Abstract
Background
Patients with cancer may progress through multiple treatments with differing adverse effect profiles. Moreover, pathways may be fixed or flexible in allowing for escalation or de-escalation of treatment depending on interim outcomes. We sought to develop a methodology capable of estimating preferences for the entirety of a pathway involving a sequence of different treatments.
Methods
Patients with early breast cancer completed an online discrete choice experiment to assess preferences for eight key early breast cancer attributes. Hierarchical Bayesian modeling was used to calculate attribute-level preference weights. Preference weights for hypothetical pathways were estimated by summing the respective weights for efficacy, flexible or fixed pathway, duration, administration regimen, and adverse event risk, the last two of which were time-adjusted by multiplying each weight by the proportion of time spent on a selected treatment.
Results
Increases in the risk of a serious adverse event were most influential in treatment pathway preferences, followed by increases in efficacy and decreases in overall pathway duration. Patients preferred a flexible pathway versus a fixed pathway. Pathway preference estimates fluctuated in a logically consistent manner. Switching from a flexible to a fixed pathway yielded a significantly lower pathway preference. For this same pathway, when adjuvant treatment was replaced with a treatment with a more favorable toxicity profile and shorter duration, it offset the negative impact of the more toxic neoadjuvant chemotherapy.
Conclusions
This novel methodology accounts for patient preference throughout a sequence of treatments, allowing for comparison of preferences across complex treatment pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For complex treatment pathways involving a sequence of treatments over time, there is a need to be able to estimate preferences not just for single treatments but for overall treatment pathways involving multiple treatments. |
A novel method has been implemented, involving estimation of treatment pathway preferences that incorporates discrete choice experiment data comprising both overall pathway and treatment-specific attribute weights. |
This methodology can potentially be applied to diseases where patients may journey through a sequence of treatments that vary in their durations, risks, and modes of administration. |
1 Introduction
A discrete choice experiment (DCE) is a quantitative method that measures the preferences of individuals and allows for the examination of the trade-offs that individuals make for different healthcare service and intervention options [1]. DCEs are increasingly used in healthcare to elicit preferences from participants without directly asking them to state their preferred options [2]. The DCE method is rooted in economic theory and is based on the principle that alternative options can be deconstructed into attributes of interest [3, 4]. The attractiveness of an option to an individual will depend on their relative preferences for these attributes, which is expressed by the frequency with which they choose options with the preferred features in a series of choice tasks. These choice frequencies are then used to estimate the predictive probabilities that will determine whether participants are more likely to choose one option over another [5].
In the health domain, a DCE is typically used to assess preferences for health status or treatments [5, 6]. DCEs that focus on treatment preferences are designed to assess respondents’ willingness to accept trade-offs among hypothetical treatment profiles providing information on key attributes that drive an individual’s treatment choice, such as efficacy, adverse events, etc. Each attribute includes several variations or ‘levels’, e.g., efficacy may be reflected with different rates, or adverse events may be reflected with different levels of risk. The selection of levels for each attribute is based on the realistic range observed across target treatments.
The selection of attributes and levels for a DCE is a relatively straightforward process when the objective is to understand preferences for a set of discrete treatment profiles. However, in several scenarios, a comparison of preferences among different treatment profiles is too limiting. Instead, a comparison of preferences for different treatment pathways, where patients may receive multiple treatments, is more useful to stakeholders. This is particularly relevant in oncology, where patients may not receive just one treatment but may instead receive a series of treatments along a pathway.
As an example, patients with early breast cancer (eBC) may undergo neoadjuvant therapy prior to surgery, as well as adjuvant therapy post-surgery. Moreover, patients may initially be presented with options that include a fixed pathway with a preselected set of treatments to be given along the pathway, versus a flexible pathway allowing for later treatments to be selected based on interim outcomes partway through the pathway, e.g., adjuvant treatment may or may not be administered, depending on the outcome of surgery. Using eBC treatments as an example, this paper outlines a methodology involving the use of a DCE to estimate preferences for a treatment pathway involving a sequence of treatments.
2 Methods
There are three steps for the estimation of preferences for treatment pathways including multiple sequential treatments: (1) implement a DCE including key attributes of the treatment pathways, including overall pathway duration as an attribute; (2) compute attribute-level preference weights based on the DCE data; and (3) sum the relevant preference weights matching to selected pathways, with time-adjustment for treatment-specific attributes.
The first step is to implement a traditional DCE that characterizes attributes of the target treatment pathways (pathway-specific attributes) as well as those that characterize attributes of the treatments that may be given along the pathways (treatment-specific attributes). The design of the DCE should follow the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines for performing conjoint analysis [7].
The study that provided the DCE data for the current analysis received exemption status from Pearl IRB on 4 August 2020 [8]. Specifically, in the current application of the proposed methodology, the first step was to conduct a DCE study in patients with eBC. All patients (N = 452) met the following eligibility criteria: stage I–IIIa breast cancer diagnosis from January 2014 to October 2020; human epidermal growth factor receptor 2-negative; completed surgery and neoadjuvant or adjuvant chemotherapy; and resided in Germany, Italy, or Japan. Table 1 presents the attribute levels and respective preference weights from this study.
To inform the selection of attributes for inclusion in the DCE, qualitative interviews were performed with eight patients with eBC from each participating country. In addition, cognitive interviews, where feedback on the draft questionnaire was obtained, were performed with five patients with eBC in each participating country. The attributes included those pertaining to the overall treatment pathway (3-year cancer-free survival rate, fixed or flexible treatment plan and pathway duration, and treatment-specific attributes [including mode of administration and risks of toxicities]). The experimental design for the DCE tasks, specifically the combinations of levels shown across treatment profiles, was based on a balanced design with minimal overlap [9]. This design optimizes overall efficiency in terms of (1) level balance (each level is shown approximately an equal number of times); (2) minimal level overlap (levels repeat within the same task); and (3) orthogonality (levels may be evaluated independently of other levels). Each respondents answered a different set of 12 DCE choice tasks. Figure 1 presents an example DCE task.
For the second step of the pathway preference estimation, preference weights are computed for the DCE data at the individual level, utilizing an appropriate statistical methodology such as Hierarchical Bayes estimation [10]. In the current analysis, Hierarchical Bayes modeling was used to calculate preference weights at the individual level, using the DCE data from the study (Table 1) [8].
Finally, treatment pathway preferences are calculated by taking the sum of the pathway-specific, attribute-level preference weights plus the treatment-specific, attribute-level preference weights, the latter of which are adjusted for the time spent on each respective treatment. Specifically, the DCE pathway estimation methodology accounts for the duration of exposure to treatment-specific attributes such as toxicity risks or administration regimen, by time-adjusting for the duration of the respective treatments along the pathway. This is done by multiplying the preference weight of the attribute level (e.g., the percentage risk of a selected adverse effect) matching to a treatment by the proportion of time spent on that treatment relative to the overall pathway duration. The time adjustment is the length of time that the treatment lasts, divided by the length of the treatment pathway.
As with traditional DCE weights, if there is interest in identifying a preference weight for a level within an attribute characterized by numeric data, the preference weight for this level can be linearly interpolated based on the available weights for other levels of the attribute. It should be noted that if the preference weights across levels for a numeric attribute do not support linearity, then it is best to linearly interpolate between the two levels used in the DCE that are closest to the target number. It is also important to note that before proceeding with pathway preference estimation, the DCE preference weights should be tested to examine whether there are any significant interactions between the attributes or main effects. A lack of significant interactions among the main effects helps to support treating the attributes as additive in our proposed summation approach. In the current study, no significant interactions were observed among the attributes in the eBC study. If significant interactions were found, then these would have been incorporated into the proposed summative equation for pathway preference estimation. Finally, it should be noted that the pathway preference weights reported in this paper were based on preference weights from a DCE that was completed by patients with stage I–IIIa breast cancer. If one is interested in assessing pathway preferences for patients at a specific disease stage (i.e., stage I, II, or IIIa), this would require generation of preference weights from that specific population.
The proposed approach to adjust the relevant attributes for time on specific treatments is consistent with a well-recognized approach for calculating quality-adjusted life-years (QALYs). The QALY approach assumes that patients’ health comprises their health-related quality of life (QoL) and the duration of this health state [11]. QALYs are calculated by multiplying QoL by the time spent in this state. The best possible QoL for patients is signified by 1.0, and the worst (i.e., death) is signified by 0.0. Thus, 1 year with a QoL value of 1.0 equates to one QALY, which is identical to 2 years with a QoL value of 0.5 [12].
This paper applies this methodology for treatment pathway preference estimation, using DCE preference weights from the previously cited DCE study in eBC [8]. The analyses examine how the estimated pathway preference weights vary depending on the changes to pathways. Flexible and fixed pathways are the two types of clinical pathway incorporated into the estimation approach (Fig. 2). A flexible pathway is one in which the decision about providing adjuvant treatment is dependent on the outcomes of surgery. In this example, adjuvant treatment would only be given if there was not a pathological complete response (pCR) after surgery. Otherwise, patients would receive no treatment but would continue to be monitored. In this respect, the pathway is flexible based on the outcome of surgery. The second pathway is fixed, whereby patients receive adjuvant treatment regardless of the result of surgery.
In addition, the likelihood estimates of preferring different selected treatment pathways compared with a flexible pathway (referenced as the base case) were computed using the preference share formula (Eq. 1):
where U0 is the summed utility of the base case used for comparison, and Ui is the summed utility of the alternative [13].
3 Results
Table 2 shows an example computation of the preference weight for a fixed pathway that includes three sequential treatments. The pathway-specific DCE weights are listed in the first column; these include the probability of being cancer-free at 3 years (87%), with a weight of 1.23, type of treatment plan (fixed), with a weight of −0.35, and total duration of pathway (18 months), with a weight of −0.83. If the overall duration of treatment is 18 months and the time spent on treatment 1, treatment 2, and treatment 3 is 3, 3, and 12 months, respectively, we time-adjust for each treatment by multiplying the sum of the treatment-specific preference weight by the proportion of the overall time spent on treatment. In this case, these proportions are 3/18 for treatment 1, 3/18 for treatment 2, and 12/18 for treatment 3. After applying these time adjustments, we arrive at a total time-adjusted preference weight for each treatment. Then, to compute the overall preference weight for the pathway, we combined the pathway weights with the time-adjusted treatment attribute weights to arrive at a pathway preference weight of 0.53.
Table 3 illustrates how the mean preference weight is computed for a flexible pathway, where neoadjuvant treatment A is given for 6 months, and adjuvant treatment C is given to those patients with residual disease post-surgery for 12 months (we have labeled this our ‘base case’). In this scenario, given that efficacy differs between patients with a pCR versus those without a pCR, the pathway preference estimates should be computed separately for these two patient groups and then combined based on the respective percentages of patients in each group. Patients with a pCR receive only neoadjuvant A and do not receive adjuvant C. As such, the pathway-specific preference weights are added to the treatment-specific weights, which do not require an adjustment for duration given that the treatment pathway includes only the 6-month neoadjuvant treatment. For those patients without a pCR, the total pathway duration is 18 months, including 6 months of neoadjuvant A and 12 months of adjuvant C. For this pathway, the pathway-specific preference weights are added to the treatment-specific weights, which are adjusted for their respective durations along the pathway. The preference weights for patients with and without pCR are then combined, adjusting for the respective percentages in these groups (32.6% and 67.4%, respectively) and arriving at a mean preference weight for this flexible pathway of 1.23 (95% confidence interval [CI] 1.10–1.36).
The proposed pathway preference estimation approach was applied to example pathways incorporating four treatment profiles, based on treatments that have been utilized in eBC (Fig. 3a). The neoadjuvant treatments included are A and B; the preference weights for the risks are worse for neoadjuvant B versus A because neoadjuvant B is reflective of more aggressive platinum chemotherapy. The adjuvant treatments are C and D; here, adjuvant D has a more favorable tolerability profile than adjuvant C.
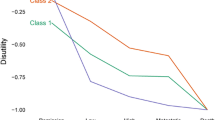
For the purposes of sensitivity analyses, the flexible pathway in Table 3 serves as the base-case pathway (mean preference weight 1.23). Figure 3b shows the mean pathway preference weights for the base case, and for separate modified pathways relative to the base case. For simplification, the assumption is that all pathways have an efficacy of 87%. If the base case is switched from a flexible to a fixed pathway, the mean pathway preference weight goes down considerably to 0.33 (95% CI 0.20–0.47), mainly because with a fixed pathway everyone proceeds onto adjuvant treatment, regardless of whether they have a pCR. If we replace neoadjuvant A with B, the more toxic chemotherapy, the mean pathway preference weight reduces even further to −0.64 (95% CI −0.77 to −0.51). For this pathway, if we replace neoadjuvant A with neoadjuvant B (the more toxic chemotherapy), the negative impact of this replacement is offset if we replace adjuvant C for 12 months with adjuvant D (which has a more favorable tolerability profile than adjuvant C) for a shorter duration of 6 months.
The pathway preference weights can be transformed into estimates of the likelihood of preferring one pathway over another using the preference share formula presented in the Methods section. Figure 3c reports the likelihood of preferring different selected pathways relative to the base-case pathway, also known as the share of preference. If the duration of neoadjuvant A is reduced from 6 to 3 months for the base case, this alternative pathway is 25% more likely to be preferred compared with the base case. Replacing neoadjuvant A with the more toxic neoadjuvant B has the most substantial impact on preferences, where patients are more than 50% likely to prefer the base case over pathways where neoadjuvant A has been replaced by neoadjuvant B.
4 Discussion
This research provides a novel method for deriving preference weights for treatment pathways involving sequential treatments. The sensitivity analysis incorporating treatment profiles matching those used in eBC shows that preference weights fluctuate in a logically consistent manner. Consistent with preference weights from the DCE, patients with eBC preferred a flexible pathway versus a fixed pathway, and the inclusion of treatments with more favorable tolerability profiles resulted in higher pathway preference weights. In addition to oncology, the pathway preference estimation approach described here may be useful in applications for other health conditions involving a sequence of treatments. For example, for some chronic conditions, patients may begin with a relatively mild therapy, but may then be switched to a more aggressive regimen depending on interim outcomes. Efficacy outcomes may be based on the frequency or severity of disease symptoms, as opposed to being disease-free, as applicable in this study.
The application of this estimation approach in eBC found that patients may prefer a pathway allowing for a flexible approach at the outset, with the ability to escalate treatment based on interim outcomes, versus a fixed approach where all treatments are preplanned. This finding parallels recommendations by the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017 [14] for more individualized treatment. The Panel favored escalating radiation therapy with regional nodal irradiation in high-risk patients, while encouraging omission of boost in low-risk patients.
The proposed pathway preference estimation approach is particularly suitable for the eBC setting, where there are various pathways involving multiple treatments. As additional systemic agents move into the adjuvant setting, it will be useful to understand how efficacy data across multiple treatments may be weighed against toxicity, dosing, duration of treatment, and other factors. However, although this methodology accounts for all treatments along a pathway, it does not account for the sequence of these treatments, i.e., the timing at which a treatment was given along the pathway. As found by Wheeler et al. [15], for some types of metastatic breast cancer, giving standard chemotherapy drugs in a specific sequence can reduce overall costs and improve the value of care while preserving QoL.
In addition, this estimation approach assumes that a patient would not deviate from a selected pathway where preferences are estimated, which may not reflect reality. For example, if a subset of patients experiences a serious adverse effect leading to hospitalization, this would likely lead to a change in treatment, and the respective treatment pathway may then change. It is also possible that experience with one treatment may affect how a patient feels about a subsequent treatment. With the proposed methodology, we cannot evaluate the potential correlation in perceptions between multiple treatments, i.e., we are assuming that preferences for treatments along a pathway do not change based on previous treatment experience within the pathway, or that preferences depend on the remaining options. Instead, this approach focuses on estimating preferences for predefined pathways. Nevertheless, clinical trials establish predefined pathways for patients; therefore, this proposed methodology is reasonable for estimating preferences for these pathways.
Similar to QALY calculations, the proposed pathway preference estimation approach accounts for the duration of time spent in a selected state; specifically, it accounts for the duration of time spent on a selected treatment. However, QALY computations may be viewed as too simplistic, as although they may account for QoL at certain points of time, they do not capture the various impacts of being on a certain treatment. These impacts may include risks of different adverse effects, as well as the emotional benefits of being on a treatment. The current proposed approach potentially enables a more comprehensive assessment of preference, by incorporating perspectives on key attributes of treatments.
A key study limitation is that the proposed pathway preference calculations do not distinguish between sequences that involve the same treatments in different order, and thus the order of the impacts of a sequence is not accounted for. This is an important topic for further research, as this limitation may also apply to studies that have incorporated health status preference measures in computations of QALYs. Although the preference weights for adverse effects of treatment are based on their risk of exposure during treatment, as opposed to experiencing the adverse effect, this is consistent with how risks of adverse effects would be discussed before treatment initiation. This study presents our best thinking about how the risks of the different components of the pathway will be presented to the patient when making a treatment decision, and how the preference weights for the risks should be aggregated into a preference weight for the entire pathway. Nevertheless, this calculation may not mimic the actual patient’s processing of the risks. Further research is needed on how risks are communicated to patients, and their perceptions of these risks. Providing patients with eBC with the full picture of possibilities and expectations along the treatment pathway may help to match them with an optimal treatment pathway.
In addition, it should be noted that the preference weights for the possible pathways are based on the assumption that a patient has made the decision to receive systemic treatment for their eBC. There may be patients who decide not to receive treatment (e.g., those who believe the toxicity is not worth the benefit) and their preferences may differ from those observed in this study. Finally, this study did not incorporate out-of-pocket costs; if out-of-pocket costs were included in the DCE, it is unknown if the relative differences observed in preference weights within and across attributes would change, affecting the final pathway preference weights.
5 Conclusions
This study presents a novel method to evaluate preference weights for clinical journeys involving sequential treatments. In addition to oncology, this methodology can potentially be applied to other diseases where patients may journey through a sequence of treatments that may vary in their durations, risks, and modes of administration. Healthcare providers can incorporate this approach when developing value-based clinical pathways that incorporate patient preferences to implement in practice.
References
Stamuli E, Corry S, Ross D, Konstantopoulou T. Advisory board participants from France, Advisory board participants from Ireland, Advisory board participants from Poland, Advisory board participants from Spain. Patient preferences for breast cancer treatments: a discrete choice experiment in France, Ireland, Poland and Spain. Future Oncol. 2022;18(9):1115–32. https://doi.org/10.2217/fon-2021-0635.
York Health Economics Consortium. Discrete Choice Experiment (DCE). 2016. Available at: https://yhec.co.uk/glossary/discrete-choice-experiment-dce/. Accessed 11 Feb 2023.
Ali S, Ronaldson S. Ordinal preference elicitation methods in health economics and health services research: using discrete choice experiments and ranking methods. Br Med Bull. 2012;103(1):21–44. https://doi.org/10.1093/bmb/lds020.
Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–26. https://doi.org/10.1007/s40273-018-0734-2.
Wang Y, Wang Z, Wang Z, Xuechun L, Pang X, Wang S. Application of discrete choice experiment in health care: a bibliometric analysis. Front Public Health. 2021;4(9):673698. https://doi.org/10.3389/fpubh.2021.673698.
Bansback N, Brazier J, Tsuchiya A, Anis A. Using a discrete choice experiment to estimate health state utility values. J Health Econ. 2012;31(1):306–18. https://doi.org/10.1016/j.jhealeco.2011.11.004.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13. https://doi.org/10.1016/j.jval.2010.11.013.
Gennari A, Jackisch C, McCutcheon S, Flood E, Murali B, Guillaume X, Will O, Shimizu C, Mokiou S. 70P Factors influencing patient treatment decisions in early breast cancer (eBC): discrete choice experiment (DCE) findings. Ann Oncol. 2022;33(Suppl 3):S154–5. https://doi.org/10.1016/j.annonc.2022.03.086.
Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, Bresnahan BW, Kanninen B, Bridges JF. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13. https://doi.org/10.1016/j.jval.2012.08.2223.
Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, IJzerman MJ, Bridges JF. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–15. https://doi.org/10.1016/j.jval.2016.04.004.
La Puma J, Lawlor EF. Quality-adjusted life-years. Ethical implications for physicians and policymakers. JAMA. 1990;263(21):2917–21. https://doi.org/10.1001/jama.263.21.2917.
Prieto L, Sacristán JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes. 2003;1:80. https://doi.org/10.1186/1477-7525-1-80.
Adams J, Bateman B, Becker F, Cresswell T, Flynn D, McNaughton R, Oluboyede Y, Robalino S, Ternent L, Gardner-Sood B, Michie S, Shucksmith J, Sniehotta FF, Wigham S. Effectiveness and acceptability of parental financial incentives and quasi-mandatory schemes for increasing uptake of vaccinations in preschool children: systematic review, qualitative study and discrete choice experiment. Health Technol Assess. 2015;19(94):1–176. https://doi.org/10.3310/hta19940.
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thürlimann B, André F, Baselga J, Bergh J, Bonnefoi H, Brucker SY, Cardoso F, Carey L, Ciruelos E, Cuzick J, Denkert C, Di Leo A, Ejlertsen B, Francis P, Galimberti V, Garber J, Gulluoglu B, Goodwin P, Harbeck N, Hayes DF, Huang CS, Huober J, Hussein K, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Osborne KC, Pagani O, Partridge AH, Pritchard K, Ro J, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao Z, Smith I, Toi M, Tutt A, Viale G, Watanabe T, Whelan TJ, Xu B. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–12. https://doi.org/10.1093/annonc/mdx308.
Wheeler SB, Rotter J, Gogate A, Reeder-Hayes KE, Drier SW, Ekwueme DU, Fairley TL, Rocque GB, Trogdon JG. Cost-effectiveness of pharmacologic treatment options for women with endocrine-refractory or triple-negative metastatic breast cancer. J Clin Oncol. 2023;41(1):32–42. https://doi.org/10.1200/JCO.21.02473.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funding source was involved in the study design, analysis, data interpretation, writing of the manuscript, and the decision to submit the manuscript for publication.
Conflicts of Interest
Kathleen Beusterien, Oliver Will, and deMauri S. Mackie are employees of Oracle, which provides consulting services to AstraZeneca. Emuella Flood and Susan McCutcheon are employees and/or stockholders of AstraZeneca. At the time of this study, Stella Mokiou was an employee and stockholder of AstraZeneca.
Availability of Data and Material
Data underlying the findings described in this manuscript cannot be shared due to the content of the informed consent forms signed by the patients. Please visit our Disclosure Commitment page for guidance on the AstraZeneca Data Sharing Policy (https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure).
Ethics Approval
The study that provided the DCE data for the current analysis received exemption status from Pearl IRB on 4 August 2020.
Consent to Participate
In the study that provided the DCE data for the current analysis, all respondents endorsed an electronic consent form.
Code Availability
Not applicable; new analytical code was not developed for this analysis.
Author Contributions
All authors contributed to study conception and design and interpretation of findings. KB, OW, and MSM also contributed to data analyses and manuscript development, and EF, SMC, and SM also reviewed the manuscript and provided feedback. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Beusterien, K., Will, O., Flood, E. et al. A Novel Approach to Computing Preference Estimates for Different Treatment Pathways: An Application in Oncology. Patient 17, 397–406 (2024). https://doi.org/10.1007/s40271-024-00680-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-024-00680-z