Abstract
Background
Invasive meningococcal disease (IMD) is a serious disease with a rapid onset, high mortality rate, and risk of long-term complications. Numerous reports in the literature conclude that IMD outbreaks are associated with substantial costs to society and significant burden on communities due to the cost associated with the prevention of secondary cases.
Objective
To systematically review the literature on the costs and public health burden associated with IMD outbreaks.
Methods
Studies were primarily identified through searching MEDLINE and EMBASE. Reports were included if they provided cost data related to the containment of an IMD outbreak after 1990 and were written in English, French, or Spanish. Costs were converted to 2010 United States dollars. Outbreaks were categorized by low-income countries (LIC) and high-income countries (HIC) based on gross domestic product per capita. Outbreak containment strategies were classified as small (e.g., targeting members of the school/institution where the outbreak occurred) or large (e.g., targeting everyone in the community).
Results
Sixteen articles reporting data on 93 IMD outbreaks fulfilled the eligibility criteria and were included. The majority of outbreaks occurred in HIC. Five studies reported the use of small containment strategies including targeted vaccination and chemoprophylaxis, all occurring in HIC. The average cost per small containment strategy was $299,641 and the average cost per IMD case was $41,857. Eight studies reported large containment strategies involving widespread vaccination targeting a specific age group or community. For HIC, the average cost per large containment strategy was $579,851 and the average cost per IMD case was $55,755. In LIC, the average cost per large containment strategy was $3,407,590 and the average cost per IMD case was $2,222.
Conclusion
IMD outbreaks were associated with substantial costs. We found that although there were numerous reports on IMD outbreaks, data on containment costs were very limited. More research in this area is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
• Invasive meningococcal disease (IMD) outbreaks are associated with substantial costs to society with a high public health burden such as IMD treatments, containment strategies, high case fatality ratios, and anxiety and disruption to the communities.
• Limited literature exists to quantify the burden associated with IMD outbreaks.
• The average cost per IMD case was $41,857 for small containment strategies and $55,755 for large containment strategies.
• Small outbreak containment strategies had approximately 50 % lower total costs compared to large outbreak containment strategies in high-income countries, yet total costs per containment strategy were highest in low-income countries.
• Cases associated with outbreaks were likely to result in higher disease burden compared to sporadic cases, due to higher case fatality ratios.
1 Background
Invasive meningococcal disease (IMD) is a bacterial infection that can be caused by Neisseria meningitides or meningococcus that causes the lining surrounding the brain and spinal cord to swell [1]. It is transmitted between humans through droplets of respiratory or throat secretions [1]. Although relatively rare in endemic areas (i.e., 1 or 2 cases per 100,000 infected individuals) [2], IMD is very serious with a rapid onset, leading to death within 24–48 h in some cases [3]. Data from a prospective cohort study in the United Kingdom (UK) found that approximately 2 % of children infected with IMD died within 5 years [4]. IMD is associated with long-term sequelae, including neurological damage and limb amputation [5]. It is one of the most feared diseases because of its potential for devastating effects on large populations [6].
Data from endemic and epidemic areas indicate that the largest number of IMD cases occur among children and adolescents in many countries [6]. A meta-analysis of carriage (i.e., individuals who carry the meningococcus bacteria without any adverse effects) indicated that 10–20 % of adolescents are asymptomatic carriers and therefore are the major transmitters of disease [7]. Vaccines have been available for over 30 years and are the best preventive strategy for children and adolescents [8].
The epidemiology and serogroup distribution of IMD varies geographically and has been found to be unpredictable and very dynamic [6]. The majority of cases due to Neisseria meningitides are caused by six serogroups: A, B, C, X, Y, and W-135 [9]. Outbreaks of IMD commonly occur in the African meningitis belt and serogroup C outbreaks (particularly those affecting university students) were key factors for introducing routine vaccination in North America and Europe [10]. It is important to note that the outbreak serogroup may not always reflect the wider epidemiology in the region.
According to the World Health Organization (WHO), an outbreak is “the occurrence of cases of disease in excess of what would normally be expected in a defined community, geographical area or season” [11]. Although IMD outbreaks are rare in endemic areas, such as North America and Europe, the case fatality ratio is higher for cases resulting from outbreaks compared to sporadic cases [12]. Furthermore, when IMD outbreaks do occur, they are associated with substantial costs to society [12, 13], as well as public panic and immense disruption to public health and communities [14–16].
IMD vaccines currently available include polysaccharide or conjugated. Both vaccine types provide protection against different combinations of serogroups A, C, W-135, and Y [8]. The advantages of conjugate vaccines over polysaccharide vaccines are well established and include longer duration of protection, no hyper-responsiveness, more immunogenicity for younger ages, and the potential protection against carriage (hence contributing to herd immunity) [8, 17–21]. A recent Cochrane review of the polysaccharide vaccines suggests that vaccine efficacy beyond the first year of vaccination could not be established [22]. Furthermore, according to WHO, use of polysaccharide vaccines might not be optimal in areas where outbreaks occur repeatedly (e.g., meningitis belt in Africa) because the protection is short lived, resulting in a substantial number of additional vaccinations required [23].
There are important health economic questions with respect to the routine versus sporadic use of vaccines to prevent meningococcal disease. However, to assess this robustly in a health economic model, the full burden of the disease needs to be assessed and included. In order to understand the true economic burden of IMD and potential benefit that could result from vaccination, reports on the cost associated with the prevention of secondary cases (e.g., containment strategies) are required. Our objective was to synthesize the costs associated with IMD outbreaks through a systematic review of the literature. Our specific systematic review question was: “Based on data from costing studies, what are the costs associated with containment strategies for IMD outbreaks?”
2 Method of Review
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was used to guide the reporting of this review [24].
2.1 Eligibility Criteria
Reports were included if they provided cost data related to the containment of an IMD outbreak. The WHO definition of an outbreak was used [11]. Only reports written in English, French, and Spanish were included because reviewers were fluent in these languages. Furthermore, only those disseminated after the year 1990 were included because the epidemiology of IMD has changed over time [9].
2.2 Information Sources and Search
Medical subject headings and text words related to IMD outbreaks were used to search MEDLINE (OVID interface, 1950 to July Week 2, 2010) and EMBASE (OVID interface, 1980 to 2010 Week 28), restricted to articles indexed after 1990 without any language or study design restrictions. To supplement the search, the reference lists of included studies were scanned and the authors’ personal files were searched. We also contacted the authors on some of the included studies by email to ensure all relevant costing studies were captured.
The search strategy for the main electronic search (MEDLINE) is presented in the Appendix. Details on the other search (EMBASE) are available upon request from the authors. An experienced information specialist, as advised in the PRISMA Statement [24], conducted all of the literature searching.
2.3 Study Selection
One reviewer screened the citations (i.e., titles and abstracts) for inclusion using a predefined relevance criteria form based on the eligibility criteria reported in Sect. 1.1. A second reviewer screened the list of excluded citations to ensure that all relevant material was captured. Discrepancies were resolved by discussion and the full-text article was obtained for the potentially relevant citations identified. Given that the reporting of costs is not always reflected by title or included in the abstract [25], the full-text article of all citations reporting an outbreak of IMD was obtained and screened independently by two reviewers to identify costing data. Discrepancies between the two reviewers were resolved by discussion or by the involvement of a third reviewer.
2.4 Data Collection Process and Data Items
The extracted data included outbreak characteristics (e.g., location, time period, serogroup, age of infected, number of infected, attack rate, hospitalization rate, case fatality ratio) and containment strategies and costs (e.g., number treated, type of treatment, volunteer costs, public health campaign costs, vaccination costs, total costs). As advised in the PRISMA Statement, a draft data extraction form was developed, piloted, and modified as necessary. Two reviewers independently abstracted data from the included studies using the standardized data extraction form.
2.5 Synthesis of Results
The systematic review results were summarized descriptively. Using information from the World Bank’s operational lending categories, the outbreaks were categorized by the country of origin’s income (e.g., low-income defined as $1,005 per capita or lower) [26]. Furthermore, the outbreak containment strategies were classified as small (i.e., targeting all members of the school where the outbreak occurred) or large (i.e., targeting everyone in the community). The costs of outbreaks were compared accounting for these two classification criteria. The average cost per containment strategy and range (minimum cost to maximum cost per containment strategy) were calculated, as well as the average cost per IMD case and range (minimum cost to maximum cost per IMD case). To compare results across the studies, costing data were converted to 2010 international United States dollars using purchasing power parities for the particular year of the costing data [27]. International United States dollars were thereafter adjusted for inflation using the consumer price index for urban consumers (medical care) [28].
3 Results
3.1 Study Selection
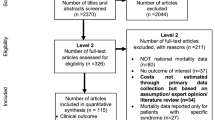
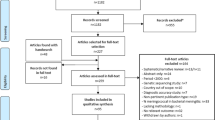
The literature search identified a total of 1,672 titles and abstracts from the literature search (Fig. 1). These were screened using the eligibility criteria reported in Sect. 1.1 and the full-text of 343 potentially relevant articles were obtained. Reasons for exclusion at the full-text level of screening included that the article did not report costing data (n = 294), did not examine the epidemiology of an outbreak (n = 30), did not examine IMD (n = 1), or was not written in English, Spanish, or French (n = 2). Seventeen articles fulfilled the eligibility criteria and were included [14–16, 29–38, 40–43], reporting information on 24 outbreaks, plus an additional 69 outbreaks reported in a review of data from the Centers for Disease Control and Prevention [12]. The review article was summarized on its own because it did not report the type of containment strategy associated with the outbreak [12]. One study [13] reported data from 7 unpublished outbreaks. Another [39] reported data from an outbreak summarized by one of the included studies [15] (i.e., a companion report) and was only used to provide supplementary data. Two of the included studies were published in French [35, 37] and the remainder were published in English.
3.2 Outbreak Characteristics among High-Income Countries
The majority of the outbreaks reported in the included studies occurred in high-income Organization for Economic Co-operation and Development (OECD) countries (or HIC; Table 1). Serogroup C was involved in all of the reported outbreaks, except for outbreaks that occurred throughout the United States between 1994 and 2002, in which the breakdown of serogroups was C (62 %), B (25 %), and Y (13 %) [12]. Twelve of the IMD outbreaks occurred among young children or adolescents.
The total number of infected per outbreak ranged from 3 to 45 individuals, with an attack rate over the entire outbreak ranging from <2 to 477 per 100,000 population. Only four of the included studies reported the number of primary and secondary cases (Table 1) [16, 32, 34, 35].
The studies with the highest number of cases occurred among socially disadvantaged individuals (e.g., ethnic minorities, low socioeconomic status) [29] and injection drug users infected with IMD [16]. Four of the studies reported the number of hospitalizations [14, 15, 32, 35], which ranged from three in an outbreak among secondary school students in Canada [32] to seven among youths in Switzerland [35]. The respective hospitalization rate was 55.6 % in an outbreak of mostly high school students in the USA [15] and 100 % in outbreaks that occurred among boys aged 3–6 years in the USA [14], secondary school students in Canada [32], and children and adolescents from Switzerland [35]. Seven of the studies reported the number of deaths [15, 16, 29–33], which ranged from zero among secondary school students in Canada [32] to nine among socially disadvantaged individuals in Washington state, USA [29]. The respective case fatality ratio ranged from 0 % among secondary school students in Canada [32] to 50 % among adolescents in the Ottawa, Ontario, and Hull, Quebec [30].
3.3 Costs for Small Outbreak Containment Strategies in High-Income Countries
Ten of the outbreaks were classified as entailing small containment strategies in HIC (Table 2). Chemoprophylaxis therapy (CPT) was offered as part of the containment strategy in three of the outbreaks. A total of 4,100 socially disadvantaged individuals in Washington, USA, were offered CPT after an outbreak including 40 confirmed cases [29]. The number of individuals offered CPT was not reported in an outbreak involving 7 confirmed cases among children in the UK [31]. Finally, an outbreak involving 3 confirmed cases resulted in CPT among 3,712 close contacts, staff, and high school students in Australia [34]. Only the study that occurred among high school students in Australia reported costs associated with CPT; the cost was $6 for 100 capsules and 3,712 capsules were administered (total cost of $228) [34]. There were 3 confirmed cases for this outbreak, at an average cost per IMD case of $74.33 for CPT [34]. One study reported the costs of antibiotics after an outbreak among adolescents in Canada [30]. The total cost for the antibiotic ceftriaxone was $12,524 (101 doses at $124/dose). Because there were 10 confirmed cases involved with this outbreak, the average cost per IMD case for ceftriaxone was $1,252 [30].
Nine reports of the small outbreak containment strategies reported the number of vaccinated. Across these reports, the total number vaccinated was 89,934 individuals, ranging from the vaccination of 656 students, close contacts, and parents after an outbreak involving 3 children in North Carolina, USA [13] to 16,000 undergraduate students after an outbreak including 9 university students in Illinois, USA [13, 40]. The average number of vaccinees across these outbreaks was 9,992 (89,934 in nine outbreaks). Of these, eight outbreaks reported costs associated with vaccination [13, 29, 31, 34, 40, 41]. The average vaccination cost per small containment strategy was $61,049 ($488,393 in eight outbreaks; range $21,588–$661,229) and the average vaccination cost per IMD case was $45,622 ($364,974 in eight outbreaks; range $8,704–$135,628).
Medical staff costs were reported in two studies as being $4,958 in one outbreak [34] and $227,267 in another [31]. None of the studies reported fire, police, or emergency medical service costs. One study reported that 37 health department staff and 4 nursing staff were involved in the public health campaign at a cost of $39,805 over 5 days, with an average cost of $13,268 per IMD case [32]. Another study reported that medical officer, nursing, administration, and media staff were associated with a cost of $5,014 [34].
Only two of the studies reported the total costs (which was undefined by the reports—they merely classified them as total costs) associated with the outbreaks, which ranged from $42,254 [34] to $557,028 [31] (Table 3). The average cost per small containment strategy was $229,641 and the average cost per IMD case was $41,857 (range $14,085–$69,629).
3.4 Costs for Large Outbreak Containment Strategies in High-Income Countries
Eleven of the outbreaks were classified as entailing large containment strategies in HIC (Table 2). CPT was offered as part of the containment strategy in three of the outbreaks [14, 15, 33]. However, only one study reported the number offered rifampicin, ciprofloxacin, or ceftriaxone (484 individuals) after an outbreak involving 7 youth cases [33]. The costs associated with CPT were not reported in any of the studies, nor was any additional information on antibiotic administration.
All of the reports of large outbreak containment strategies reported the number vaccinated. Across these reports, the total number vaccinated was 201,479 individuals, ranging from the vaccination of 3,500 direct contacts of 4 boys aged 3–6 years in Illinois, USA [14], to the vaccination of 55,250 residents of south central Phoenix aged 2–9 years after an outbreak involving 31 individuals in Arizona, USA [13]. The average number of vaccinees across these outbreaks was 18,316 (201,479 in 11 outbreaks). Of these, 9 reported costs associated with vaccination [13, 15, 33, 35, 42, 43]. The average vaccination cost per large containment strategy was $783,640 ($7,052,756 in 9 outbreaks, range $230,361–$1,477,511) and the average vaccination cost per IMD case was $73,931 (range $26,327–$164,168).
Medical staff costs were reported as $98,995 [33] and $54,483 [35] in two of the large containment strategies occurring in HIC. Public health costs were only reported in one study, which totaled $29,431 [33]. None of the studies reported emergency medical service costs.
Four of the studies reported the total costs associated with the outbreaks (which was undefined by the reports), ranging from $105,484 [14] to $1,081,627 over 94 days [16] (Table 3). The average cost per large containment strategy was $579,851 and the average cost per IMD case was $55,755 (range $26,371–$91,046).
3.5 Review of Outbreaks: July 1994 to June 2002 in the United States
One of the included studies was a review of IMD outbreaks occurring between July 1994 and June 2002 in the United States, mostly using data from health departments and the Centers for Disease Control and Prevention [12]. Because this study only reported aggregate data, we were unable to include it with the other results. Characteristics of outbreaks were compared with sporadic cases identified through population-based surveillance. A total of 69 outbreaks were identified, including 229 patients, with a median of 9.5 outbreaks per year (range 3–14). The majority of outbreaks were due to IMD serogroup C (62 %), while B and Y caused 25 and 13 % of the outbreaks, respectively. The majority of the outbreaks occurred in communities (25/69, 36 %), while 29 % occurred in primary and secondary schools, 17 % occurred in college and university settings, and 12 % occurred in nursing homes. Vaccination campaigns were implemented for less than half of the outbreaks, with an estimated total cost of more than $15,705,224 (based on a unit cost of $88 per vaccine). The calculated vaccine cost per IMD case was $67,272. A total of 44 individuals died due to IMD and the case fatality ratio was 16 %.
3.6 Outbreak Characteristics Among Low-Income Countries
Three of the studies reported outbreaks that occurred in low-income countries (LIC) in Africa (i.e., $1,005 per capita or lower) [36–38] (Table 1). The IMD serogroup was not reported in one study [37], but was reported as type A and C in one study [36] and type W135 in another [38]. None of these studies reported the proportion of cases by age.
One outbreak occurring in Guinea in 1992 to 1993 resulted in 2,435 probable cases, with an attack rate of 142 per 100,000 [36]. The age range of infected individuals was not reported. The number of hospitalizations was not reported but there were 319 deaths (case fatality ratio of 13 %) [36]. Another outbreak occurring in Senegal, West Africa, involved 33,047 infected in 1995 (attack rate 27,771 per 100,000) and 153,655 infected in 1996 (attack rate of 59,461 per 100,000) [37]. The age range of cases was not reported, nor was the number of hospitalizations or deaths. The third outbreak occurred in Nanoro, Burkina Faso, in 2002 and resulted in 1,500 probable cases (estimated attack rate of 4,474 per 100,000) [38]. The age range of the infected was not reported, nor was the number of hospitalizations. The authors reported 300 deaths (case fatality ratio of 20 %) associated with this outbreak [38].
3.7 Costs for Large Outbreak Containment Strategies in Low-Income Countries
In one study, the number of infected was 33,047 in 1995 and 153,655 in 1996, resulting in 85,925 individuals vaccinated at a total vaccine cost of $39,526 [37]. Volunteer and medical staff costs were $4,360 in this outbreak [37]. Another study reported 2,435 infected, which resulted in the vaccination of 629,913 individuals with a total vaccine cost of $258,108 [36]. The third study reported 1,500 cases, resulting in the vaccination of 135,000 individuals at a total vaccination cost of $135,000 [38]. The average vaccination cost per large containment strategy in Africa was $144,211 ($432,634 in three outbreaks, range $39,526–$258,108) and the average vaccination cost per IMD case was $66 (range $1.20–$106).
The total costs related to the outbreaks ranged from $58,363 [37] to donations of $9,726,937 [38] (Table 3). Based on these three African outbreaks, the average cost per containment strategy was $3,407,590 and the average cost per IMD case was $2,222 (range $0.31–$6,465).
4 Discussion
We performed a systematic review to assess the costs of IMD outbreaks, which occurred in HIC and LIC in Africa and entailed small (direct contacts) and large (community level) containment strategies. Sixteen articles reporting data on 93 outbreaks fulfilled the eligibility criteria and were included after screening 343 full-text articles and 1,672 citations. Five studies reported the use of small containment strategies including targeted vaccination and chemoprophylaxis, which all occurred in HIC. Eight studies reported large containment strategies involving widespread vaccination targeting a specific age group or community. Based on the total costs reported by four studies in HIC, the average cost per large containment strategy was $579,851 (range $105,484–$1,081,627) and the average cost per IMD case was $55,755 (range $26,371–$91,046). In LIC in Africa, the average cost per large containment strategy was $3,407,590 (range $58,363–$9,726,937) and the average cost per IMD case was $2,222 (range $0.31–$6,465) based on data from three studies. These results demonstrate the economic impact of IMD outbreaks on healthcare systems internationally.
We found that total costs associated with IMD outbreaks were highest in LIC in Africa when compared with HIC. In HIC, small outbreak containment strategies had approximately 50 % lower total costs compared to large outbreak containment strategies. The average cost per IMD case was similar for both small and large containment strategies in HIC, yet was the lowest in Africa even after purchasing power parity adjustment. Some explanations for the differences between LIC and HIC include the use of cheaper vaccines (e.g., polysaccharide vaccine) and fewer community interventions in Africa.
Many of the outbreaks in HIC occurred in children and adolescents. This supports recent decisions in USA, Canada, and other countries to vaccinate with A, C, W, Y vaccines routinely in adolescents, even though the incidence is low. It is believed that by vaccinating adolescents (the major transmitters of the disease) [7] with conjugate vaccines, herd effects may also result in protection against carriage and disease in unvaccinated individuals [17]. In addition, a review of IMD outbreaks reported that cases from outbreaks had over three times the odds of dying versus sporadic cases (odds ratio 3.3, 95 % confidence interval 2.0–5.5) [12]. They concluded that although outbreaks were rare, the case fatality ratio was higher for cases resulting from outbreaks compared to sporadic cases [12]. Cases associated with outbreaks are therefore associated with a higher disease burden. This is an important element for consideration in health economic studies.
An important health economic question is: does the cost of routine vaccination for a very low-incidence disease offset the costs public health would spend in outbreak situations, and is routine vaccination cost-effective? It’s a difficult question to answer given the unpredictability of the disease, and in addition for one to truly assess this, they would need to also account for the societal impact of outbreaks, which is typically not included in health economic models.
A framework has been published in PharmacoEconomics on important elements that should be reported in studies that analyze costs [44]. The purpose of the study, perspective, time horizon, and discounting of future costs should be reported. The types of resources used in the analysis should be reported and classified. The measures of the resources used should be described including unit of measurement, sources of data, adjustments of measures, and the level of uncertainty in the costing measures. The resource valuation that is applied to each of the units should be reported, including the measures used to obtain the costs, adjustments to the costs, and uncertainty of the costs. Finally, it should be reported whether the costs are per patient, comparator, or overall costs. None of the included costing studies fulfilled the majority of these criteria.
Another important finding of this systematic review was that the burden of outbreaks goes beyond economic impact. Nearly every article commented on the tremendous and immediate disruption to the communities and the public at large, as well as mass public fear and anxiety largely driven by the media [12, 15, 33]. Given the unpredictable nature of IMD, most communities were not prepared for such containment initiatives. This raises important economic questions about opportunity costs and the impact from a societal perspective. Given this societal burden, another important question needs to be addressed: what is the overall societal value in terms of economic benefit and beyond such as on the quality of life or the disruption to the affected community in avoiding these outbreaks? This is difficult to quantify and is never taken into account in health economic studies, and, therefore, the societal value of these vaccines is underestimated, but a key factor in decision making [45].
It is also worth noting that many outbreaks were not reported in the literature [13], and therefore the impact and costs of outbreaks are likely underestimated. Furthermore, none of the studies reported costs related to emergency medical services and few reported public health campaign costs. This suggests that our costing estimates are conservative.
Over 300 full-text published articles relevant to meningococcal disease outbreaks were identified and examined in depth. However, only a small proportion adequately reported relevant costs and very few (n = 3) indicated in the title/abstract that costs were examined. Individuals conducting studies on outbreaks who examine costs should report that they examined costs in their title or abstract. Similarly, systematic reviewers conducting reviews of the cost of outbreaks will need to be cautious and recognize that these studies are poorly reported. Furthermore, comparing the attack rate across the studies was difficult, because some used the entire population as the denominator while others used the target population as the denominator. The systematic review process was limited because not all languages were included. However, we did include studies written in English, Spanish, and French, which decreases the potential for language bias. Furthermore, we calculated an average cost per IMD case, although this was based on very little data and probably cannot be generalized. However, these costs give a range of what the economic burden can be per case based on age groups affected and region.
5 Conclusion
IMD outbreaks were associated with substantial costs. Most of the studies noted a high public health burden with respect to public fear, anxiety, and immediate disruption to the affected communities. Small outbreak containment strategies had approximately 50 % lower total costs compared to large outbreak containment strategies in HIC, yet total costs were highest in LIC. The average cost per IMD case was similar for both small and large containment strategies in HIC, yet was the lowest for LIC even after purchasing power parity adjustment. This might be due to the use of cheaper vaccines in LIC (e.g., polysaccharide vaccine) versus HIC. Cases associated with outbreaks were likely to result in higher disease burden compared to sporadic cases, due to higher case fatality ratios. Numerous reports on IMD outbreaks were identified, but few reported on the containment costs. More research in this area is warranted.
References
Racloz VN, Luiz SJ. The elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiology. BMC Infect Dis. 2010;10:175.
Barquet N, Domingo P, Cayla JA, Gonzalez J, Rodrigo C, Fernandez-Viladrich P, et al. Meningococcal disease in a large urban population (Barcelona, 1987–1992): predictors of dismal prognosis. Barcelona Meningococcal Disease Surveillance Group. Arch Intern Med. 1999;159(19):2329–40.
Branco RG, Amoretti CF, Tasker RC. Meningococcal disease and meningitis. J Pediatr (Rio J). 2007;83(2 Suppl):S46–53.
Bedford H, de Louvois J, Halket S, Peckham C, Hurley R, Harvey D. Meningitis in infancy in England and Wales: follow up at age 5 years. BMJ. 2001;323(7312):533–6.
Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–28.
Harrison LH, Pelton SI, Wilder-Smith A, Holst J, Safadi MA, Vazquez JA, et al. The global meningococcal initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29(18):3363–71.
Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61.
Poland GA. Prevention of meningococcal disease: current use of polysaccharide and conjugate vaccines. Clin Infect Dis. 2010;50(Suppl 2):S45–53.
Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63.
O’Donovan D, Iversen A, Trounce J, Curtis S. Outbreak of group C meningococcal infection affecting two preschool nurseries. Commun Dis Public Health. 2000;3(3):177–80.
World Health Organization. Health topics: disease outbreaks. http://www.who.int/topics/disease_outbreaks/en/. 2011. Cited 1 Jan 2010.
Brooks R, Woods CW, Benjamin DK Jr, Rosenstein NE. Increased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994–2002. Clin Infect Dis. 2006;43(1):49–54.
Jackson LA, Schuchat A, Reeves MW, Wenger JD. Serogroup C meningococcal outbreaks in the United States. An emerging threat. JAMA. 1995;273(5):383–9.
Austin CC, Fingar AR, Langkop C. Outbreak of serogroup C meningococcal disease among preschool-aged children: Illinois, 1996. Am J Public Health. 1998;88(4):685.
Osterholm MT. How to vaccinate 30,000 people in three days: realities of outbreak management. Public Health Rep. 2001;116(Suppl 2):74–8.
Weiss D, Stern EJ, Zimmerman C, Bregman B, Yeung A, Das D, et al. Epidemiologic investigation and targeted vaccination initiative in response to an outbreak of meningococcal disease among illicit drug users in Brooklyn, New York. Clin Infect Dis. 2009;48(7):894–901.
Bermal N, Huang LM, Dubey AP, Jain H, Bavdekar A, Lin TY, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin. 2011;7(2):239–47.
Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8(7):851–61.
Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, et al. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J. 2011;30(4):e56–62.
Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159(10):907–13.
Pichichero M, Casey J, Blatter M, Rothstein E, Ryall R, Bybel M, et al. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide–diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr Infect Dis J. 2005;24(1):57–62.
Patel M, Lee CK. Polysaccharide vaccines for preventing serogroup A meningococcal meningitis. Cochrane Database Syst Rev. 2005;(1):CD001093.
World Health Organization. Cost of reactive campaigns: a reactive vaccination campaign is expensive and provides no long-term protection. http://www.meningvax.org/financial-impact.php. 2012. Cited 1 Jan 2011.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Getsios D, Caro I, El-Hadi W, Caro JJ. Assessing the economics of vaccination for Neisseria meningitidis in industrialized nations: a review and recommendations for further research. Int J Technol Assess Health Care. 2004;20(3):280–8.
The World Bank. Country and Lending Groups. http://data.worldbank.org/about/country-classifications/country-and-lending-groups#Low_income. 2011. Cited 1 Jan 2010.
Organisation for Economic Co-operation and Development. Purchasing power parities (PPP). http://www.oecd.org/.../0,3746,en_2649_34347_45854149_1_1_1_1,00.html. 2011. Cited 1 Jan 2010.
United States Department of Labor. Consumer price index. http://www.bls.gov/cpi/. 2011. Cited 1 Jan 2010.
Houck P, Patnode M, Atwood R, Powell K. Epidemiologic characteristics of an outbreak of serogroup C meningococcal disease and the public health response. Public Health Rep. 1995;110(3):343–9.
Banerji A, King WJ, MacDonald N, Li M. Use of single dose ceftriaxone in the emergency department during an outbreak of serogroup C meningococcal disease. Pediatr Infect Dis J. 1995;14(10):904–5.
Irwin DJ, Miller JM, Milner PC, Patterson T, Richards RG, Williams DA, et al. Community immunization programme in response to an outbreak of invasive Neisseria meningitidis serogroup C infection in the Trent region of England 1995–1996. J Public Health Med. 1997;19(2):162–70.
Tolomeo O, Buffett C, Richardson E. Management of a cluster of cases of invasive group C Neisseria meningitidis infections in the Hamilton-Wentworth region—Ontario. Can Commun Dis Rep. 1998;24(15):122–6.
Krause G, Blackmore C, Wiersma S, Lesneski C, Gauch L, Hopkins RS. Mass vaccination campaign following community outbreak of meningococcal disease. Emerg Infect Dis. 2002;8(12):1398–403.
Robinson P, Taylor K, Tallis G, Carnie J, Rouch G, Griffith J, et al. An outbreak of serogroup C meningococcal disease associated with a secondary school. Commun Dis Intell. 2001;25(3):121–5.
Renevey F, Demierre G, Chuard C, Regamey C. Invasive meningococcal infections in the Fribourg canton. Reasons for and development of a vaccination program in Gruyere in February 2001. Rev Med Suisse Romande. 2001;121(8):569–72.
Varaine F, Caugant DA, Riou JY, Konde MK, Soga G, Nshimirimana D, et al. Meningitis outbreaks and vaccination strategy. Trans R Soc Trop Med Hyg. 1997;91(1):3–7.
da Silva A, Parent dC I, Beckr GA, Dompnier JP, Seck I. Microeconomic evaluation of a mass preventive immunisation campaign against meningococcal meningitis and yellow fever in Senegal in 1997. Sante. 2003;13(4):215–23.
Ahmad K. Vaccination halts meningitis outbreak in Burkina Faso. Lancet. 2004;363(9417):1290.
Siegel B. Meningitis outbreak: mother nature sends a scary message. Med Econ. 1995;72(16):173–80, 185.
Imrey PB, Jackson LA, Ludwinski PH, England AC 3rd, Fella GA, Fox BC, et al. Outbreak of serogroup C meningococcal disease associated with campus bar patronage. Am J Epidemiol. 1996;143(6):624–30.
Edmond MB, Hollis RJ, Houston AK, Wenzel RP. Molecular epidemiology of an outbreak of meningococcal disease in a university community. J Clin Microbiol. 1995;33(8):2209–11.
Oakes P. Meningococcal outbreak. Miss Morb Rep. 1991;9:1.
Wenger JD, Jackson LA, Raj P, Tonelli MJ. Issues in the control of outbreaks of group C meningococcal disease in the United States. Infect Dis Clin Pract. 1994;3:136–40.
Jacobs P, Ohinmaa A, Brady B. Providing systematic guidance in pharmacoeconomic guidelines for analysing costs. Pharmacoeconomics. 2005;23(2):143–53.
Kauf TL. Methodological concerns with economic evaluations of meningococcal vaccines. Pharmacoeconomics. 2010;28(6):449–61.
Funding
GlaxoSmithKline Biologicals SA funded this review and was involved in all stages of research conduct, including the development and publication of this manuscript. ACT is funded by a Canadian Institutes for Health Research/Drug Safety and Effectiveness Network New Investigator Award in Knowledge Synthesis.
Conflict of interest
AA was an employee of the GlaxoSmithKline group of companies and she is currently an employee of Abbott Laboratories, Diagnostics Division. AV and ND are employees of the GlaxoSmithKline group of companies, and ND has stock options in the GlaxoSmithKline group of companies. ACT has received fees from GlaxoSmithKline group of companies for consulting and lectures, for writing the present manuscript, and for presenting the results from other research at conferences. GW has received consulting fees from GlaxoSmithKline group of companies.
Authorship contribution
AA, ACT, and ND performed the analysis and interpreted the data; AV commented on the design during draft development; GW and ACT reviewed the literature and abstracted the data; ACT and AA drafted the manuscript; all authors provided scientific advice; all authors reviewed and commented on drafts, and approved the final manuscript.
Acknowledgements
The authors thank William Witteman for conducting the literature searches, David Hallett for verifying the data, and Abdelilah Ibrahimi (XPE Pharma & Science on behalf of GlaxoSmithKline Vaccines), Cédric Laloyaux, and Jennifer Dorts (Business & Decision Life Sciences on behalf of GlaxoSmithKline Vaccines) for managing the publication.
Author information
Authors and Affiliations
Corresponding author
Appendix: Search strategy for MEDLINE
Appendix: Search strategy for MEDLINE
-
1
exp Neisseria meningitidis/
-
2
“invasive meningococcal disease”.mp.
-
3
(invasive adj2 (meningococcal or meningitis or meningococcal or meningitidis)).mp.
-
4
(neisseria adj2 (meningitis or meningitidis)).mp.
-
5
exp Meningococcal Infections/
-
6
exp Meningitis, Meningococcal/
-
7
exp Disease Outbreaks/
-
8
(disease adj2 outbreaks).mp.
-
9
or/1-6
-
10
or/7-8
-
11
9 and 10
-
12
limit 11 to (humans and yr = "1990-Current”)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited. The exclusive right to any commercial use of the article is with Springer.
About this article
Cite this article
Anonychuk, A., Woo, G., Vyse, A. et al. The Cost and Public Health Burden of Invasive Meningococcal Disease Outbreaks: A Systematic Review. PharmacoEconomics 31, 563–576 (2013). https://doi.org/10.1007/s40273-013-0057-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0057-2