Abstract
Introduction
The purpose of the study was to conduct a cost-effectiveness analysis and budget impact analysis comparing lopinavir with ritonavir (LPV/r) and atazanavir plus ritonavir (ATV+RTV) for antiretroviral-naïve patients with a baseline CD4+ T-cell distribution and total cholesterol (TC) profile as reported in the CASTLE study.
Methods
This decision analysis study used a previously published Markov model of HIV disease, incorporating coronary heart disease (CHD) events to compare the short- and long-term budget impacts and CHD consequences expected for the two regimens.
Results
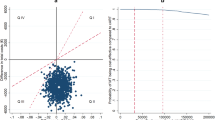
Patients were assumed to have a baseline CHD risk of 4.6% (based on demographic data) and it was also assumed that 50% of the population in the CASTLE study were smokers. The CHD risk differences (based on percent of patients with TC >240 mg/dL) in favor of ATV+RTV resulted in an average improvement in life expectancy of 0.031 quality-adjusted life years (QALYs) (11 days), and an incremental cost-effectiveness ratio of $1,409,734/QALY. Use of the LPV/r regimen saved $24,518 and $36,651 at 5 and 10 years, respectively, with lifetime cost savings estimated at $38,490. A sensitivity analysis using a cohort of all smokers on antihypertensive medication estimated an average improvement in life expectancy of 31 quality-adjusted days in favor of ATV+RTV, and a cost-effectiveness ratio of $520,861/QALY: a ratio that is still above the acceptable limit within the US.
Conclusion
The use of an LPV/r-based regimen in antiretroviral-naïve patients with a baseline CHD risk similar to patients in the CASTLE study appears to be a more cost-effective use of resources compared with an ATV+RTV-based regimen. The very small added CHD risk predicted by LPV/r treatment is more than offset by the substantial short- and long-term cost savings expected with the use of LPV/r in antiretroviral-naïve individuals with average to moderately elevated CHD risk.
Similar content being viewed by others
References
Chen RY, Accortt NA, Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010.
Simpson KN, Luo M, Chumney ECG, King MS, Brun S. Cost-effectiveness of lopinavir/ritonavir compared to atazanavir in antiretroviral-naïve patients: modeling the combined effects of HIV and heart disease. Clin Drug Invest. 2007;27:67–74.
D’Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000;139:273–281.
Friis-Moller N, Reiss P, El-Sadr W, et al. Exposure to PIs and NNRTI and risk of myocardial infarction: results of the D:A:D study. Presented at: 13th Conference on Retroviruses and Opportunistic Infections; February 5–8, 2006; Denver, Colorado. Session 35. Oral abstract 144.
Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naïve HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655.
Ghani AC, de Wolf F, Ferguson NM, et al. Surrogate markers for disease progression in treated HIV infection. J Acquir Immune Defic Syndr. 2001;28:226–231.
Ghani AC, Henley WE, Donnelly CA, Mayer S, Andersen RM. Comparison of the effectiveness of non-nucleoside reverse transcriptase inhibitor-containing and protease inhibitor-containing regimens using observational databases. AIDS. 2001;15:1133–1142.
Mocroft A, Ruiz L, Reiss P, et al. Virological rebound after suppression on highly active antiretroviral therapy. AIDS. 2003;17:1741–1751.
Castiel D, Herve C, Gaillard M, et al. Cost-utility analysis of early thrombolytic therapy. Pharmacoeconomics. 1992;1:438–442.
Fleming T, ed. Red Book 2007: Pharmacy’s Fundamental Reference. Montvale NJ: Thompson Healthcare; 2007.
Simpson KN, Luo MP, Chumney EG, Sun E, Brun S, Ashraf T. Cost effectiveness of using lopinavir/ritonavir vs. nelfinavir as the first highly active antiretroviral regimen for HIV infection. HIV Clin Trials. 2004;5:294–304.
Simpson KN, Roberts G, Hicks CB, et al. Cost-effectiveness of tipranavir in treatment-experienced HIV patients in the United States. HIV Clin Trials. 2008;9:224–236.
Dolan P. Modeling valuations for EuroQoL health states. Med Care. 1998;35:1095–1108.
Mrus JM, Yi MS, Freedberg KA, et al. Utilities derived from visual analog scale scores in patients with HIV/AIDS. Med Decis Making. 2003;23: 414–421.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simpson, K.N., Rajagopalan, R. & Dietz, B. Cost-effectiveness analysis of lopinavir/ritonavir and atazanavir+ritonavir regimens in the CASTLE study. Adv Therapy 26, 185–193 (2009). https://doi.org/10.1007/s12325-008-0141-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-008-0141-8