Abstract
Objective
This study aimed to elicit preferences for attributes of current and novel long-acting antiretroviral therapy for human immunodeficiency virus treatment.
Methods
Primary survey data were collected (July–October 2022) on a sample of 333 people living with human immunodeficiency virus in Germany from a patient recruitment agency. Respondents were invited by e-mail to respond to a web-based questionnaire. After performing a systematic literature review, we conducted qualitative semi-structured interviews to identify and select the key attributes of drug therapy for patients’ preferences for human immunodeficiency virus treatment. Based on this, a discrete choice experiment survey elicited preferences for long-acting antiretroviral therapy characteristics, including the type of medication, frequency of dosing, the location of treatment, the risk of both short-term and long-term side effects, as well as possible interactions with other medications or (party) drugs. A statistical data analysis was performed using multinomial logit models. An additional latent class multinomial logit was performed to evaluate subgroup differences.
Results
Overall, 226 respondents (86% male, mean age 46.1 years) were included in the analysis. The frequency of dosing (36.1%) and the risk of long-term side effects (28.2%) had the greatest influence on preferences. The latent class analysis identified two patient groups. While the first class (n = 135; 87% male, mean age 44.4 years) found the frequency of dosing (44.1%) to be most important, the second class (n = 91; 85% male, mean age 48.6 years) focused on the risk of long-term side effects (50.3%). The evaluation of structural variables showed that male respondents, those living in small cities or villages, and those with better health status results were significantly more likely to be assigned to the second class (p < 0.05 each).
Conclusions
All attributes included in our survey were important to participants when choosing an antiretroviral therapy. We found evidence that the frequency of dosing as well as the risk of long-term side effects have a particular impact on the acceptance of novel therapy regimens and should be considered in order to optimize adherence and satisfaction.
Similar content being viewed by others
Targeted antiretroviral therapy for human immunodeficiency virus treatment is important. Therefore, this study provides evidence on patient preferences for current and novel long-acting antiretroviral therapy attributes. |
Based on a discrete choice experiment, the frequency of dosing (36.1%) and the risk of long-term side effects (28.2%) were most important for patients. The latent class analysis demonstrated significant differences between two patient groups; while the first class found the frequency of dosing (44.1%) to be most important, the second group focused on the risk of long-term side effects (50.3%). |
The results may inform policymakers, physicians, research institutions, and other stakeholders about patients’ preferences of novel long-acting antiretroviral therapies for human immunodeficiency virus treatment. |
1 Introduction
The human immunodeficiency virus (HIV) is a pathogen that damages the immune system, which weakens the ability to fight everyday infections and diseases [1]. As the virus destroys and impairs the function of immune cells, people with HIV gradually become immunodeficient. The most advanced stage of HIV infection is the acquired immunodeficiency syndrome. Acquired immunodeficiency syndrome is defined by the development of certain cancers, infections, or other severe long-term clinical manifestations [2]. To date, despite intensive studies, neither curative drugs nor preventive vaccines are available [2,3,4]. However, with increasing access to effective antiretroviral therapies (ARTs) enabling a longer life expectancy for people living with HIV, the infection has become a manageable chronic health condition [2]. Current ARTs suppress viral replication and allow an individual’s immune system recovery to strengthen and regain the capacity to fight off opportunistic infections and some cancers [2].
Human immunodeficiency virus continues to be a major global public health issue; for example, in 2021, 1.5 million people became newly infected with HIV and 650,000 people died from HIV-related causes [2, 3]. In this regard, the United Nations Program for HIV/Acquired Immunodeficiency Syndrome (called UNAIDS) had set itself three treatment goals (“90-90-90 target”) to be reached by 2020: (1) 90% of all people living with HIV will know their HIV status; (2) 90% of all people with diagnosed HIV infection will receive sustained ART; and (3) 90% of all people receiving ART will have viral suppression [5]. In Germany, the total number of people living with HIV was estimated to be approximately 90,800 in 2021, with 1800 people who became newly infected with HIV [6]. Currently, approximately 96% of all people both living and diagnosed with HIV in Germany are accessing ART (79,100 out of 82,100) [6]. Thereby, 96% of all people receiving ART will have viral suppression (i.e., having a viral load of <200 copies/mm3) [7]. In 2020, all three treatment values were reached in Germany for the first time, with the second and third target values even being far exceeded (90%, 97%, and 96%, respectively) [7].
At the same time, a widening of the spectrum of available drug therapies regarding both the type of medication and the frequency of dosing can be observed. Innovative and novel therapeutic regimens are accompanied by new active principles (e.g., an attachment inhibitor [fostemsavir/Rukobia®] or a monoclonal antibody [ibalizumab/Trogarzo®], additional forms of doses (e.g., depot injection [cabotegravir/Vocabria® in conjunction with Rilpivirine /Rekambys®]), and longer application intervals (e.g., monthly or bimonthly). These research advances specifically comprise the availability of single-tablet daily regimens for HIV [8], injection-based therapies (e.g., under the skin, into muscle) [9], longer acting formulations of pill-based or injection-based regimens [9], and others. Achievements might have the potential to modify current treatment routines for certain patient groups; for example, for those with suboptimal adherence, confidentiality/privacy concerns, as well as the emotional burden and other barriers of daily dosing [8, 9]. Thereby, the emergence of novel long-acting therapy regimens requires both patients and providers to navigate a complex array of ART characteristics (e.g., pill burden, side effects, drug interactions, long-term toxicities, out-of-pocket costs) moving away from a “one-size-fits-all model” [10, 10]. This may have an impact on patient satisfaction, ART initiation, adherence, treatment effectiveness, and the quality of life of people living with HIV [8, 9, 12, 13]. Therefore, it seems necessary to elicit patient preferences for both current and novel long-acting ART regimens to evaluate their acceptance. The results from this study could thus improve our understanding of patient preferences for innovations in HIV therapy regimens [10] as well as enable better matching of treatments to patient expectations [11].
Previous studies have already explored patient preferences for HIV treatment [1, 9,10,11, 13,14,15,16,17,18]. For example, Mühlbacher and colleagues have conducted a discrete choice experiment (DCE) in a German population in 2009/2010, and demonstrated a high impact of quality-of-life-related attributes on patient preferences for the selection of treatments. In particular, the emotional quality of life (i.e., disease not obvious for others) was most important followed by the physical quality of life (i.e., diarrhea, nausea less frequent) and the social quality of life (i.e., participation in social life possible) [17]. Another study showed that people living with HIV in the USA valued minimizing side effects and long-term toxicities over dosing and administration characteristics [13]. Another preference-revealing study is being carried out in the USA to learn more about preferences for both current and novel HIV treatment regimens; with the results still to be published [8]. So far, to the best of our knowledge, DCEs have not yet been conducted to elicit patient preferences for both current and novel HIV treatment regimens in Germany.
Therefore, the present study aims to elicit patient preferences for both current and novel HIV treatment regimens in Germany and to explore in greater detail the heterogeneity in preferences of people living with HIV. The latter might increase the understanding of how preferences might diverge depending on characteristics (e.g., age, gender) [10, 13, 18].
2 Methods
This study used a mixed-methods approach. After performing a systematic literature review, we conducted qualitative semi-structured interviews to identify and select the most important attributes for patients’ preferences for HIV treatment in Germany. Based on this, a DCE was developed and performed to elicit patients’ preferences for HIV treatment relevant attributes and to quantitatively determine their relative value. Instead of ranking or rating different attributes, as is done in traditional importance elicitation formats, DCEs perform a pairwise comparison of hypothetical alternatives (i.e., differently configured HIV treatments) and ask the participants to choose between them [19]. Based on the decisions, DCEs can help understand which characteristics (termed attributes) are preferred by consumers and determine the relative value of each attribute [20, 21]. The design and analysis of the DCE were based on standardized research practices for undertaking a conjoint analysis of the ISPOR Conjoint Analysis Good Research Practices Task Force [22,23,24].
2.1 Systematic Search Procedure
In a first step, we conducted a systematic search procedure on MEDLINE (via PubMed), the Cochrane Library, and PsycINFO to identify studies assessing patients’ preferences for HIV treatment. The search was carried out in March 2022 and aimed at identifying English and German language literature published since 2010 (Electronic Supplementary Material [ESM]). In addition, we screened reference lists of identified research articles for further articles. The review complied with the Guideline from the Cochrane Collaboration [25]. Our search strategy was segmented into three components. The first component referred to patient preferences (e.g., priorities, expectations, perceptions, attitude), the second component to drug therapy respectively anti-HIV agents (e.g., anti-HIV agents, drug therapy, ART), and the third component to HIV infections (e.g., HIV, acquired immunodeficiency syndrome) [see ESM for a detailed information of the search procedure]. As a result, 8608 potentially relevant papers were identified in the electronic databases. After eliminating duplicates and judging titles and abstracts in a first step as well as full papers in a second step, 43 studies were considered relevant. Those studies investigated patients’ preferences for HIV treatment using qualitative research (n = 11), quantitative research (n = 16), mixed-methods studies (n = 3), systematic reviews/meta-analysis (n = 3), as well as DCEs (n = 10) [see ESM 3 for an overview of the included studies]. In sum, we derived 34 different attributes from the 43 studies (ESM). We added two further attributes (e.g., suitability for daily use, ease of use) based on German HIV expert consultations. To reduce the number of attributes, we discussed the relevance of each attribute regarding the context within the German healthcare system (e.g., the cost of services [26, 27] does not play an important role in the German setting from the patient’s perspective), discussed the attributes with the involvement of HIV experts, and qualitatively ranked the importance of each attribute. Following this, nine attributes remained for the next qualitative step.
2.2 Qualitative Steps
2.2.1 Semi-Structured Interviews
In total, we qualitatively surveyed (May to June 2022) 15 randomly selected people with confirmed HIV status from a patient recruitment agency (Liberating Research, LR) living in Germany (40% female, mean age 42.9 years). Written informed consent was obtained from all participants. Respondents were mailed a short survey before conducting the semi-structured interviews. This enabled us to learn more about the HIV history of the interviewees and their initial assessments of the relevance of the nine attributes. For example, each respondent was asked to rate each attribute on a 1–5 scale (1 = not all important, 5 = extremely important). The aim of the semi-structured interviews was to discuss the previously identified attributes and their relevance from the perspective of people living with HIV in the German healthcare setting, ask for possibly missing attributes, clarify the wording, specify both level characteristics as well as short descriptions, and to evaluate the comprehensibility of hypothetical choice tasks for the DCE (see below). The interviews were conducted online via MS Teams, recorded, transcribed, and analyzed verbatim by using MAXQDA (Release 2020.4.2). Individuals who completed the qualitative study received 30 Euros. We did not collect personal data such as addresses, names, or phone numbers. Based on all steps, six attributes were derived that were of major importance for HIV treatment from the patients’ perspective, which are as follows: the type of medication, the frequency of dosing, the location of treatment, the risk of short-term side effects, the risk of long-term side effects, as well as possible interactions with other medication or (party) drugs (see Table 1 for an overview of all attributes and corresponding levels). In contrast, the following three attributes were not included in the DCE because of a lower relevance from the patients’ perspective: the suitability for daily use, the ease of use, and the risk of HIV-related stigma (privacy concerns).
2.3 Quantitative Steps
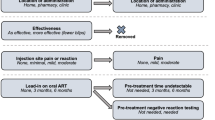
We used Sawtooth Software (Lighthouse Studio Version 9.14.0) to both program and conduct the survey. The questionnaire began with demographic and self-reported health status questions, and ended with HIV-related questions after the DCE choice sets. The experiment was designed as a full profile design and was generated using the balanced overlap method to permit estimation of the main effects and interactions, as well as D-optimal procedures to maximize statistical efficiency [28]. [The strength of design for our research model is 940.53503.] In this context, a full profile design refers to a design that ensures the inclusion of all possible attributes across the choice sets presented to survey respondents. We set forced-choice tasks (i.e., choice sets had no option to opt out, as opting for no treatment is not a rational option [1, 29]) and two hypothetical HIV treatments. Thus, respondents were forced to make trade-offs between attributes and their levels. The method offers practical advantages such as closeness to reality as trade-off decisions are part of everyday life [19]. This can provide valuable insights into how patients prioritize and trade-off different attributes or levels when making HIV-treatment related decisions. As stated above, HIV treatments differed in six attributes with between two and five levels (see Table 1). In DCE research, four to eight attributes per choice set are seen as appropriate [30, 31]. Figure 1 provides one hypothetical choice task as an example. The questionnaire was pilot tested for clarity and comprehensibility with 15 people living with HIV in Germany and slightly modified accordingly (e.g., we slightly modified the wording of two questions to improve understanding). The latter included a comprehensive qualitative discussion with the respondents about the final version of the survey as well as a quantitative analysis based on the 15 responses to detect implausible responses. [Please note that the final wording of all attributes, levels, and corresponding descriptions had already been examined during the qualitative part of this study.]
Orme’s often-used rule-of-thumb calculates a sample of 104 participants for a DCE having our design specifications (i.e., 12 choice tasks per respondent, two alternatives, two to five levels per attribute) [32]. This sample size was discussed as being the lowest limit for a main effects estimation. However, we aimed at doubling this number (i.e., to include at least 208 participants) following more advanced recommendations for statistical robustness [33].
2.4 Study Sample
To be included in either the qualitative or quantitative part of our study, patients had to be at least 18 years of age, to be a current resident in Germany, to have a confirmed HIV status, to be taking ART, and to have appropriate language skills in German. Final questionnaires were sent by e-mail to 333 randomly selected respondents with confirmed HIV status from a patient recruitment agency (LR) between July and November 2022. On registering, all patients provide LR with both their e-mail address and phone number to ensure direct contact for survey participation purposes. LR, which maintains the panel, invited eligible members and referred them to the online survey. The registered panel of people living with HIV in Germany at LR is representative in terms of gender and age for all patients in Germany and was also surveyed in other HIV-related research [9]. As an incentive, patients received between 20 Euros and 40 Euros, with at least 10% of the incentive being donated to a German HIV charity.
2.5 Survey Administration
In total, the survey consisted of four parts (ESM). First, we collected general sociodemographic information on participants (e.g., age, gender) before asking for HIV-treatment related issues (e.g., the year of both diagnosis and first treatment, current HIV treatment) in the second part. Afterwards, respondents were presented with information on all six attributes as well as corresponding levels and were asked to rate each attribute on a 1–5 scale (1 = not all important; 5 = extremely important) as well as to select the single most important attribute for the therapy choice. In the following, participants had to answer 12 DCE choice tasks, in which they were asked to choose between two treatment options. In the fourth part, respondents were asked to respond to further preference-related items regarding the different types of therapy regimens.
2.6 Statistical Methods
Analyses of general survey questions were performed using SPSS (released 2019, IBM SPSS Statistics for Windows, Version 26.0; IBM Corp. Armonk, NY, USA). Descriptive statistics were used to examine demographic and experience-related variables. Discrete choice experiment analyses were performed using R Statistical Software (Version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) and the corresponding mlogit-packages by Croissant (2020) [34] and “gmnl” by Sarrias and Daziano [35].
In our analysis, we tested different MNL and random parameter logit (RPL) models to identify the best-fitting model (ESM). As a benchmark, we used a MNL model with attributes as explanatory variables and assumed homoscedastic errors. In alternative models, we included heteroscedastic errors and additional explanatory variables. [We consider it relevant to test for heteroscedasticity, as unnoticed heteroscedasticity may bias the estimates of nonlinear models (e.g., logit models) [36]]. Overall, we did not find improvements in using the variations. Both, Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) point at the benchmark model to be the best-fitting model. Despite significant scaling factors, heteroscedastic errors were rejected by the Wald test, likelihood ratio test, and Lagrange multiplier test. Including an intercept or sociodemographic variables (i.e., age, gender, education, place of living, sexual orientation, and health state) as additional explanatory variables did not detect statistically significant changes. In order to test for unobserved heterogeneity, we analyzed the data by using an RPL model. We assumed that random parameters were normally distributed and uncorrelated with each other. We chose R = 1000 random draws, which is sufficiently large for convergence. However, including random parameters on all attributes did not lead to statistically significant additional findings apart from those related to the attribute “Interaction with other medication or (party) drugs” (we also applied uniform distribution without any meaningful changes). Additionally, no information criteria supported the use of the RPL model compared with the benchmark estimation (see also the likelihood ratio test and Wald test). [Please note: When we restricted random parameters to the attribute “Interaction with other medication or (party) drugs” as the only attribute with significant random parameters, the RPL model performs slightly better, and the Wald test reports that the remaining random parameters were relevant. Yet, the benchmark MNL model remains superior and we conclude that including random parameters did not provide additional benefits.] Based on this, we analyzed the DCE by performing the initial MNL model (see above).
We applied effect coding to all attributes so that the levels were expressed as (1, 0, − 1); thereby, (− 1) indicating the reference category. We chose effect coding over dummy coding because with dummy coding the parameter estimate for the (omitted) baseline category is equal to zero and cannot be recovered. Hence, the estimates of the other levels are expressed relative to the benchmark level. Instead, with effect coding, we can calculate the parameter of the baseline category from the negative sum of the included categories and the standard error from the covariances of the included categories. This enables us to compare all levels of the attributes against the corresponding mean value indicating a positive or negative impact compared to the mean of the attribute and the magnitude specifying the size of the effect [1, 24]. For all analyses, p values less than 0.05 were considered statistically significant [28].
2.7 Latent Class Analysis
With a latent class analysis, groups of respondents with similar preferences may be identified. In contrast to a cluster analysis, respondents are not assigned to different segments in a discrete (all-or-nothing) manner, but have probabilities of membership in each group [37]. A latent class analysis assumes that the underlying distribution for the parameters is discrete. This includes the assumption that there are different decision-making strategies used by groups (labeled classes) of respondents in a sample but that within each of these classes, responses are homogeneous [38]. More specifically, respondents are assigned to a class based on their highest posterior probability of class membership [38, 39]. In order to identify differences between potential subgroups, we conducted a latent class multinomial logit model. Using the gmnl package by Sarrias and Daziano [35], we compared models with two to five classes. We included sociodemographic variables as regressors (see above) in order to capture their impact on class membership. Again, we applied effect coding on all variables except for age. As recommended for analyses with a small sample size (< 300) as well with the intention to provide a probable allocation of the participants per group, we examined AIC, BIC, and sample-size adjusted BIC to select the best-fitting model [40,41,42]. The information criteria led to heterogenous recommendations (ESM). However, if incorrect, BIC and adjusted BIC are likely to underestimate the number of classes while AIC could be shown to be more likely to overestimate the number of classes, especially for studies with a small sample size [46, 47]. Based on this, we chose a model with two classes for the following analysis and provide the results of the model with three classes in the ESM for transparency reasons.
3 Results
3.1 Participant Characteristics
Of 333 eligible people living with HIV in Germany who were contacted, a total of 237 respondents returned the survey (71.2% response rate). We report on the n = 226 participants who fully completed the DCE component of the questionnaire (Table 2). The mean age of respondents was 46.09 years (standard deviation 11.20 years). Most respondents (85.8%) were male and most respondents (38.0%) stated intermediate secondary school or less as the highest educational level. Overall, 179 respondents (79.2%) rated their current health as good or better. The year of both diagnosis and first treatment ranged between 1984 and 2020. Regarding HIV treatment-related issues, most participants report a pill-based therapy (96.5%), take one HIV-related pill daily (75.2%), have been 12 months or more under the current HIV-therapy regimen (88.0%), and report to be (very) satisfied with the current HIV therapy (91.2%). Because each respondent made 12 choices, the final samples consisted of 2712 (226 times 12) cases to be analyzed.
3.2 Patients’ Preferences: Descriptive Findings
Respondents’ assessments of the importance of different attributes for the HIV-related treatment choice are presented in the ESM. First, each treatment attribute was rated on a 1–5 scale (1 = not all important; 5 = extremely important). As shown, participants rated the risk of long-term side effects (4.70 ± 0.68) as most important. In addition, the risk of short-term side effects (4.25 ± 0.95), the location of treatment (4.24 ± 0.98), and the frequency of dosing (4.23 ± 0.97) was rated slightly more important than the type of medication (4.14 ± 0.99). In contrast, possible interactions with other medication or (party) drugs seemed to be less important (3.64 ± 1.40). Second, the survey results for the single most important information item for the treatment choice revealed the risk of long-term side effects (35.4%) and the frequency of dosing (31.4%) as most relevant.
3.3 Patients’ Preferences: Findings from the DCE Analysis
Table 3 presents the results of multinomial logit models of 226 participants’ choices, totaling 2712 comparisons (see above). Overall, patients preferred a pill-based treatment with less frequent dosing regimens, which can be administered at home and further characterized by lower risks of both short-term and long-term side effects as well as by no interactions with other medications or (party) drugs. We showed statistically significant coefficients for all levels except for “Subcutaneous injection” and “Increased risk of reduced effect due to (party) drugs”. The coefficient values within an attribute add up to zero because of the effect coding (Table 3). The range of coefficient values within an attribute shows the importance of an attribute on a choice decision. We calculated the relative importance of each attribute by each attribute’s coefficient range (i.e., the difference between the coefficients of the highest and lowest level of each attribute) expressed as a share of the total range across attributes. As shown in Fig. 2, the frequency of dosing (36.1%; level range of 1.664) as well as the risk of long-term side effects (28.2%; level range of 1.298) had the strongest influence on preferences. In contrast, the type of medication (11.2%; level range of 0.514), the risk of short-term side effects (10.7%; level range of 0.494), the location of treatment (7.4%; level range of 0.340), and possible interactions with other medications or (party) drugs (6.4%; level range of 0.294) were less important.
3.4 Latent Class Analysis
Figure 3 displays the level estimates and the mean relative importance of attributes for both subgroups (see ESM for a detailed overview). Thereby, slightly more respondents were assigned to class 1 (n = 135; 59.7%) than to class 2 (n = 91; 40.3%). Regarding the preference pattern of class 1, the frequency of dosing (44.1%; level range of 1.990) is weighted highest. Furthermore, the type of medication (16.8%; level range of 0.759) was ranked second in this class. The remaining attributes risk of long-term side effects (10.8%; level range of 0.486), location of treatment (10.4%; level range of 0.470), possible interactions with other medications or (party) drugs (9.0%; level range of 0.408), and the risk of short-term side effects (8.8%; level range of 0.399) were rated less important in this class. Regarding the preference pattern of class 2, the risk of long-term side effects (50.3%; level range of 3.645) was weighted highest, followed by the frequency of dosing (27.4%; level range of 1.987). The attribute risk of short-term side effects (14.1%; level range of 1.022) was ranked third in this class. The remaining three attributes, type of medication (3.9%; level range of 0.280), location of treatment (2.9%; level range of 0.212), and possible interactions with other medications or (party) drugs (1.4%; level range of 0.105) were less important.
Graphic display of level estimates with the 95% confidence interval (discrete choice experiment) and mean relative importance of attributes for both subgroups (based on each attribute’s coefficient range, i.e., the difference between the coefficients of the highest and lowest level of each attribute)
The evaluation of structural variables for both classes shows that age, educational attainment, and sexual orientation did not show any significant impact on the estimated class probabilities (p > 0.05 each) [ESM]. For class 1, further sociodemographic variables (i.e., gender, place of living, and health status) did not show any significant impact either. In contrast, male respondents, those living in small cities or villages, and those with higher health status results were significantly more likely to be assigned to class 2. Table 4 presents the participant characteristics of both subgroups. As shown, we found significant differences between both groups for all characteristics. For example, members of class 1 were more likely to be younger (p < 0.001), have a lower educational level (p < 0.001), live in small cities or villages (p < 0.001), and are less satisfied with the current HIV therapy (p = 0.013) compared with members of class 2.
4 Discussion
This study provides new insights into preference patterns that people living with HIV in Germany place on certain attributes of current and novel long-acting ART regimens. Thereby, the study’s DCE-based findings appear broadly consistent with the results from both the rating-based and ranking-based survey results and may allow conclusions to be drawn for targeting therapy regimens: based on our findings, the frequency of dosing and the risk of long-term side effects had the greatest influence on preferences. The corresponding attributes were also the most important to the study participants when asked about the most significant information items for choosing a certain HIV treatment. In addition, consistent with these findings, study participants rated the risk of long-term side effects as most important when rating all attributes on a scale of 1–5.
As mentioned above, previous studies have also explored patient preferences for HIV treatment in different parts of the world [1, 9,10,11, 13,14,15,16,17,18]. For example, Mühlbacher and colleagues have surveyed 218 people living with HIV in Germany in 2009/2010 and have shown that the attribute “Emotional quality of life (disease not obvious for others)” was most important, followed by “Physical quality of life (diarrhoea, nausea less frequent)” and “Social quality of life (participation in social life possible)”. Less important but still statistically significant were the attributes “Life expectancy,” “Flexibility of dosing,” and “Long term side effects improbable” [17]. The study is comparable to ours in that it included two similar attributes and one attribute that refers to the flexibility of dosing. It identified similar preference structures for people living with HIV in Germany. For example, the possibility of taking medication at home is also a significant attribute in our study, although it was only ranked the fifth most important attribute. However, the “Frequency of dosing” and “Risk of long-term side effects” were the two most significant factors in our study, whereas they were the two least significant factors in the mentioned study.
Another study including respondents from Germany (along with those from France, Italy, and the UK) was conducted by Akinwunmi and colleagues addressing in detail the importance of the attribute “Frequency of dosing” [9]. Here, the authors assessed whether people living with HIV as well as physicians would be interested in trying and offering a novel long-acting regimen (LAR) for HIV treatment that requires a dosing every 2 months instead of daily. As a result, it could be shown that two thirds of all respondents were very strongly interested in trying LAR. As one main reason, people living with HIV viewed the described LAR as addressing several unmet needs, such as suboptimal adherence, confidentiality/privacy concerns, and the emotional burden from taking pills every day. Further regression results demonstrated that the odds of being interested in trying LAR were 4.21 times higher among those reporting versus not reporting that taking their HIV treatment less often “would reduce the shame or stigma I feel for having HIV” [9]. Even though the study has applied a different design it does confirm, at least to some extent, the absolute importance of the attribute “Frequency of dosing” as one major attribute when choosing HIV therapy regimens.
In another European study, Brégigeon-Ronot and colleagues surveyed 101 people living with HIV in France in 2014 to elicit relative preferences for attributes of ARTs [11]. The sample is comparable to ours regarding age, gender, as well as therapy-based characteristics (e.g., educational level, current health status, year of both diagnosis, and first treatment). As a result from the DCE analysis, it appears that patients placed significant value on the avoidance of both short-term side effects (i.e., diarrhea) as well as long-term health problems, such as cardiovascular disease and kidney disease [11]. Also similar to our findings, patients were concerned about the potential risk of drug–drug interactions and demonstrated a strong preference for a treatment with limited drug–drug interactions. Patients also preferred to avoid problems associated with treatment failure or therapy that left them with a higher viral load after the first weeks of treatment. Even though some attributes evaluated in this study were different from those in ours, in both studies, we detected limited drug–drug interactions and both short-term and long-term health problems to be significant attributes for patients’ preferences in ART regimens.
In addition, we identified two DCE studies from the USA with the intention to identify patient preferences for novel ART regimens. First, Ostermann and colleagues surveyed 403 people living with HIV in the USA in 2017/2018. They included four attributes in their DCE but those were similar to ours, namely dosing (i.e., the number of pills per dose and the dosing frequency), administration (i.e., meal requirement and pill size), short-term side effects (e.g., diarrhea, dizziness), and long-term side effects (e.g., risk of heart attack, risk of new or worse kidney problems) [13]. As a result, they could show that people living with HIV valued minimizing short-term side effects (relative importance: 44%) and long-term risks (32%) over dosing (17%) and administration characteristics (8%) [13]. Those findings are partly similar, except that in our study, the long-term side effects and the frequency of dosing were most important. In contrast, the short-term side effects were less relevant and the administration was not among the most relevant attributes in our study. Second, another study is currently being carried out in the USA to learn more about preferences for both current and novel HIV treatment regimens; with the results still to be published [8]. The findings of this study will enable interesting comparisons, as the attributes identified as relevant in this study are partly very similar to ours (e.g., location, frequency of dosing, side effects), but partly also address alternative aspects (e.g., late dose leeway).
In another interesting DCE study, Tieosapjaroen and colleagues surveyed 335 people living with HIV in Melbourne, Australia in 2021 to examine the relative importance of antiretroviral medication side effects so as to improve patient satisfaction, adherence, and treatment. In particular, the authors focused on weight gain as a possible side effect of HIV treatment. As a result, it could be shown that weight gain was the second most important short-term side effect following depression and the third most important undesirable long-term side effect, following risks of heart attack and kidney problems. Interestingly, the authors could show that respondents who weighed more than 85 kg preferred medications not causing weight gain while respondents with a lower weight preferred medications causing weight gain [43].
We have identified further studies that have been conducted in developing countries (e.g., Colombia [1, 16], Zambia [15], Kenya [10], urban Zimbabwe [18]). However, these studies seem to be less comparable to ours compared with studies from other European countries (e.g., France) and the USA, as they are likely to focus on other aspects of care [3, 5, 44]. For example, Dommaraju and colleagues have surveyed 104 persons living with HIV in Kenya to elicit preferences for differentiated care models. Here, the authors concluded that clinically stable patients expressed a preference for facility-based care provided clinical visits were extended to biannually. Here, the most important attributes were the location of clinical review (24.1%), location of ART refills (21.6%), the frequency of clinical visits (15.2%), and the frequency of ART refills (13.5%), followed by the person providing ART (9.7%), adherence support (8.2%), and refill pick-up/delivery time (7.8%) [10].
As mentioned above, patients preferred a pill-based treatment with less frequent dosing regimens, which can be administered at home and further characterized by lower risks of both short-term and long-term side effects as well as by no interactions with other medications or (party) drugs. In line with the current HIV healthcare delivery in the USA [8], most people living with HIV in Germany take one or more pills daily as well. Based on our results, more than 86% take one or two HIV-related pills daily combining two or more antiretroviral medications for treatment (see above). Using the example of frequency of dosing, it appears evident that the reality of care and the preferences of people living with HIV diverge, at least to some extent.
Finally, our latent class analysis-based findings have demonstrated meaningful differences between both subgroups. Interestingly, we detected one attribute to be of major importance in both groups. While the frequency of dosing was most important in class 1, the risk of long-term side effects was most important in class 2. The relative weight of those attributes accounted almost for 50% of all attributes. This means that these criteria were almost as important as the other five criteria combined. In both groups, we found one additional attribute to be of higher importance; while the type of medication was relevant in class 1, the frequency of dosing was weighted important in class 2. In contrast, the three attributes location of treatment, risk of short-term side effects, and the interaction with other medications or (party) drugs did not play an important role in either class.
With this in mind, our findings on the therapy preferences of people living with HIV have several implications: first, the results are directed to pharmaceutical researchers, who should consider them when developing innovative and novel therapy regimens. It should be noted that the desideratum of drug research and development corresponding to preferences is currently already being met, at least to some extent. Present research advances including the availability of single-tablet daily regimens for HIV [8], injection-based therapies (e.g., under the skin, into muscle), longer acting formulations of pill-based regimens, and others might have the potential to modify current treatment routine, at least for some patients; for example, for patients with suboptimal adherence, confidentiality/privacy concerns, as well as the emotional burden and other barriers of daily dosing [8, 9]. Thus, the emerging treatment options already correspond, in part, to patient preferences emerging from the present study. This applies, for example, to longer application intervals. As mentioned above, new long-acting therapy regimens might then have the potential to increase ART initiation and adherence [8, 12].
Second, our findings are relevant to professional societies, which should consider them when revising therapeutic guidelines or therapy regimens. Third, the results should also be considered by actors and institutions entrusted with regulatory tasks and responsible for priority setting. This applies, for example, to benefit assessments or health economic evaluations and thus also to pricing as well as decisions on reimbursement or inclusion in the benefit catalog of health insurance funds. Fourth, the results are directed at the medical profession, as they can contribute to joint decision making with patients when discussing different therapy options so as to increase therapy adherence. This, in turn, would also have a positive impact on both the outcomes and economic aspects of HIV treatment. For example, the frequency of treatment administration could be shown to be a key predictor of patient adherence [45].
In summary, taking into account the preferences of people living with HIV could further improve decisions and medical care. In addition, a strengthened role for preferences should be regarded as a form of participation that helps increase trust in health systems as a whole that may also strengthen the legitimacy of decisions within that system.
4.1 Limitations
As with any study, there are several limitations that have to be considered when interpreting the results of our research. First, our surveyed study respondents were recruited by a patient recruitment agency (LR). The registered panel of people living with HIV in Germany at LR is representative in terms of gender and age for all patients in Germany and was also surveyed in other HIV-related research [9]. In our study in particular, the proportion of male participants was slightly above the average in Germany (85.8% vs 80.1% [6]). Overall, it must be stated that there is little sociodemographic information available of people living with HIV in Germany [46]. Therefore, we cannot ensure that our sample is representative of the German population with respect to other characteristics as well (e.g., education, marital status, place of living). In addition, the recruitment via LR could have influenced the study population with respect to individual parameters, which could not be examined in total [47]. However, based on the sociodemographic data, it seems that our study population is similar to those of other studies (see above), at least to a certain extent. For example, the sociodemographic data showed a similar pattern to other studies in HIV-related preference research [9, 11, 17]. Second, it must be noted that the selected attributes for the DCE referred to both currently already available as well as novel, innovative HIV treatment regimens. Therefore, we cannot exclude that the participants in the experiment were more familiar with the already available therapy regimens and therefore chose those primarily. Third, the selection of the levels for the attribute “Frequency of Dosing” was associated with some challenges as related novel therapy regimens are currently not yet available. In the qualitative steps, however, we aimed to assure not to integrate any unrealistic characteristics (i.e., levels) but those that are actually currently being investigated in research. Therefore, the pool of experts from HIV research provided support in this regard. Fourth, the participants surveyed in this study had a mean year of first diagnosis of 2006, with the earliest diagnosis in 1984 and the latest diagnosis in 2020. Because of this relatively broad range, a different amount of experience of the participants can be assumed. Preferences of patients who have received one or more treatments and preferences of those who are newly diagnosed might vary as preferences might change over time [48]. Therefore, it should be taken into account that preferences of individual patients might change in the future. However, our results shall be interpreted as a current snapshot of preferences of people living with HIV in Germany [48]. Fifth, the generalizability (external validity) of our results to other settings should also be mentioned. As described above, studies from other European countries (Germany [17], France, Italy, and the UK [9], France [11]) or the USA [8, 13] tend to have included more similar attributes and the samples surveyed appear more comparable to our study. Therefore, our results are more likely to be transferable to these countries. In contrast, studies that have been conducted in developing countries (e.g., Colombia [1, 16], Zambia [15], Kenya [10], Urban Zimbabwe [18]) are likely to focus on other aspects of care as well [3, 5, 44]. Here, the transferability is likely to be lower. Sixth, patients received a financial incentive for participating in the quantitative part of our study. It should be mentioned that the incentive has been increased over time from 20 Euros to 40 Euros to improve the response rate. Please note that the average paid incentive was calculated to be 28.32 Euros. Finally, it should be noted that we did not randomly allocate the order of the presentation of attributes between participants. We refrained from doing so, as previous research has shown that the ordering of the attributes was found to have no statistically significant effect on the importance attached to them by respondents [49, 50]. Nevertheless, we follow Norman and colleagues by recognizing that in some situations (e.g., relatively difficult choice tasks, unfamiliar choice task), respondents may have the tendency to rely on simplifying heuristics such as focusing on the earlier or later dimensions [49]. However, in our study, we felt that the choice tasks were neither very difficult nor unfamiliar to the respondents because we surveyed only people with confirmed HIV status.
5 Conclusions
This study provides new insights into preference patterns that people living with HIV in Germany place on certain attributes of current and novel ART regimens. Thereby, the study’s DCE-based findings appear broadly consistent with the results from both the rating-based and ranking-based survey results. One of the major results is the importance placed by people living with HIV in Germany on the characteristics of HIV-related therapy regimens related to the frequency of dosing and the risk of long-term side effects. The latter had the greatest influence on peoples’ preferences. This should be considered when further developing HIV-related therapy regimens in order to increase treatment adherence and the satisfaction of people living with HIV.
References
Sijstermans E, Cheung KL, Goossens AJM, et al. A discrete choice experiment to assess patients’ preferences for HIV treatment in the urban population in Colombia. J Med Econ. 2020;23(8):812–8. https://doi.org/10.1080/13696998.2020.1735399.
World Health Organization. HIV: key facts 2022. https://www.who.int/news-room/fact-sheets/detail/hiv-aids. Accessed 4 Jul 2023.
Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV & AIDS statistics: fact sheet 2022. https://www.unaids.org/en/resources/fact-sheet. Accessed 4 Jul 2023.
German Center for Infection Research. HIV: HIV scientists concentrate their research on both remission and cure. 2022. https://www.dzif.de/en/hiv. Accessed 4 Jul 2023.
Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: an ambitious treatment target to help end the AIDS epidemic 2014. https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed 4 Jul 2023.
Robert Koch-Institut (RKI). HIV/AIDS in Deutschland: Eckdaten der Schätzung. Epidemiologische Kurzinformation des Robert Koch-Instituts, Stand: Ende 2021. Robert Koch-Institut (RKI): Berlin; 2022.
Robert Koch-Institut (RKI). Epidemiologisches Bulletin: HIV in Deutschland 2020. Erfassung der SARS-CoV-2-PCR-Testzahlen. Robert Koch-Institut (RKI): Berlin; 2021.
Barthold D, Brah AT, Graham SM, et al. Improvements to survey design from pilot testing a discrete-choice experiment of the preferences of persons living with HIV for long-acting antiretroviral therapies. Patient. 2022;15(5):513–20. https://doi.org/10.1007/s40271-022-00581-z.
Akinwunmi B, Buchenberger D, Scherzer J, et al. Factors associated with interest in a long-acting HIV regimen: perspectives of people living with HIV and healthcare providers in four European countries. Sex Transm Infect. 2021;97(8):566–73. https://doi.org/10.1136/sextrans-2020-054648.
Dommaraju S, Hagey J, Odeny TA, et al. Preferences of people living with HIV for differentiated care models in Kenya: a discrete choice experiment. PLoS ONE. 2021;16(8):e0255650. https://doi.org/10.1371/journal.pone.0255650.
Brégigeon-Ronot S, Cheret A, Cabié A, et al. Evaluating patient preference and satisfaction for human immunodeficiency virus therapy in France. Patient Prefer Adherence. 2017;11:1159–69. https://doi.org/10.2147/PPA.S130276.
Cohen J, Beaubrun A, Bashyal R, et al. Real-world adherence and persistence for newly-prescribed HIV treatment: single versus multiple tablet regimen comparison among US medicaid beneficiaries. AIDS Res Ther. 2020;17(1):12. https://doi.org/10.1186/s12981-020-00268-1.
Ostermann J, Mühlbacher A, Brown DS, et al. Heterogeneous patient preferences for modern antiretroviral therapy: results of a discrete choice experiment. Value Health. 2020;23(7):851–61. https://doi.org/10.1016/j.jval.2020.03.007.
Eshun-Wilson I, Kim H-Y, Schwartz S, et al. Exploring relative preferences for HIV service features using discrete choice experiments: a synthetic review. Curr HIV/AIDS Rep. 2020;17(5):467–77.
Eshun-Wilson I, Mukumbwa-Mwenechanya M, Kim H-Y, et al. Differentiated care preferences of stable patients on antiretroviral therapy in Zambia: a discrete choice experiment. J Acquir Immune Defic Syndr. 2019;81(5):540–6.
Goossens AJM, Cheung KL, Sijstermans E, et al. A discrete choice experiment to assess patients’ preferences for HIV treatment in the rural population in Colombia. J Med Econ. 2020;23(8):803–11. https://doi.org/10.1080/13696998.2020.1735398.
Mühlbacher AC, Stoll M, Mahlich J, et al. Patient preferences for HIV/AIDS therapy: a discrete choice experiment. Health Econ Rev. 2013;3(1):14. https://doi.org/10.1186/2191-1991-3-14.
Strauss M, George G, Mantell JE, et al. Optimizing differentiated HIV treatment models in urban Zimbabwe: assessing patient preferences using a discrete choice experiment. AIDS Behav. 2021;25(2):397–413.
Mühlbacher AC, Bethge S. Patients’ preferences: a discrete-choice experiment for treatment of non-small-cell lung cancer. Eur J Health Econ. 2015;16(6):657–70. https://doi.org/10.1007/s10198-014-0622-4.
Vass C, Rigby D, Payne K. The role of qualitative research methods in discrete choice experiments. Med Decis Making. 2017;37(3):298–313. https://doi.org/10.1177/0272989X16683934.
Naik Panvelkar P, Armour C, Saini B. Community pharmacy-based asthma services: what do patients prefer? J Asthma. 2010;47(10):1085–93.
Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13. https://doi.org/10.1016/j.jval.2010.11.013.
Hauber AB, González JM, Groothuis-Oudshoorn CGM, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–15. https://doi.org/10.1016/j.jval.2016.04.004.
Higgins J, Thomas J, Chandler J, et al editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: Wiley; 2019.
Opuni M, Bishai D, Gray GE, et al. Preferences for characteristics of antiretroviral therapy provision in Johannesburg, South Africa: results of a conjoint analysis. AIDS Behav. 2010;14(4):807–15. https://doi.org/10.1007/s10461-009-9584-4.
Kruk ME, Riley PL, Palma AM, et al. How can the health system retain women in HIV treatment for a lifetime? A discrete choice experiment in Ethiopia and Mozambique. PLoS ONE. 2016;11(8):e0160764. https://doi.org/10.1371/journal.pone.0160764.
Marshall DA, Deal K, Conner-Spady B, et al. How do patients trade-off surgeon choice and waiting times for total joint replacement: a discrete choice experiment. Osteoarthr Cartil. 2018;26(4):522–30.
Veldwijk J, Lambooij MS, de Bekker-Grob EW, et al. The effect of including an opt-out option in discrete choice experiments. PLoS ONE. 2014;9(11):e111805. https://doi.org/10.1371/journal.pone.0111805.
Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. 2003;2(1):55–64.
Sawtooth Software. The CBC system for choice-based conjoint analysis. In: Sawtooth Software, Inc., editor. CBC: technical paper. Technical Paper Series. Sawtooth Software, Inc.: Washington DC; 2008.
Orme BK. Getting started with conjoint analysis: strategies for product design and pricing research. Research Publishers LLC: USA; 2020.
Orme BK, Chrzan K. Becoming an expert in conjoint analysis: choice modeling for pros. Sawtooth Software, Inc.: North Orem (UT); 2017.
Croissant Y. Estimation of random utility models in R The mlogit Package. J Stat Soft. 2020;95(11):1–41.
Sarrias M, Daziano R. Multinomial logit models with continuous and discrete individual heterogeneity in R The gmnl Package. J Stat Soft. 2017;79(2):1–46.
White H. Estimation, inference, and specification analysis. Cambridge: Cambridge University Press; 1994.
Sawtooth Software, Inc. Technical paper series: the latent class technical paper V4.8. Utah; 2021.
Street AE, Street DJ, Flynn GM. Who gets the last bed? A discrete-choice experiment examining general population preferences for intensive care bed prioritization in a pandemic. Med Decis Making. 2021;41(4):408–18. https://doi.org/10.1177/0272989X21996615.
Greene WH, Hensher DA. A latent class model for discrete choice analysis: contrasts with mixed logit. Transp Res B-Meth. 2003;37(8):681–98.
Mühlbacher AC, Sadler A, Dippel F-W, et al. Treatment preferences in Germany differ among apheresis patients with severe hypercholesterolemia. Pharmacoeconomics. 2018;36(4):477–93.
Mühlbacher AC, Bethge S. Reduce mortality risk above all else: a discrete-choice experiment in acute coronary syndrome patients. Pharmacoeconomics. 2015;33(1):71–81.
Sinha P, Calfee CS, Delucchi KL. Practitioner’s guide to latent class analysis: methodological considerations and common pitfalls. Crit Care Med. 2021;49(1):e63-79.
Tieosapjaroen W, Fairley CK, Chow EPF, et al. Preferences for weight gain compared with other antiretroviral therapy side effects in people living with HIV: a discrete choice experiment. J Acquir Immune Defic Syndr. 2022;91(3):305–11.
Byanyima W. In danger: UNAIDS global AIDS update 2022: foreword. UNAIDS: Geneva; 2022.
Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open. 2013. https://doi.org/10.1136/bmjopen-2013-003028.
Robert Koch-Institut (RKI). Epidemiologisches Bulletin: HIV in Deutschland 2021. Robert Koch-Institut (RKI): Berlin; 2022.
Mühlbacher A, Bethge S. What matters in type 2 diabetes mellitus oral treatment? A discrete choice experiment to evaluate patient preferences. Eur J Health Econ. 2016;17(9):1125–40. https://doi.org/10.1007/s10198-015-0750-5.
Mühlbacher AC, Junker U, Juhnke C, et al. Chronic pain patients’ treatment preferences: a discrete-choice experiment. Eur J Health Econ. 2015;16(6):613–28. https://doi.org/10.1007/s10198-014-0614-4.
Norman R, Kemmler G, Viney R, et al. Order of presentation of dimensions does not systematically bias utility weights from a discrete choice experiment. Value Health. 2016;19(8):1033–8. https://doi.org/10.1016/j.jval.2016.07.003.
Farrar S, Ryan M. Response-ordering effects: a methodological issue in conjoint analysis. Health Econ. 1999;8(1):75–9.
Acknowledgments
The authors thank all survey participants.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was financed by Gilead Sciences GmbH, Planegg, Germany.
Conflicts of interest/competing interests
Martin Emmert, Stefan Rohrbacher, Jennifer Jahn, Katharina Fernando, and Michael Lauerer have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Ethics approval for this study was not necessary according to the Ethics Committee from the University of Bayreuth, Germany.
Consent to participate
All respondents were informed about the study and its potential risks and benefits prior to participation. Respondents had to sign an informed consent to participate in the study, and to have their data used to develop the results contained in this paper. In addition, participation was voluntarily and the participant could stop at any time.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
ME designed and directed this study as the principal investigator. ME and ML conceived and planned the experimental design of the study. ME and SR performed the analysis on all samples. All authors contributed to the interpretation of the results. JJ and KF both conducted and analyzed the qualitative interviews. ME took the lead in preparing the manuscript. All authors provided critical feedback and commented on the manuscript. Finally, all authors approved the latest version of the manuscript to be published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Emmert, M., Rohrbacher, S., Jahn, J. et al. Preferences of People Living with HIV for Long-Acting Antiretroviral Treatment in Germany: Evidence from a Discrete Choice Experiment. Patient 16, 537–553 (2023). https://doi.org/10.1007/s40271-023-00641-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-023-00641-y