Abstract
Objective
Our objective was to describe the efficacy and safety of furosemide and chlorothiazide combination continuous infusion (FCCCI) in children in a pediatric intensive care unit (ICU), including postoperative cardiac patients.
Methods
This was a retrospective cohort study in a pediatric ICU within a tertiary care teaching hospital. Children aged < 18 years admitted from 1 January 2010 to 31 December 2019 were included if they received a furosemide infusion for at least 6 h and then transitioned to FCCCI. Each patient acted as their own control.
Results
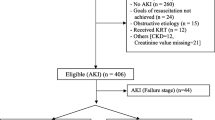
A total of 203 patients (107 [53%] postoperative cardiac) met the study inclusion criteria. The study population was 55% male and 74% Caucasian, with a median age of 4.9 months. Of the total patients, 143 (70.4%) required mechanical ventilation and 39 (19.2%) required dialysis. The median duration of furosemide and FCCCI was 24.6 h (interquartile range [IQR] 12.4–54) and 41 h (IQR 15–162), respectively. Urine output increased by 52% with FCCCI (mean increase of 2.2 mL/kg/h [95% confidence interval {CI} 1.8–2.5]; p < 0.01). The change to FCCCI led to a net negative daily fluid balance (mean difference − 301.9 mL/day [95% CI − 390.9 to − 212.9]; p < 0.01). FCCCI resulted in a greater requirement for potassium bolus supplementation (mean increase of 12.8 boluses [95% CI 8.5–17.2]; p < 0.01) and a small but statistically significant increase in serum creatinine (mean difference 0.1 mg/dL [95% CI 0.06–0.14]; p < 0.01) with a resultant decrease in estimated glomerular filtration rate (mean difference − 13.5 [95% CI 9.7–17.4]; p < 0.01). Of the furosemide-refractory patients, 78.9% were responsive to FCCCI. Younger patients and patients who underwent cardiothoracic surgery were more likely to be responsive. Nonresponders to FCCCI had slightly higher mortality (21 vs. 6.6%, p = 0.05).
Conclusions
FCCCI resulted in a significant improvement in diuresis with achievement of negative fluid balance in pediatric ICU patients. FCCCI is a reasonable approach to aggressive diuresis in the pediatric patient, particularly in patients with limited access. Serum potassium should be routinely monitored during such therapy.

Similar content being viewed by others
References
Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
Sinitsky L, Walls D, Nadel S, et al. Fluid overload at 48 hours is associated with respiratory morbidity but not mortality in a general PICU: retrospective cohort study. Pediatr Crit Care Med. 2015;16(3):205–9.
Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–80.
Carpenter RJ, Kouyoumjian S, Moromisato DY, et al. Lower-dose, intravenous chlorothiazide is an effective adjunct diuretic to furosemide following pediatric cardiac surgery. J Pediatr Pharmacol Ther. 2020;25(1):31–8.
Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527–34.
Congenital Heart Surgery Public Reporting [Internet]. Chicago (IL): The Society of Thoracic Surgeons. 2021. https://publicreporting.sts.org/chsd-exp. Accessed 13 Mar 2021.
McIntosh AM, Tong S, Deakyne SJ, et al. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med. 2017;18(8):750–7.
Schwartz GJ, Haycock GB, Edelmann CM Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–63.
Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–61.
2020 ASP Drug Pricing Files [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services. 2020. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2020-asp-drug-pricing-files. Accessed 13 Mar 2021.
Klinge J, Scharf J, Hofbeck M, et al. Intermittent administration of furosemide versus continuous infusion in the postoperative management of children following open heart surgery. Intensive Care Med. 1997;23(6):693–7.
Singh N, Kissoon N, Bennett M, et al. Comparison of continuous versus intermittent furosemide administration in postoperative pediatric cardiac patients. Crit Care Med. 1992;20(1):17–21.
Moffett BS, Tsang R, Kennedy C, et al. Efficacy of sequential nephron blockade with intravenous chlorothiazide to promote diuresis in cardiac intensive care infants. Cardiol Young. 2017;27(6):1104–9.
Cies JJ, Moore WS, Chopra A, et al. Stability of furosemide and chlorothiazide stored in syringes. Am J Health Sys Pharm. 2015;72(24):2182–8.
Thomas CA, Morris JL, Sinclair EA, et al. Implementation of a diuretic stewardship program in a pediatric cardiovascular intensive care unit to reduce medication expenditures. Am J Health Sys Pharm. 2015;72(12):1047–105.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
Summer Record, Aaron Harthan, and Sandeep Tripathi have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
University of Illinois College of Medicine institutional review board (IRB #1576231, 04/29/20)
Consent to participate
The need for informed consent was waived by the institutional review board.
Consent for publication
Not applicable.
Availability of data and material
Data are available upon request from the authors.
Code availability
Not applicable.
Authors' contributions
Summer Record participated in researching previous literature, collecting data, analysis, methodology, and writing. Aaron Harthan participated in conceptualization, analysis, methodology, researching previous literature, reviewing, and editing. Sandeep Tripathi participated in project administration, conceptualization, analysis, methodology, supervision, writing, reviewing, and editing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Record, S.R., Harthan, A.A. & Tripathi, S. Use of Furosemide and Chlorothiazide Combination Continuous Infusion in Furosemide-Refractory Patients in the Pediatric Intensive Care Unit: A Retrospective Cohort Study. Pediatr Drugs 23, 575–582 (2021). https://doi.org/10.1007/s40272-021-00472-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00472-0