Abstract
Objective
To evaluate the effects of furosemide administration in critically ill children on the progression of acute kidney injury (AKI) and its outcomes compared to those not receiving furosemide.
Method
A retrospective cohort study involving children aged 1 month (corrected) to 13 years admitted to the pediatric intensive care unit (PICU) and who were diagnosed with AKI within 24 h was screened for enrollment. Those who received furosemide are classified as the furosemide group, and others as no-furosemide group. The primary outcome was the proportion of patients with AKI (risk or injury stage) progressing to a higher stage. The secondary outcomes were kidney replacement therapy (KRT), fluid balance (%FO), urine output, multi-organ dysfunction, kidney recovery, length of mechanical ventilation, hospital stay including PICU, and all-cause mortality (PICU and hospital).
Results
Three hundred sixty-two patients’ data [furosemide group, n = 182; no-furosemide group, n = 180] were enrolled. The median (IQR) pediatric risk of mortality–III score was similar between groups [10, 4–16 vs. 10, 4–16; p = 0.244]. The primary outcome occurred in 51 (28%) in the furosemide and 36 (20%) in the no-furosemide group. The difference was not statistically significant [RR = 1.40, 95% CI 0.96 to 2.04, p = 0.074]. Higher mean (SD) urine output (ml/kg/hr) was noted in the furosemide group [2.3 (0.9) vs. 1.4 (0.6); p = < 0.001). Significantly higher mean (SD) organ dysfunction score [10 (4) vs. 8.3 (4.4); p < 0.001) and increased median (IQR) length of stay in mechanical ventilation [4, 3–6 vs. 3, 2–6 days; p < 0.001] and hospital [8, 5–11 vs. 6, 5–8 days; p < 0.001] and lower kidney recovery at discharge was noted in the furosemide group [n = 86, 47.3% vs. n = 104, 57.8%; RR = 0.80, 95% CI 0.64 to 0.99; p = 0.044]. No difference was noted in all-cause mortality, fluid balance, and KRT requirement.
Conclusion
Furosemide infusion in AKI management did not reduce the progression to a higher stage of AKI. Nevertheless, it was associated with higher morbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About one-fourth of children admitted to the pediatric intensive care unit (PICU) develop acute kidney injury (AKI), with incidence ranging from 5 to 82% [1,2,3]. The etiology of AKI has shifted from primary kidney disease to multifactorial causes, particularly in critically ill children, and AKI is independently associated with increased mortality and resource utilization [1, 3, 4]. AKI management is primarily supportive care. Furosemide, a sulfonamide, a potent, short-acting loop diuretic, is used extensively in critical care settings [5,6,7]. It is secreted into proximal convoluted tubules and decreases the activity of the Na–K-Cl cotransporter in the apical membranes of tubular epithelial cells in the thick ascending loop of Henle [6, 7]. However, furosemide is commonly used to decrease fluid overload in PICU [5, 6]. It may help convert oliguric AKI to non-oliguric AKI and helps identify AKI patients at risk of progression as long as volume status is maintained [6, 8].
Nevertheless, furosemide use is not beneficial in preventing and managing AKI if kidney-related endpoints are considered, and the Kidney Disease Improving Global Outcomes (KDIGO) guideline recommends not using furosemide to prevent and manage AKI [6, 8]. Systematic reviews involving adults and limited pediatric studies have confirmed the lack of evidence for mortality benefits and mixed results in other outcomes [5, 7]. The present study aimed to evaluate the effects of furosemide administration in critically ill children on AKI’s progression, recovery, and related outcomes. We hypothesized that administering furosemide would be associated with a lower proportion of progression to a higher stage during a PICU stay.
Materials and methods
The study was a retrospective cohort study conducted in the PICU of tertiary care academic institute from July 2017 to June 2019. The patient’s medical records were screened for eligibility from January 2015 to July 2017. Children aged 1 month (corrected) to 13 years, diagnosed with AKI within 24 h of admission, and achieved immediate resuscitation targets were included in the study. The institutional ethics committee approved the study with a waiver of written informed consent. The immediate resuscitation goals were defined as directed by the treating physician, which included one or more of the following: fluid resuscitation and/or vasoactive therapy to achieve (i) capillary refill of ≤ 2 s, (ii) > 5th percentile mean arterial blood pressure (MABP), (iii) normal pulse volume with no differential peripheral and central pulse, (iv) central venous pressure (CVP) ≥ 8 cmH2O (if measured), (v) central venous oxygen saturation (ScvO2) ≥ 70% (if measured), and (vi) cardiac index between 3.3 and 6.0 L/min/m2 (if measured). AKI was defined by pediatric-Risk, Injury, Failure, Loss, End-stage kidney disease (p-RIFLE) criteria (either serum creatinine or urine output criteria or both) [2]. Children with any of the following conditions were excluded: (i) stage 4 or more chronic kidney disease (CKD); (ii) end-stage kidney disease on kidney replacement therapy (KRT); kidney transplantation, or already received KRT in PICU; (iii) obstructive etiology for AKI; (iv) severe malnutrition; and (v) death within 24 h of admission. Serum creatinine was estimated by the modified Jaffe method using an auto-analyzer (Olympus® AU 680, Beckman Coulter, California, USA).
Creatinine clearance was calculated using the modified Schwartz formula (= length in cm × 0.413 ÷ serum creatinine in mg/dL). If a patient had a documented lowest creatinine value in the past 3 months, the lowest value was taken as the baseline; otherwise, 100 ml/min/1.73 m2 was taken as the baseline [2]. According to unit protocol, patients initially received normal saline and/or 5% dextrose in normal saline at the fluid rate of 80% maintenance by the Holliday Segar formula. The furosemide infusion dose used in our unit was 0.05 to 0.4 mg/kg/h. Those who received furosemide infusion were classified as the furosemide group, and those who not received furosemide were classified as the no-furosemide group. Baseline characteristics, severity score (PRISM-III), organ failure score (Pediatric Logistic Organ Dysfunction—PeLOD), diagnosis, investigation(s) detail, intervention(s) received, nephrotoxic medication(s), fluid balance (FO%), and outcomes data were collected in standard case-report form. Kidney recovery was defined by the return of serum creatinine to within 10% of baseline levels and a spontaneous urine output ≥ 1.0 mL/kg/h for a minimum of 24 h independent of KRT [9]. The fluid balance was calculated by using the fluid overload percentage (FO%) = [(total fluid input in liter – total fluid output in liter ÷ weight at admission in kilogram) × 100] formula [10]. Malnutrition was defined as per Indian standards (weight for age-matched) [10].
The study’s primary outcome was the proportion of patients with AKI (risk or injury stage) progressing to a higher stage. The secondary outcomes were KRT, fluid balance (%FO) (72 h and cumulative), urine output, multi-organ dysfunction, kidney recovery at discharge, length of mechanical ventilation, hospital (including PICU stay), and all-cause mortality (PICU and hospital).
Statistical analysis
The incidence of AKI was 17.2% to 82.3% in critically ill children [2, 11]. With the assumption that furosemide infusion achieves the primary endpoint from 30% to 15%, power of 90% with a two-sided alpha of 5%, and a ratio of 1:1 (unexposed to exposed), the total sample size of 180 is required in each group including the 10% attrition rate. The normality of data was checked with Kolmogorov–Smirnov z test. Continuous variables were compared by Student’s t test if normally distributed or Mann–Whitney U test if the data were non-normally distributed. The proportion was compared by chi-square test (Fisher’s exact test when cell frequencies were < 5). Kaplan–Meier and log-rank test followed by Cox proportional model adjusted for age, gender, and severity were used for time-to-event data. Relative risk and hazard ratio with a 95% confidence interval were calculated wherever appropriate. All tests were two-tailed, and a p value of < 0.05 was considered statistically significant. Data were analyzed using IBM SPSS Statistics (Version 26.0. Armonk, NY).
Results
The study flow is depicted in Fig. 1. AKI at admission was present in 406 patients, and the 362 patients (risk, n = 169, injury, n = 193) data were enrolled. All the patients met creatinine criteria, and 80 (22.1%) met both creatinine and urine output criteria. Baseline characteristics are given in Table 1. Baseline and admission serum creatinine significantly differed between the two groups, but e-GFR, urine output, and severity score were comparable. One-fourth of patients received nephrotoxic medications. More than half of the patients had malnutrition (210/362, 58%). The most common system involved was the respiratory system (124/362, 34.3%), followed by the central nervous system (51/362, 14%). Twenty-six patients (7.1%) had culture-positive sepsis. The most common organism was Pseudomonas aeruginosa (6/26), followed by Staphylococcus aureus (5/26), Klebsiella pneumoniae (2/26), Enterococcus faecalis (2/26), Salmonella Typhi (2/26), and Candida albicans (2/26).
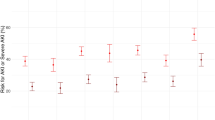
Eighty-seven patients (24%) progressed to the higher stage of AKI. In the furosemide group, a higher proportion of patients progressed to the higher stage of AKI than the no-furosemide group (51/182, 28% vs. 36/180, 20%). However, the difference was not statistically significant (RR = 1.40, 95% CI 0.96 to 2.04, p = 0.074) (Table 2). Among AKI-progressed patients, a significantly higher proportion of patients progressed to the failure stage in the furosemide group and to the injury stage in the no-furosemide group (p = 0.003) (Table 2). No significant difference was noted in the hazard of progression to a higher stage between study groups (adjusted hazard ratio = 1.19, 95% CI 0.76 to 1.86; p = 0.439) (Fig. 2). Subgroup analysis, in the risk stage (n = 169), no significant difference was noted in progression to the higher stage (30/84, 35.7% vs. 31/85, 36.5%, p = 0.918), and in the injury stage (n = 193), a significant difference was noted in progression to a higher stage in furosemide group (21/98, 21.4% vs. 5/95, 5.3%, p = < 0.001) as compared to a no-furosemide group, respectively (Table 2). Mean (SD) cumulative urine output (mL/kg/h) was significantly higher in the furosemide group as compared to the no-furosemide group (p = < 0.001) (Table 2). Significantly higher organ dysfunction scores and increased length of stay in mechanical ventilation, PICU, and hospital were noted in the furosemide group compared to the no-furosemide group (Table 2). Kidney recovery at discharge was significantly lower in the furosemide group (47.3%) as compared to the no-furosemide group (57.8%) (RR = 0.80, 95% CI 0.64 to 0.99; p = 0.044). No significant differences were noted in the two groups’ fluid balance (FO%), the proportion of patients who required KRT, mechanical ventilation, and all-cause PICU, and hospital mortality (Table 2).
Discussion
The current study shows that no significant difference was noted in the progression of AKI to a higher stage in the furosemide and no-furosemide groups. However, significant progression was present in the injury stage to the failure stage in those who received furosemide. Further, furosemide infusion was not associated with mortality benefit but associated with increased urine output, organ dysfunction, length of stay in mechanical ventilation, PICU, and hospital, and decreased kidney recovery at discharge.
Furosemide is widely used to prevent or treat AKI in critically ill children. The kidney medulla receives less blood flow than the cortex, and the high metabolic demand makes it more susceptible to hypoxic damage [12, 13]. Furosemide inhibits sodium transport in the loop of Henle, thus reducing oxygen consumption and ischemic damage to the tubules [7, 13]. It might wash out the necrotic debris and improve kidney function. Furosemide converts oliguric AKI to non-oliguric AKI, thereby makes the fluid administration and nutrition support liberal [7]. Retrospective studies involving post-pediatric cardiac surgery reported the prediction of AKI primarily by using the furosemide stress test (FST). Low urine output after the furosemide dose was independently associated with the development of AKI and prolonged mechanical ventilation and hospital stay. However, no mortality benefit was noted [14,15,16]. Hence, furosemide is not beneficial in treating AKI and may convert oliguric AKI to non-oliguric AKI in certain conditions, namely fluid overload and as long as euvolemia is maintained [6, 7]. Furosemide might aggravate AKI and increase mortality by reducing kidney perfusion, precipitation of Tamm–Horsfall protein in acidic urine, and furosemide-induced vacuolar degeneration of tubular epithelial cell [6, 17].
Urine output response to furosemide varies depending upon administration methods (bolus vs. continuous infusion). Systematic review and meta-analysis found that loading dose followed by continuous infusion was associated with a greater diuretic effect and a net decrease in body weight compared to bolus alone and continuous infusion alone. Nevertheless, it was also associated with a prolonged hospital stay, and there was no mortality or change in kidney function (creatinine and e-GFR) benefits. These effects are seen mainly in adult studies, not pediatric ones [18, 19]. In our unit, furosemide was administrated as a continuous infusion to avoid bolus dose-related complications and was found to have significantly higher urine output. We also found no significant difference in the progression of AKI to the higher stage. However, it was associated with increased organ dysfunction, significantly lower recovery of kidney function, prolonged stay in ventilation and hospital, including PICU stay, and no mortality benefit. The earlier controlled study from the author center also reported that furosemide infusion did not reduce the progression of AKI to the higher stage compared to the placebo [7]. The exact mechanism was not precise. Furosemide is hypothesized to lower oxygen and adenosine triphosphate (ATP) consumption by inhibiting the highly active sodium transporter in the ischemia model [20]. However, the etiology of AKI in PICU is multifactorial and involves complex pathogenesis rather than a simple hypoxia–ischemia model. The mere inhibition of the sodium pump alone might not result in a beneficial effect in a complex AKI environment.
In this study, furosemide significantly increased the risk of progression to a higher stage in the subgroup of patients in the injury stage. Several mechanisms might explain this phenomenon. Furosemide-induced vacuolar degeneration of tubular epithelial cells, activation of the renin–angiotensin–aldosterone system and sympathetic stimulation, and reduction in preload with intense kidney vasoconstriction compromise the kidney medullary blood flow, thereby worsening the AKI [6, 17]. Animal studies also showed preferential blood flow to the kidney cortex, compared to the medulla after furosemide administration [20]. Similar to our study, Levi et al. demonstrated that furosemide increased (68.4%) the chance of developing AKI in patients with sepsis/septic shock [21]. Another study by Wu et al. found that one-fourth of AKI was caused only by furosemide, and glomerular basement membrane injury was higher in a larger dose of furosemide. The most common significant lesion was vacuolar degeneration of tubular epithelial cells [17].
All-cause mortality and KRT requirement were not different between the two groups in this study. Similar to our study results, metanalysis by Bove et al. [22] and Krzych et al. [23] reported that furosemide impacts neither mortality nor the KRT requirement. However, furosemide decreased the mortality in the subpopulation of critically ill adults when it was used as a preventive measure [22]. In contrast, few studies reported increased mortality on furosemide use in AKI [23, 24]. The pathogenesis of AKI is complex, and the conflicting results might be due to differences in the population included, usage as prevention or treatment of AKI, and underlying comorbidities.
Our study is one of the few pediatric studies exploring furosemide’s role in critically ill children with AKI. The sample size is large with varieties of etiology. There are a few limitations. It is a retrospective cohort study. The diuretic effect might be blunted in severe AKI, and a high diuretic dose might be required to produce the same urine output [25]. Because of the retrospective design, the toxicity profile could not be studied. Our study involved patients with heterogenous etiology for AKI and the overall effect was not beneficial. There might be a subpopulation in which diuretics might be beneficial. We did not follow up with the patients for long-term outcomes. Recently, kidney biomarkers for early identification of AKI have emerged. Future prospective controlled studies focusing on diuretic use and emerging biomarkers on early identification of AKI with long-term follow-up are required.
Conclusion
The study concludes that Furosemide infusion in AKI management did not reduce the progression to a higher stage of AKI. Nevertheless, it was associated with higher morbidities.
Availability of data and materials
RR is the guarantor of the paper. RR has full access to data which shall be shared upon request.
References
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators AWARE (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/NEJMoa1611391
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035. https://doi.org/10.1038/sj.ki.5002231
Sanchez-Pinto LN, Goldstein SL, Schneider JB, Khemani RG (2015) Association between progression and improvement of acute kidney injury and mortality in critically ill children. Pediatr Crit Care Med 16:703–710. https://doi.org/10.1097/PCC.0000000000000461
Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, Goldstein SL (2015) AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10:554–561. https://doi.org/10.2215/CJN.01900214
Lethaby D, Cyriac J, Bockenhauer D (2015) Question 1: is the use of furosemide beneficial in the treatment of acute kidney injury in the paediatric population including neonates? Arch Dis Child 100:713–715. https://doi.org/10.1136/archdischild-2015-308472
Patschan D, Patschan S, Buschmann I, Ritter O (2019) Loop diuretics in acute kidney injury prevention, therapy, and risk stratification. Kidney Blood Press Res 44:457–464. https://doi.org/10.1159/000501315
Abraham S, Rameshkumar R, Chidambaram M, Soundravally R, Subramani S, Bhowmick R, Sheriff A, Maulik K, Mahadevan S (2021) Trial of furosemide to prevent acute kidney injury in critically ill children: a double-blind, randomized, controlled trial. Indian J Pediatr 88:1099–1106. https://doi.org/10.1007/s12098-021-03727-3
Kellum JA, Lameire N; KDIGO AKI Guideline Work Group (2013) Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 17:204. https://doi.org/10.1186/cc11454
Bagshaw SM, Gibney RT, McAlister FA, Bellomo R (2010) The SPARK Study: a phase II randomized blinded controlled trial of the effect of furosemide in critically ill patients with early acute kidney injury. Trials 11:50. https://doi.org/10.1186/1745-6215-11-50
Bhanudeep S, Rameshkumar R, Chidambaram M, Selvan T, Mahadevan S (2021) Prospective inverse probability of treatment-weighting analysis of the clinical outcome of red blood cell transfusion practice in critically ill children. Indian J Pediatr 88:985–990. https://doi.org/10.1007/s12098-021-03740-6
Al-Jboor W, Almardini R, Al Bderat J, Frehat M, Al Masri H, Alajloni MS (2016) Acute kidney injury in critically ill child. Saudi J Kidney Dis Transpl 27:740–747. https://doi.org/10.4103/1319-2442.185236
Kramer HJ, Schüürmann J, Wassermann C, Düsing R (1980) Prostaglandin-independent protection by furosemide from oliguric ischemic renal failure in conscious rats. Kidney Int 17:455–464. https://doi.org/10.1038/ki.1980.53
Aravindan N, Aravindan S, Riedel BJ, Weng HR, Shaw AD (2007) Furosemide prevents apoptosis and associated gene expression in a rat model of surgical ischemic acute renal failure. Ren Fail 29:399–407. https://doi.org/10.1080/08860220701263671
Penk J, Gist KM, Wald EL, Kitzmiller L, Webb TN, Li Y, Cooper DS, Goldstein SL, Basu RK (2019) Furosemide response predicts acute kidney injury in children after cardiac surgery. J Thorac Cardiovasc Surg 157:2444–2451. https://doi.org/10.1016/j.jtcvs.2018.12.076
Kakajiwala A, Kim JY, Hughes JZ, Costarino A, Ferguson J, Gaynor JW, Furth SL, Blinder JJ (2017) Lack of furosemide responsiveness predicts acute kidney injury in infants after cardiac surgery. Ann Thorac Surg 104:1388–1394. https://doi.org/10.1016/j.athoracsur.2017.03.015
Borasino S, Wall KM, Crawford JH, Hock KM, Cleveland DC, Rahman F, Martin KD, Alten JA (2018) Furosemide response predicts acute kidney injury after cardiac surgery in infants and neonates. Pediatr Crit Care Med 19:310–317. https://doi.org/10.1097/PCC.0000000000001478
Wu X, Zhang W, Ren H, Chen X, Xie J, Chen N (2014) Diuretics associated acute kidney injury: clinical and pathological analysis. Ren Fail 36:1051–1055. https://doi.org/10.3109/0886022X.2014.917560
Ng KT, Velayit A, Khoo DKY, Mohd Ismail A, Mansor M (2018) Continuous infusion versus intermittent bolus injection of furosemide in critically ill patients: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 32:2303–2310. https://doi.org/10.1053/j.jvca.2018.01.004
Alqahtani F, Koulouridis I, Susantitaphong P, Dahal K, Jaber BL (2014) A meta-analysis of continuous vs intermittent infusion of loop diuretics in hospitalized patients. J Crit Care 29:10–17. https://doi.org/10.1016/j.jcrc.2013.03.015
Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML (1994) Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int 45:981–985. https://doi.org/10.1038/ki.1994.132
Levi TM, Rocha MS, Almeida DN, Martins RT, Silva MG, Santana NC, Sanjuan IT, Cruz CM (2012) Furosemide is associated with acute kidney injury in critically ill patients. Braz J Med Biol Res 45:827–833. https://doi.org/10.1590/s0100-879x2012007500093
Bove T, Belletti A, Putzu A, Pappacena S, Denaro G, Landoni G, Bagshaw SM, Zangrillo A (2018) Intermittent furosemide administration in patients with or at risk for acute kidney injury: Meta-analysis of randomized trials. PLoS One 13:e0196088. https://doi.org/10.1371/journal.pone.0196088
Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Nacedo E, Gibney N, Tolwani A, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators, (2004) Diuretics and mortality in acute renal failure. Crit Care Med 32:1669–1677. https://doi.org/10.1097/01.ccm.0000132892.51063.2f
Mehta RL, Pascual MT, Soroko S, Chertow GM; PICARD Study Group (2002) Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 288:2547–2553. https://doi.org/10.1001/jama.288.20.2547
Ho KM, Walters S, Faulke D, Liang J (2003) Clinical predictors of acute renal replacement therapy in critically ill patients with acute renal impairment. Crit Care Resusc 5:97–102 (PMID: 16573466)
Acknowledgements
We acknowledge the contribution of Mrs. S. Raja Deepa B. Com, MCA (Puducherry, India), for the review and editing of the manuscript; Mr. Rakesh Mohindra (Punjab University, Chandigarh, India), and Miss. Thenmozhi M (M.Sc, Ph.D., Senior Demonstrator, CMC, Vellore, India), for helping with the statistical analysis. Rameshkumar R. is the guarantor of the paper.
Code availability
Data were analyzed using IBM SPSS Statistics (Version 26.0. Armonk, NY), and the code shall not be shared because of restricted access.
Funding
Supported in part by the institutional and departmental funds.
Author information
Authors and Affiliations
Contributions
RR and SM were involved in the management of the patients. SV and PK collected the data, reviewed the literature, and drafted the first manuscript. SM and CGDK contributed to the protocol development and review of the literature and the manuscript. MM, MC, TS, and CGDK interpretation of the analysis, drafting of the manuscript, and critical review. RR conceptualized the study, reviewed the literature, and critically reviewed the manuscript. RR and SM supervised the study. The authors approved the final version of the manuscript. RR is the guarantor of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Name of Institute where the work was performed: Division of Pediatric Critical Care, Department of Pediatrics, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India-605006. Institute Ethics Committee approved the study with a waiver of written consent (Approval Number: JIP/IEC/2017/0201).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vivek, S., Rameshkumar, R., Muthu, M. et al. Furosemide in the management of acute kidney injury in the pediatric intensive care unit—retrospective cohort study. Intensive Care Med. Paediatr. Neonatal 1, 6 (2023). https://doi.org/10.1007/s44253-023-00010-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-023-00010-5