Abstract
Background
Chronic hepatitis C virus (HCV) infection represents a crucial health problem in children that greatly influences their quality of life. Many efforts have been directed toward investing in effective drugs with a high safety profile and oral administration for better compliance.
Objectives
This study aims to assess the safety of a fixed-dose combination of ledipasvir/sofosbuvir plus drug efficacy and sustained virologic response (SVR) at 12 weeks after treatment discontinuation.
Method
One tablet (90 mg ledipasvir, 400 mg sofosbuvir) was administered to treatment-naïve children aged 12–18 years weighing at least 35 kg with chronic HCV infection for 6 months, genotype 4. Patients were divided into 2 groups, (1) without comorbidities (24 patients) and (2) with comorbidities (26 patients).
Results
At the end of the therapy, all patients (100%) had SVR and a significant reduction of liver enzymes with mild tolerable side effects.
Conclusion
Ledipasvir/sofosbuvir fixed-dose combination is a safe and highly effective therapeutic option in Egyptian children aged ≥ 12 years, with chronic HCV infection, genotype 4, either without or with comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pediatric HCV patients with comorbidities such as cancer, epilepsy, hemophilia, thalassemia, and nephrotic syndrome are neglected. |
Pediatric HCV infection has an aggressive course due to associated chronic disease or medications received. |
Ledipasvir–sofosbuvir fixed-dose combination is safe and effective in this special population. |
1 Introduction
Hepatitis C virus (HCV) infection in children continues to be a serious public health concern, with an estimated global prevalence of 0.13%, equating to 3.26 million viremic children worldwide. Pakistan, China, India, and Nigeria accounted for more than half of all viremic HCV infections [1]. Hepatitis C virus antibodies and HCV RNA were found in 0.4 % and 0.2 % of Egyptian children aged 1–14 years, respectively, rising to 1 % and 0.8 % in teenagers aged 15–19 years [2].
Hepatitis C virus infection in children is frequently asymptomatic and has a slower progression with an unpredictable histological course than in adults [3]. Cirrhosis is documented in 1–2% of persistently infected adolescents and children, including decompensation, even though histology may show a normal liver [4], and there are few case reports of hepatocellular carcinoma [5]. Advanced liver disease and decompensated cirrhosis have been diagnosed in children aged as young as 3 years and as early as 1 year after infection [6]. Patients with longer follow-up and infection duration have more evidence of disease progression, and progression is more likely 10 years after the start of infection [7]. In addition, HCV infection has been linked to poor quality of life and cognitive impairment in children [8, 9].
Thanks to the development of direct-acting antivirals (DAAs), chronic hepatitis C infection has been transformed from a condition requiring complex therapies with unsatisfactory outcomes to one that can be easily treated with few adverse effects [10]. In 2017, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) approved the use of the fixed-dose combination of ledipasvir/sofosbuvir and also the combination of sofosbuvir and ribavirin for treatment of adolescents (aged 12–17 years or weighing > 35 kg) with chronic HCV genotype 1, 4, 5, and 6 and genotype 2 and 3 infections, respectively [11].
Recently, the HCV recommendation panel of the 2019 American Association for the Study of Liver Diseases and Infectious Diseases Society of America supported DAA therapy for all children and adolescents (aged ≥ 3 years) with HCV infection. Age, treatment status, indications of cirrhosis, and HCV genotype all play a role in determining which medication combination is best for the patient: ledipasvir/sofosbuvir and sofosbuvir/ribavirin for 12 weeks are approved for those aged ≥ 3 years in HCV genotypes 1, 4, 5 and 6, and genotypes 2 and 3, respectively. In March 2020, the FDA expanded the approval of sofosbuvir/velpatasvir for 12 weeks to children aged 6 to < 18 years with any genotype. Also, the combination of glecaprevir/pibrentasvir was approved for children aged ≥ 3 with any genotype for a duration of 8 weeks [12].
Comorbidities such as hematological illnesses with iron overload, obesity, cancer, and viral co-infections such as human immunodeficiency virus (HIV) and hepatitis B virus (HBV) might hasten the development of hepatic fibrosis in adolescents and children [4]. For example, many patients with inherited blood disorders have been infected with HCV because of the many transfusions they receive. Transfusion-associated liver iron overload leads to the advancement of liver fibrosis and exposure to complications of chronic liver disease, including cirrhosis, end-stage liver disease, and hepatocellular carcinoma [13]. In these patients, pegylated interferon (pegIFN) and ribavirin were contraindicated because of the risk of ribavirin-associated anemia [14].
Hepatitis C virus infection is a neglected disease in patients with cancer [15]. It is more aggressive in these patients because of the concurrent use of cytotoxic chemotherapy, which increases the risk of fibrosis progression, early cirrhosis, and hepatocellular carcinoma [16]. Case studies have reported the need for dosage reduction or cessation of chemotherapy in 80 % of pediatric acute leukemia cases who are anti‐HCV positive due to hepatotoxicity. Safety and effectiveness data among immunosuppressed populations are limited, despite the recent clearance of DAA for use in pediatrics [17]. The current study was conducted just after the beginning of the Egyptian protocol for treating chronic HCV infection in pediatrics to confirm the high efficacy and safety profile of sofosbuvir/ledipasvir in the Delta region. We emphasize in this study the use of DAA in chronic HCV pediatric populations with comorbidities because of the above-mentioned aggressive natural history and complications of the associated chronic illness and medications with limited research on these individuals.
2 Patients and Methods
This is a prospective therapeutic clinical trial study (over 18 months from March 2018 to August 2019) that evaluated the safety and efficacy of combined sofosbuvir/ledipasvir in treating chronic HCV infection in treatment-naïve children.
Fifty treatment-nave children with chronic HCV genotype 4 infection diagnosed by positive HCV RNA PCR test [18] were recruited from the gastroenterology and hepatology outpatient clinic at Mansoura University Children’s Hospital (MUCH). All patients and their parents were informed about the safety, efficacy, and adverse effects of the drugs before the start of the study. Written informed consent was obtained from all parents or legal guardians of the included patients. All were aged between 12 and 18 years or weighed at least 35 kg, with chronic HCV infection ≥ 6 months, without or with compensated cirrhosis, and no fibrosis. Those with certain comorbidities, such as decompensated heart failure [19], severe renal impairment [glomerular filtration rate (GFR) < 30] [20], uncontrolled diabetes mellitus (DM) [21], concomitant HBV [22] or HIV infection [23] and those who were maintained on beta-blockers or amiodarone were excluded.
2.1 Study Groups
Patients were divided into two groups:
-
The group without comorbidities included 24 patients (16 males, 8 females).
-
The group with comorbidities included 26 patients (20 males, 6 females).
The group with comorbidities included 1 patient with ankylosing spondylitis, 7 patients with thalassemia, 1 patient with hemophilia A, 14 patients with previously treated malignancies (5 had leukemia, 4 had lymphoma, 3 had medulloblastoma, 1 had Langerhans cell histiocytosis, and 1 had retinoblastoma), 1 patient had nephrotic syndrome and 2 had epilepsy.
2.2 Methods
Relevant data from all included patients were obtained, including risk factors for HCV infection, date and route of acquisition, and medications, including previous antiviral therapy. Hepatitis C virus genotyping was done before treatment, revealing GT 4 in all included patients. Fibroscan, which was done for all patients and showed no fibrosis. Before treatment, a basic electrocardiogram (ECG) and abdominal ultrasonography were performed. Concomitant HBV infection was excluded by HBsAg [24] and anti-HBc antibody for fear of fulminant hepatitis B infection [25]. Human immunodeficiency virus screening was done prior to treatment. Basic investigations were conducted prior to therapy, including: complete blood count (CBC), serum creatinine (SCr), blood urea nitrogen (BUN), random blood glucose (RBG), and liver function tests (LFT), including serum albumin, total and direct bilirubin, and international normalized ratio (INR). For 12 weeks, all patients were given a fixed-dose combination of ledipasvir–sofosbuvir [Harvoni “90 mg ledipasvir, 400 mg sofosbuvir”] orally once daily at a fixed time with or without food. Regular visits were arranged at the pediatric hepatology unit at 4, 8, and 12 weeks with easy access to the treating physician if any urgent problem arose between the visits. Some patients with comorbidities were maintained on medications. Drug interactions were checked using the application (Liverpool HEP ichart), and no interactions were found before or during the treatment. At every visit, CBC, SCr, BUN, RBG, and LFTs were checked. Adverse events (after excluding other possible causes) were reported for the drug’s safety profile. Regarding the efficacy of the drug, HCV-RNA was assessed by quantitative real-time PCR [18] at 4 weeks and after the end of the treatment course (12 weeks).
2.3 Statistical Analysis
The SPSS software (Statistical Package for the Social Sciences, version 24), was used for data processing. Continuous variables were presented as mean ± standard deviation (SD) for parametric data and median and inter-quartile range (IQR) for non-parametric data. The study groups were compared using Student t-test for parametric data and Mann-Whitney test for non-parametric data, while paired groups were compared by the paired t-test (parametric) and Wilcoxon signed rank test (nonparametric). The p values less than 0.05 were considered significant.
3 Results
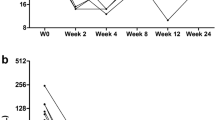
The 50 children included were all aged 12–18 years except 4 patients who were aged less than 12 years but weighed more than 35 kg (1 without and 3 with comorbidities). As shown in Table 1, there was no significant difference between either group with regard to age or sex distribution. At the end of treatment, we found that only 2 patients had mild hepatomegaly, and both had thalassemia. In both groups, 10 patients had elevated liver enzymes at the start of the treatment compared to only 4 patients at the end of the treatment; 2 from 10 patients (1 without comorbidity and the other with) showed improved INR. The reduction of liver enzymes reached statistical significance, but INR did not. On the other hand, total bilirubin increased, which can be explained by non-compliance with ursodeoxycholic acid by some patients (Tables 2 and 3). Platelet count decreased in HCV patients with comorbidities but was still in the normal range, so this may be related to the chronic conditions of the patients (Table 3).
A comparison between patients without comorbidities and those with comorbidities was made before and at the end of the treatment. Pretreatment comparison showed no statistical difference except for hemoglobin (HB) and INR; this was explained by the chronic situation of patients with comorbidities (Table 4). End of treatment comparison showed no statistical difference between groups (Table 5).
All adverse effects and frequencies observed among our patients were summarized in Table 6 to determine the drug’s safety profile. The most encountered adverse effects were headaches, drowsiness, fatigue, abdominal pain, nausea, vomiting, chest pain, and diarrhea. Headache was the most common side effect observed in 24 % of cases, followed by drowsiness in 18 %, and fatigue in 12 %.
Assessment of HCV RNA by real-time PCR was performed one and three months after the end of therapy to assess drug efficacy. It was reported that all included patients turned negative after one month, and all had SVR. This indicates 100 % efficacy of the drug.
4 Discussion
The current study evaluated the safety and efficacy of a combined sofosbuvir/ledipasvir regimen in children with chronic HCV, genotype 4 infections. Our patients who received 12 weeks of a single daily tablet of ledipasvir/sofosbuvir (90/400 mg) showed statistically significant improvement in laboratory parameters of liver function, such as a decline in the levels of liver enzymes ALT and AST in both groups.
The most common adverse effects observed among our patients were headache, drowsiness, and fatigue. A few patients complained of nausea, vomiting, chest pain, and abdominal pain. Fortunately, most documented side effects were transient during the first two weeks of therapy and were well tolerated by those affected. None required drug discontinuation. Eleven patients without comorbidity and 12 with comorbidities did not experience side effects or discomfort. Ledipasvir/sofosbuvir is a safe and well-tolerated drug, similar to that observed in adolescents in other studies [26][26]. A systematic review of a meta-analysis from 2020 showed that headache was the most common side effect in children and adolescents (19.9 %), followed by fatigue (13.3 %) [28].
After assessing HCV RNA by PCR one and three months after the end of protocol therapy, it was clear that SVR was achieved in 100 % of our patients in both groups. This agrees with another Egyptian study published in 2018, which included 40 children (associated comorbid conditions in 27 patients) with chronic HCV infection genotype 4 and reported an overall SVR-12 rate in 100 % of cases with a reduction in liver enzymes and mild adverse effects. The age and weight criteria in the included children was the same as the current study. However, they included both treatment-naïve and treatment-experienced patients [29].
Another multicenter study in 2017 concluded that use of a combination tablet of 90 mg ledipasvir and 400 mg sofosbuvir once daily for 12 weeks achieved SVR in 98 % of patients. The two patients who did not achieve SVR were lost during follow-up. The most observed adverse events were headache (27 % of patients), diarrhea (14 %), and fatigue (13 %). No serious adverse events were reported. The study included adolescents aged between 12 and 17 years but with genotype 1 [26].
Similar results were reported from a prospective multicenter study on chronic HCV, genotype 4 patients, with SVR-12 observed in 142/144 patients (99 %) and minimal tolerable adverse effects [27].
Another study in 2019 reported 100 % efficacy of the same drug regimen in adolescents. However, abdominal pain was the most common side effect, unlike our study, where the headache was the most common [30].
An Egyptian study carried out in 2019 included 157 patients randomly assigned to two groups according to the duration of therapy: one group for 8 weeks (73 patients) and the second group for 12 weeks (84 patients). Patients had the same age and weight criteria as in our but were either treatment-naïve or experienced. Sustained virologic response-12 was 98.6 % in patients treated for 8 weeks and 97.6 % in patients treated for 12 weeks. In all patients, no serious adverse effects led to treatment discontinuation, death, or hepatic decompensation [31].
The same drug regimen was applied to a cohort of treatment-naïve patients chronically infected with HCV, with various genotypes 1a, 1b, or 4, with or without cirrhosis. All patients exhibited SVR 12 weeks after completion of treatment with minimal side effects reported [32].
Further studies that were carried out on younger age groups, 3–6 years and lighter weights (< 35 kg), revealed similar safety and efficacy. They used a combination tablet of 45 mg ledipasvir and 200 mg sofosbuvir; the efficacy was similar between 8 and 12 weeks of therapy, with 100 % SVR-12 rates of 100 %. Nonspecific side effects were observed in all patients [33].
A recent prospective study that included 30 treatment-naïve children without cirrhosis aged 4–10 years with chronic HCV infection revealed SVR-12 in 100 % of cases after a short 8-week course, with minimal side effects [34].
Schwartz et al in 2020 conducted a multicenter study on patients aged 3–6 years with chronic HCV infections, genotypes 1, 3, 4, 5, or 6. The duration of treatment was 12 weeks for all patients except those with cirrhosis secondary to genotype 1 infection who received treatment for 24 weeks. Patients received weight-based doses of ledipasvir/sofosbuvir fixed-dose combination granules once daily. Overall, 97 % of patients achieved SVR-12 with mild side effects [35].
There are several studies like ours that specifically target the population of chronic HCV with comorbidities. Fourteen patients with thalassemia who had chronic HCV genotype 3 and were treatment-naïve without cirrhosis received sofosbuvir 400 mg/daclatasvir 80 mg daily for 12 weeks. All patients had achieved 100 % SVR, with substantial reductions in liver enzymes [36]. Makhlouf et al recruited adolescents with and without hematological disorders who had chronic HCV and received sofosbuvir 400 mg/ledipasvir 90 mg daily for 12 weeks. All patients attained SVR-12 100 % [37]. Many studies performed on adults diagnosed with chronic HCV with blood disorders showed the efficacy and safety of DAA [13, 38, 39].
Patients with malignancies show ALT flares during chemotherapy and have been associated with more rapid liver disease progression and a higher risk of malignancy relapse due to chemotherapy interruption. Jakhar et al reported a case of an HCV-infected child with acute leukemia treated with sofosbuvir/velpatasvir simultaneously with the chemotherapy regimen. The child tolerated the full dose of chemotherapy along with DAA for 12 weeks and was in remission with SVR [17]. An Egyptian study had recruited patients aged 12–18 years with chronic HCV genotype 1 or 4 infection receiving chemotherapy for hematological malignancies who were given sofosbuvir/ledipasvir (400 mg/90 mg) once daily for 12 weeks. All patients completed treatment and achieved SVR-12, 100 % [40]. Another Egyptian prospective, uncontrolled, open-label multicenter study recruited 20 children infected with chronic HCV, genotype 4, who had been in continuous complete remission from leukemia and lymphoma for at least one year. All patients were treated with combined sofosbuvir/daclatasvir for 12 weeks, and all achieved 100 % SVR-12 [41].
5 Limitations
The limitations of our study are the relatively small number of patients and the short-term follow-up duration. The patients with comorbidities are a large population and should be divided into subgroups according to their chronic disease to study each special population. Further long-term follow-up studies for adolescents are recommended to observe rare side effects of the new antiviral drugs and to detect the expected high risk of reinfection in the setting of horizontal transmission.
6 Conclusion
Treatment with ledipasvir/sofosbuvir fixed-dose combination of one tablet (90 mg ledipasvir, 400 mg sofosbuvir) was well tolerated in Egyptian children and adolescents aged 12 to 18 years or weighing at least 35 kg with treatment-naïve, chronic HCV infection genotype 4 either with or without underlying chronic disease and it achieved 100 % SVR-12 with negligible side effects.
The World Health Organization (WHO) has targeted the eradication of hepatitis C by 2030, as a public health issue [42]. Unfortunately, access to anti-HCV drugs will be vital in low- and middle-income countries, such as Egypt, where the virus is most widespread, to achieve this target [1]. A micro-elimination approach in pediatric patients with comorbidities may be a strategy. These individuals may have a high prevalence rate, especially in resource-limited settings, and are more prone to spread infection through nosocomial transmission and are at risk for consequences that develop more rapidly [40].
Change history
17 October 2022
Missing Open Access funding information has been added in the Funding Note
References
Schmelzer J, Dugan E, Blach S, Coleman S, Cai Z, DePaola M, Estes C, Gamkrelidze I, Jerabek K, Ma S, Montoya S, Razavi-Shearer D, Razavi-Shearer K, Robbins-Scott S, Razavi H, El Sayed MH. Global prevalence of hepatitis C virus in children in 2018: a modelling study. Lancet Gastroenterol Hepatol. 2020;5(4):374–92. https://doi.org/10.1016/S2468-1253(19)30385-1.
El-Zanaty F, Ministry of Health and Population [Egypt], El-Zanaty and Associates [Egypt], ICF International, Egypt health issues survey 2015. 2015, Ministry of Health and Population, ICF International Cairo, Rockville. 2015.
Bortolotti F, Verucchi G, Cammà C, Cabibbo G, Zancan L, Indolfi G, Giacchino R, Marcellini M, Marazzi MG, Barbera C, Maggiore G, Vajro P, Bartolacci S, Balli F, Maccabruni A, Guido M, IOfHIaHCi Children. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology. 2008;134(7):1900–7. https://doi.org/10.1053/j.gastro.2008.02.082.
Indolfi G, Guido M, Azzari C, Resti M. Histopathology of hepatitis C in children, a systematic review: implications for treatment. Expert Rev Anti Infect Ther. 2015;13(10):1225–35. https://doi.org/10.1586/14787210.2015.1070668.
González-Peralta RP, Langham MR, Andres JM, Mohan P, Colombani PM, Alford MK, Schwarz KB. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr. 2009;48(5):630–5. https://doi.org/10.1097/MPG.0b013e318170af04.
Rumbo C, Fawaz RL, Emre SH, Suchy FJ, Kerkar N, Morotti RA, Shneider BL. Hepatitis C in children: a quaternary referral center perspective. J Pediatr Gastroenterol Nutr. 2006;43(2):209–16. https://doi.org/10.1097/01.mpg.0000228117.52229.32.
Indolfi G, Easterbrook P, Dusheiko G, El-Sayed MH, Jonas MM, Thorne C, Bulterys M, Siberry G, Walsh N, Chang MH, Meyers T, Giaquinto C, Wirth S, Chan PL, Penazzato M. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4(6):477–87. https://doi.org/10.1016/S2468-1253(19)30046-9.
Nydegger A, Srivastava A, Wake M, Smith AL, Hardikar W. Health-related quality of life in children with hepatitis C acquired in the first year of life. J Gastroenterol Hepatol. 2008;23(2):226–30. https://doi.org/10.1111/j.1440-1746.2007.04859.x.
Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, Murray KF, Narkewicz MR, Rosenthal P, Smith LJ, Schwarz KB, Robuck P, Barton B, González-Peralta RP. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr. 2009;48(3):341–7. https://doi.org/10.1097/MPG.0b013e318185998f.
Indolfi G, Serranti D, Resti M. Direct-acting antivirals for children and adolescents with chronic hepatitis C. Lancet Child Adolesc Health. 2018;2(4):298–304. https://doi.org/10.1016/S2352-4642(18)30037-3.
Indolfi G, Hierro L, Dezsofi A, Jahnel J, Debray D, Hadzic N, Czubkowski P, Gupte G, Mozer-Glassberg Y, van der Woerd W, Smets F, Verkade HJ, Fischler B. Treatment of chronic hepatitis C virus infection in children: a position paper by the hepatology committee of European Society of paediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):505–15. https://doi.org/10.1097/MPG.0000000000001872.
American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) HCV Guidance. Recommendations for Testing, Managing, and Treating Hepatitis C. 2021. www.hcvguidelines.org. Accessed 23 Apr 2022.
Ruiz I, Fourati S, Ahmed-Belkacem A, Rodriguez C, Scoazec G, Donati F, Soulier A, Demontant V, Poiteau L, N’Debi M, François M, Chevaliez S, Pawlotsky JM. Real-world efficacy and safety of direct-acting antiviral drugs in patients with chronic hepatitis C and inherited blood disorders. Eur J Gastroenterol Hepatol. 2021;33(1):e191–6. https://doi.org/10.1097/MEG.0000000000002003.
Di Marco V, Capra M, Angelucci E, Borgna-Pignatti C, Telfer P, Harmatz P, Kattamis A, Prossamariti L, Filosa A, Rund D. Management of chronic viral hepatitis in patients with thalassemia: recommendations from an international panel. Blood J Am Soc Hematol. 2010;116(16):2875–83.
Torres HA, Mahale P, Blechacz B, Miller E, Kaseb A, Herlong HF, Fowler N, Jiang YR II, Kontoyiannis DP. Effect of hepatitis C virus infection in patients with cancer: addressing a neglected population. J Natl Compr Canc Netw. 2015;13(1):41–50. https://doi.org/10.6004/jnccn.2015.0007.
Torres HA, Shigle TL, Hammoudi N, Link JT, Samaniego F, Kaseb A, Mallet V. The oncologic burden of hepatitis C virus infection: a clinical perspective. CA Cancer J Clin. 2017;67(5):411–31. https://doi.org/10.3322/caac.21403.
Jakhar N, Gera A, Mittal R, Mehndiratta SS, Singh A. Treatment of Hepatitis C in a Case Of Pediatric B-cell acute leukemia. J Glob Infect Dis. 2020;2020:35-37. https://doi.org/10.4103/jgid.jgid_1_21.
Gupta E, Bajpai M, Choudhary A. Hepatitis C virus: screening, diagnosis, and interpretation of laboratory assays. Asian J Transfus Sci. 2014;8(1):19–25. https://doi.org/10.4103/0973-6247.126683.
Hagiwara S, Nishida N, Watanabe T, Sakurai T, Ida H, Minami Y, Takita M, Minami T, Iwanishi M, Chishina H, Ueshima K, Komeda Y, Arizumi T, Kudo M. Outcome of combination therapy with sofosbuvir and ledipasvir for chronic type C liver disease. Oncology. 2017;92(Suppl 1):3–9. https://doi.org/10.1159/000451010.
Maruyama A, Partovi N, Yoshida EM, Erb SR, Azalgara VM, Hussaini T. A review of direct-acting antivirals for the treatment of hepatitis C in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2017;32(1):35–41. https://doi.org/10.1093/ndt/gfv361.
Premji R, Roopnarinesingh N, Qazi N, Nylen ES. New-onset diabetes mellitus with exposure to ledipasvir and sofosbuvir. J Investig Med High Impact Case Rep. 2015;3(4):2324709615623300. https://doi.org/10.1177/2324709615623300.
Yanny BT, Latt NL, Saab S, Han S, Choi G, Kramer J, Sahota AK. Risk of Hepatitis B virus reactivation among patients treated with ledipasvir-sofosbuvir for hepatitis C virus infection. J Clin Gastroenterol. 2018;52(10):908–12. https://doi.org/10.1097/MCG.0000000000000986.
Berenguer J, Calleja JL, Montes ML, Gil Á, Moreno A, Bañares R, Aldámiz-Echevarría T, Albillos A, Téllez MJ, Olveira A, Domínguez L, Fernández I, García-Samaniego J, Polo BA, Álvarez B, Ryan P, Barrio J, Devesa MJ, Benítez L, Santos I, Buey LG, Sanz J, Poves E, Losa JE, Fernández-Rodríguez C, Jarrín I, Calvo MJ, González-García J. HIV Coinfection Predicts Failure of Ledipasvir/Sofosbuvir in Treatment-Naïve Noncirrhotic Patients With HCV Genotype 1. Open Forum Infect Dis. 2019;6(5):ofz214. https://doi.org/10.1093/ofid/ofz214.
Gencay M, Hübner K, Gohl P, Seffner A, Weizenegger M, Neofytos D, Batrla R, Woeste A, Kim HS, Westergaard G, Reinsch C, Brill E, Thu Thuy PT, Hoang BH, Sonderup M, Spearman CW, Pabinger S, Gautier J, Brancaccio G, Fasano M, Santantonio T, Gaeta GB, Nauck M, Kaminski WE. Ultra-deep sequencing reveals high prevalence and broad structural diversity of hepatitis B surface antigen mutations in a global population. PLoS ONE. 2017;12(5): e0172101. https://doi.org/10.1371/journal.pone.0172101.
Mitchell AH, Pannell MA, Arbury S, Thomas R, Hodgson MJ. Bloodborne pathogens standard enforcement at the occupational safety and health administration: the first twenty-five years. New Solut. 2019;29(2):172–85. https://doi.org/10.1177/1048291119840077.
Balistreri WF, Murray KF, Rosenthal P, Bansal S, Lin CH, Kersey K, Massetto B, Zhu Y, Kanwar B, German P, Svarovskaia E, Brainard DM, Wen J, Gonzalez-Peralta RP, Jonas MM, Schwarz K. The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12–17 years old with hepatitis C virus genotype 1 infection. Hepatology. 2017;66(2):371–8. https://doi.org/10.1002/hep.28995.
El-Khayat HR, Kamal EM, El-Sayed MH, El-Shabrawi M, Ayoub H, RizK A, Maher M, El Sheemy RY, Fouad YM, Attia D. The effectiveness and safety of ledipasvir plus sofosbuvir in adolescents with chronic hepatitis C virus genotype 4 infection: a real-world experience. Aliment Pharmacol Ther. 2018;47(6):838–44. https://doi.org/10.1111/apt.14502.
Indolfi G, Giometto S, Serranti D, Bettiol A, Bigagli E, De Masi S, Lucenteforte E. Systematic review with meta-analysis: the efficacy and safety of direct-acting antivirals in children and adolescents with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2020;52(7):1125–33. https://doi.org/10.1111/apt.16037.
El-Karaksy H, Mogahed EA, Abdullatif H, Ghobrial C, El-Raziky MS, El-Koofy N, El-Shabrawi M, Ghita H, Baroudy S, Okasha S. Sustained viral response in genotype 4 chronic hepatitis C virus-infected children and adolescents treated with sofosbuvir/ledipasvir. J Pediatr Gastroenterol Nutr. 2018;67(5):626–30. https://doi.org/10.1097/MPG.0000000000002101.
Fouad HM, Ahmed Mohamed A, Sabry M, Abdel Aziz H, Eysa B, Rabea M. The Effectiveness of ledipasvir/sofosbuvir in youth with genotype 4 hepatitis C virus: a single Egyptian center study. Pediatr Infect Dis J. 2019;38(1):22–5. https://doi.org/10.1097/INF.0000000000002189.
El-Khayat H, Kamal EM, Yakoot M, Gawad MA, Kamal N, El Shabrawi M, Sameh Y, Haseeb A, Fouad Y, Attia D. Effectiveness of 8-week sofosbuvir/ledipasvir in the adolescent chronic hepatitis C-infected patients. Eur J Gastroenterol Hepatol. 2019;31(8):1004–9. https://doi.org/10.1097/MEG.0000000000001360.
Quintero J, Juampérez J, Julio E, Cabello V, Mercadal-Hally M, Soler-Palacín P, Segarra Ó, Rodrigo C. Ledipasvir/sofosbuvir combination for chronic hepatitis C infection in children and adolescents. An Pediatr (Engl Ed). 2019;90(3):141–7. https://doi.org/10.1016/j.anpedi.2018.07.007.
Kamal EM, El-Shabrawi M, El-Khayat H, Yakoot M, Sameh Y, Fouad Y, Attia D. Effects of sofosbuvir/ledipasvir therapy on chronic hepatitis C virus genotype 4, infected children of 3–6 years of age. Liver Int. 2020;40(2):319–23. https://doi.org/10.1111/liv.14308.
Behairy BE, El-Araby HA, El-Guindi MA, Basiouny HM, Fouad OA, Ayoub BA, Marei AM, Sira MM. Safety and efficacy of 8 weeks ledipasvir/sofosbuvir for chronic hepatitis C genotype 4 in children aged 4–10 years. J Pediatr. 2020;219:106–10. https://doi.org/10.1016/j.jpeds.2019.12.034.
Schwarz KB, Rosenthal P, Murray KF, Honegger JR, Hardikar W, Hague R, Mittal N, Massetto B, Brainard DM, Hsueh CH, Shao J, Parhy B, Narkewicz MR, Rao GS, Whitworth S, Bansal S, Balistreri WF. Ledipasvir-sofosbuvir for 12 weeks in children 3 to <6 years old with chronic hepatitis C. Hepatology. 2020;71(2):422–30. https://doi.org/10.1002/hep.30830.
Padhi S, Maharshi S, Gupta GK, Garg K, Nijhawan S. Efficacy and safety of direct acting antiviral therapy for chronic hepatitis C in thalassemic children. J Pediatr Hematol Oncol. 2018;40(7):511–4. https://doi.org/10.1097/MPH.0000000000001217.
Makhlouf NA, Abdelmalek MO, Ibrahim ME, Abu-Faddan NH, Kheila AE, Mahmoud AA. Ledipasvir/sofosbuvir in adolescents with chronic hepatitis C Genotype 4 with and without hematological disorders: virological efficacy and impact on liver stiffness. J Pediatric Infect Dis Soc. 2021;10(1):7–13. https://doi.org/10.1093/jpids/piaa006.
Hézode C, Colombo M, Bourlière M, Spengler U, Ben-Ari Z, Strasser SI, Lee WM, Morgan L, Qiu J, Hwang P, Robertson M, Nguyen BY, Barr E, Wahl J, Haber B, Chase R, Talwani R, Marco VD, C-EIS Investigators. Elbasvir/grazoprevir for patients with hepatitis C virus infection and inherited blood disorders: a phase III study. Hepatology. 2017;66(3):736–45. https://doi.org/10.1002/hep.29139.
Mehta R, Kabrawala M, Nandwani S, Desai P, Bhayani V, Patel S, Parekh V. Safety and efficacy of sofosbuvir and daclatasvir for hepatitis C virus infection in patients with β-thalassemia major. J Clin Exp Hepatol. 2018;8(1):3–6.
El-Sayed MH, Ebeid FSE, Zekri AR, Massetto B, Kersey K, Zhang F, Gaggar A, Elsayed W, El-Haddad A. Ledipasvir-sofosbuvir in adolescents with chronic hepatitis C and hematological malignancies undergoing chemotherapy. J Pediatr Gastroenterol Nutr. 2022. https://doi.org/10.1097/mpg.0000000000003406.
El-Shabrawi MH, Sherief LM, Yakoot M, Kamal NM, Almalky MA, AbdElgawad MM, Mahfouz AA, Helmy S, Kamal EM, Attia D, El-Khayat HR. Effects of dual sofosbuvir/daclatasvir therapy on, chronic hepatitis C infected, survivors of childhood malignancy. World J Clin Cases. 2019;7(16):2247–55. https://doi.org/10.12998/wjcc.v7.i16.2247.
World Health Organization, Global hepatitis report. 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Conflicts of interest
Othman AbouBakr, Mohammed Ezz El Regal, Amr Ali Sarhan, Maysaa El Sayed Zaki, and Ahmed Noaman have no relevant financial or non-financial interests to disclose.
Availability of data and material
The data presented in this study are available on request from the corresponding author.
Code availability
Not applicable.
Authors’ contributions
All share the idea, concept, and design in addition of Othman AbouBakr: performed the clinical trial, procedures, and writing. Amr Ali Sarhan: guidance, supervision, and revision of the article. Mohammed Ezz El Regal: shared concept, design of the study, and revision of the article. Maysaa El Sayed Zaki: follow-up materials and equipment and review of laboratory results. Ahmed Noaman: performed the clinical trial, procedures, and writing.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Mansoura University-Faculty of Medicine (Date 16/12/2017/No MD/17.11.92).”
Consent to participate
Written informed consent was obtained from the parents of the patients.
Consent for publication
Not applicable.
Additional information
The original online version of this article was revised: The Open Access funding information was missed and published in the original version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
AbouBakr, O., Ezz El Regal, M., Sarhan, A.A. et al. Safety and Efficacy of Ledipasvir/Sofosbuvir in the Treatment of Chronic Hepatitis C Virus Infection in Treatment-Naïve Children without and with Comorbidities. Pediatr Drugs 24, 529–537 (2022). https://doi.org/10.1007/s40272-022-00522-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00522-1