2). http://www.europeanaidsclinicalsociety.org. Accessed 6 Sep 2010.
Panel on Antiretroviral Guidelines for Adults and Adolescents. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; 2013. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. Accessed 18 Mar 2014.
Tibotec Press Release. Once-daily Prezista (darunavir) for treatment-naïve adults with HIV-1 receives approval in European Union as part of combination therapy; 2009. http://www.tibotec.com/news/list.jhtml. Accessed 31 Aug 2010.
U.S. Food and Drug Administration (FDA). Antiretroviral drugs used in the treatment of HIV infection; 2010. http://www.fda.gov/ForConsumers/byAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118915.htm. Accessed 31 Aug 2010.
Johnson & Johnson Press Release. Health Canada approves Prezista once daily as part of combination therapy for treatment-naïve adults with HIV-1; 2009. http://www.jnj.com/connect/news/all/20090407_900000. Accessed 31 Aug 2010.
Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naïve HIV-1-infected patients at week 48. AIDS. 2008;22:1389–97.
Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naïve, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23:1679–88.
Orkin C, DeJesus E, Khanlou H. ARTEMIS: 192-week efficacy and safety of once-daily darunavir/ritonavir (DRV/r) vs lopinavir/r (LPV/r) in treatment-naïve HIV-1-infected adults [Poster]. Presented at: 10th International Congress on HIV and Drug Therapy in HIV Infection. Glasgow, United Kingdom, 7–11 Nov 2010.
Common Drug Review (CDR). Canadian Expert Drug Advisory Committee (CEDAC) final recommendation; darunavir; new indication: HIV-1 treatment-naïve; 2009. http://www.cadth.ca/media/cdr/complete/cdr_complete_Prezista_HIV_October-14-2009.pdf. Accessed 9 Sep 2010.
Mauskopf J, Brogan A, Martin S, Smets E. Cost effectiveness of darunavir/ritonavir in highly treatment-experienced, HIV-1-infected adults in the USA. Pharmacoeconomics. 2010;28(Suppl 1):83–105.
Brogan A, Mauskopf J, Talbird SE, Smets E. US cost effectiveness of darunavir/ritonavir 600/100 mg bid in treatment-experienced, HIV-infected adults with evidence of protease inhibitor resistance included in the TITAN Trial. Pharmacoeconomics. 2010;28(Suppl 1):129–46.
Mauskopf J, Brogan AJ, Talbird SE, Martin S. Cost-effectiveness of combination therapy with etravirine in treatment-experienced adults with HIV-1 infection. AIDS. 2012;26:355–64.
Mauskopf J, Brogan A, Malmberg C, Hwang P. Cost-effectiveness of darunavir for the management of HIV-infected, treatment-experienced adults in Canada [Poster]. Presented at: 16th Annual Canadian Conference on HIV/AIDS Research. Toronto, Ontario, 26–29 Apr 2007.
Deeks SG, Barbour JD, Grant RM, Martin JN. Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. AIDS. 2002;16:201–7.
Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62.
Tibotec, data on file. Analyses of ARTEMIS clinical trial data. Mechelen, Belgium; 2008.
Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370:49–58.
Tibotec, data on file. Analyses of TITAN clinical trial data. Mechelen, Belgium; 2008.
Lazzarin A, Campbell T, Clotet B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:39–48.
Madruga JV, Cahn P, Grinsztejn B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:29–38.
Katlama C, Haubrich R, Lalezari J, et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS. 2009;23:2289–300.
Tibotec, data on file. Analyses of pooled DUET 1 and DUET 2 clinical trial data. Mechelen, Belgium; 2008.
Cooper DA, Gatell J, Rockstroh J, et al. 48-week results from BENCHMRK-1, a phase III study of raltegravir (RAL) in patients failing antiretroviral therapy (ART) with triple-class resistant HIV-1 [Poster]. Presented at: 15th Conference on Retroviruses and Opportunistic Infections. Boston, Massachusetts; 3–6 Feb 2008.
Steigbigel R, Kumar P, Eron J, et al. 48-week results from BENCHMRK-2, a phase III study of raltegravir (RAL) in patients failing antiretroviral therapy (ART) with triple-class resistant HIV-1 [Poster]. Presented at: 15th Conference on Retroviruses and Opportunistic Infections. Boston, Massachusetts, 3–6 Feb 2008.
Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–54.
Isentress (raltegravir) prescribing information. Whitehouse Station: Merck & Co., Inc.; 2008.
Selzentry (maraviroc) prescribing information. New York: Pfizer, Inc.; 2007.
Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–41.
Nelson M, Girard PM, Demasi R, et al. Suboptimal adherence to darunavir/ritonavir has minimal effect on efficacy compared with lopinavir/ritonavir in treatment-naïve, HIV-infected patients: 96 week ARTEMIS data. J Antimicrob Chemother. 2010;65:1505–9.
Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S123–7.
Viciana P, Rubio R, Ribera E, et al. Longitudinal study on adherence, treatment satisfaction, and effectiveness of once-daily versus twice-daily antiretroviral therapy in a Spanish cohort of HIV-infected patients (CUVA study). Enferm Infecc Microbiol Clin. 2008;26:127–34.
De Meyer S, Azijn H, Surleraux D, et al. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49:2314–21.
Tarwater PM, Margolick JB, Jin J, et al. Increase and plateau of CD4 T-cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:168–75.
Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–95.
Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–15.
Smith CJ, Sabin CA, Lampe FC, et al. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. AIDS. 2003;17:963–9.
Garcia F, de Lazzari E, Plana M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr. 2004;36:702–13.
Cook J, Dasbach E, Coplan P, et al. Modeling the long-term outcomes and costs of HIV antiretroviral therapy using HIV RNA levels: application to a clinical trial. AIDS Res Hum Retroviruses. 1999;15:499–508.
Simpson KN, Luo MP, Chumney E, et al. Cost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infection. HIV Clin Trials. 2004;5:294–304.
Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–46.
Olsen CH, Gatell J, Ledergerber B, et al. Risk of AIDS and death at given HIV-RNA and CD4 cell count, in relation to specific antiretroviral drugs in the regimen. AIDS. 2005;19:319–30.
Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–9.
Lewden C, Bouteloup V, De Wit S, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥ 500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41:433–45.
Statistics Canada. Deaths, 2009 (Table 4). 25 Jul 2012. http://www.statcan.gc.ca/pub/84f0211x/84f0211x2009000-eng.htm. Accessed 26 Feb 2014.
Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99–106.
Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355–65.
Van der Ryst E, Cooper D, Konourina I, et al. Efficacy of maraviroc in combination with at least one other potent new antiretroviral drug: 24-week combined analysis of the MOTIVATE 1 and 2 studies [Poster]. Presented at: 4th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention. Sydney, Australia, 22–25 Jul 2007.
Ontario Ministry of Health and Long-Term Care. Ontario Drug Benefit Formulary. 27 Jan 2014. http://www.health.gov.on.ca/english/providers/program/drugs/odbf_mn.html. Accessed 27 Jan 2014.
Association Québécoise des Pharmaciens Propriétaires pharmacist list in Quebec. 2010. http://www.aqpp.qc.ca. Accessed 9 Sep 2010.
Common Drug Review (CDR). Canadian Expert Drug Advisory Committee (CEDAC) final recommendation and reasons for recommendation; maraviroc. 12 Nov 2008. http://www.cadth.ca/media/cdr/complete/cdr_complete_Celsentri_Final_Nov-12-08.pdf. Accessed 4 Feb 2009.
Common Drug Review (CDR). Canadian Expert Drug Advisory Committee (CEDAC) final recommendation and reasons for recommendation; tipranavir; 2006. http://www.cadth.ca/media/cdr/complete/cdr_complete_Aptivus_May-17-06.pdf. Accessed 2 Aug 2006.
Yip B, Linia VD, Burke TA, et al. Non-drug health care resource utilization and costs of managing HIV/AIDS patients prescribed antiretroviral therapy: evidence from a contemporary HIV practice in Canada [Poster]. Presented at: 11th European AIDS Conference. Madrid, Spain, 24–27 Oct 2007.
London Health Science Center, Canada (LHSC). Specific analysis for cost data, fiscal 2006/07. Case costing. London Health Science Center, Canada; 2008.
Montaner J, Yip B, Hogg R. Resource utilization by HIV-positive patients in British Columbia, Canada [Unpublished Report]; 2008.
Druyts EF, Yip B, Lima VD, et al. Health care services utilization stratified by virological and immunological markers of HIV: evidence from a universal health care setting. HIV Med. 2009;10:88–93.
Statistics Canada. The Consumer Price Index for Canada, major components and special aggregates, not seasonally adjusted, historical data, health and personal care (Table 7); 2014. http://www.statcan.gc.ca/pub/62-001-x/62-001-x2014001-eng.htm. Accessed 25 Feb 2014.
Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Ottawa: CADTH; 2006. www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. Accessed 12 Mar 2010.
Moulton D. Provincial squeeze on generic prices continues. CMAJ. 2011;183:E1049–50.
Mauskopf J. A methodological review of models used to estimate the cost effectiveness of antiretroviral regimens for the treatment of HIV infection. Pharmacoeconomics. 2013;31:1031–50.
Simpson KN, Pei PP, Moller J, et al. Lopinavir/ritonavir versus darunavir plus ritonavir for HIV infection: a cost-effectiveness analysis for the United States. Pharmacoeconomics. 2013;31:427–44.
Möller J, Desai K, Simpson K, et al. Cost-minimization comparison of darunavir plus ritonavir and lopinavir/ritonavir in HIV-1 infected treatment-naïve women of childbearing age. J Med Econ. 2014;17:250–8.
Colombo GL, Colangeli V, di Biagio A, di Matteo S, Viscoli C, Viale P. Cost-effectiveness analysis of initial HIV treatment under Italian guidelines. Clinicoecon Outcomes Res. 2011;3:197–205.
Simpson KN, Rajagopalan R, Dietz B. Cost-effectiveness analysis of lopinavir/ritonavir and atazanavir + ritonavir regimens in the CASTLE study. Adv Ther. 2009;26:185–93.
Sax PE, Losina E, Weinstein MC, et al. Cost-effectiveness of enfuvirtide in treatment-experienced patients with advanced HIV disease. J Acquir Immune Defic Syndr. 2005;39:69–77.
Hornberger J, Kilby JM, Wintfeld N, Green J. Cost-effectiveness of enfuvirtide in HIV therapy for treatment-experienced patients in the United States. AIDS Res Hum Retroviruses. 2006;22:240–7.
Simpson KN, Roberts G, Hicks CB, Finnern HW. Cost-effectiveness of tipranavir in treatment-experienced HIV patients in the United States. HIV Clin Trials. 2008;9:225–37.
Phillips AN, Lundgren JD. The CD4 lymphocyte count and risk of clinical progression. Curr Opin HIV AIDS. 2006;1:43–9.
Hill A, Sawyer W, Gazzard B. Effects of first-line use of nucleoside analogues, efavirenz, and ritonavir-boosted protease inhibitors on lipid levels. HIV Clin Trials. 2009;10:1–12.
Hill A, Balkin A. Risk factors for gastrointestinal adverse events in HIV treated and untreated patients. AIDS Rev. 2009;11:30–8.
Tramarin A, Parise N, Campostrini S, et al. Association between diarrhea and quality of life in HIV-infected patients receiving highly active antiretroviral therapy. Qual Life Res. 2004;13:243–50.
O’Brien ME, Clark RA, Besch CL, et al. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003;34:407–14.
Feasey NA, Healey P, Gordon MA. Review article: the aetiology, investigation and management of diarrhoea in the HIV-positive patient. Aliment Pharmacol Ther. 2011;34:587–603.
Sherman DS, Fish DN. Management of protease inhibitor-associated diarrhea. Clin Infect Dis. 2000;30:908–14.
Lundgren JD, Battegay M, Behrens G, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9:72–81.
Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–27.
Simpson KN. Economic modeling of HIV treatments. Curr Opin HIV AIDS. 2010;5:242–8.
Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94.
Conseil du Médicament Québec (CdM). Capsules CdM, Fuzeon; 2008. http://www.cdm.gouv.qc.ca/site/116.963.0.0.1.0.phtml. Accessed 22 Mar 2010.
Acknowledgments
The authors would like to thank the participants of the ARTEMIS trial and their families for their participation and support during the study. The authors also thank the ARTEMIS study team and their co-investigators for their collaboration. In addition, the authors would like to acknowledge Tom Van de Casteele at Tibotec Pharmaceuticals, a subsidiary of Johnson & Johnson, for providing assistance with requests for statistical analyses of clinical trial data.
Funding for this study was provided to RTI Health Solutions by Johnson & Johnson Pharmaceutical Services, LLC. During the conduct of this study, Erik Smets was an employee of Johnson & Johnson Pharmaceutical Services, LLC. He is currently an employee of UCB Pharma SA. Ines Adriaenssen is an employee of Johnson & Johnson Pharmaceutical Services, LLC. Sarah Manuel is an employee of Janssen Inc. Both Johnson & Johnson Pharmaceutical Services, LLC and Janssen Inc. are subsidiaries of Johnson & Johnson. Anita Brogan and Josephine Mauskopf are employees of RTI Health Solutions, an independent research organization, and maintained independent scientific control over the study, including data analysis and interpretation of final results. All authors jointly conceptualized the study, contributed to the model design, and interpreted the results. Anita Brogan and Josephine Mauskopf also had primary responsibility for input parameter identification and model programming. Anita Brogan will act as the overall guarantor for the content.
Aggregated data used in this manuscript were taken from clinical studies that were approved by the appropriate ethics committees and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and later amendments. All persons in the clinical studies gave their informed consent prior to their inclusion in the studies. Neither subject-level data nor details that might disclose the identities of the subjects were provided to the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brogan, A.J., Smets, E., Mauskopf, J.A. et al. Cost Effectiveness of Darunavir/ritonavir Combination Antiretroviral Therapy for Treatment-Naive Adults with HIV-1 Infection in Canada. PharmacoEconomics 32, 903–917 (2014). https://doi.org/10.1007/s40273-014-0173-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0173-7