Abstract
Background and Objective
Patient support programs aim to provide solutions beyond the medication itself, by enhancing treatment adherence, improving clinical outcomes, elevating patient experience, and/or increasing quality of life. As patient support programs increasingly play an important role in assisting patients, numerous observational studies and pragmatic trials designed to evaluate their impact on healthcare have been conducted in recent years. This review aims to characterize these studies.
Methods
A systematic literature review, supplemented by a broad search of gray literature, was conducted following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and Cochrane recommendations. Observational studies and pragmatic trials conducted in Europe to evaluate the impact of patient support programs, published in English or Spanish between 17/03/2010 and 17/03/2020, were reviewed. Two patient support program definitions were applied starting with Ganguli et al.’s broad approach, followed by the European Medicines Agency definition, narrowed to Marketing Authorization Holders organized systems and their medicines. The quality of publications was assessed using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement 22-item checklist.
Results
Of the 49 identified studies following the Ganguli et al. definition, 20 studies met the European Medicines Agency definition and were reviewed. Patient support program impact was evaluated based on a wide range of methodologies: 70% assessed patient support program-related patient-reported outcomes, 55% reported clinical outcomes, and 25% reported economic impacts on health resources. Only 45% conducted a comparative analysis. Overall, 75% of the studies achieved their proposed objectives.
Conclusions
The heterogeneity of the observational studies reviewed reflects the complexity of patient support programs that are built ad hoc for specific diseases, treatments, and patients. Results suggest that patient support programs play a key role in promoting treatment effectiveness, clinical outcomes, and satisfaction. However, there is a need for standardizing the definition of patient support programs and the methods to evaluate their impact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We appraised 49 studies aimed at evaluating patient support programs conducted in Europe in the last 10 years. Among them, 40% were sponsored by a marketing authorization holder and related to medicinal products. |
In general, studies showed a positive impact of patient support programs on patients’ adherence to medication, satisfaction, or health-related quality of life. In addition, patient support programs ameliorate clinical outcomes or reduce the use of resources and costs. |
However, the heterogeneity of the observational studies in terms of design and outcomes makes it difficult to determine to what extent patient support programs provide additional value to the standard of care. Therefore, there is a need to standardize the definition of patient support programs and the methods to evaluate their impact on health outcomes. |
1 Introduction
Patient support programs (PSPs) aim to provide solutions beyond the medication itself, adding complementary value to medicinal products by supporting patient care. Historically, PSPs have been defined as enhanced self-management support programs that include interventions such as individualized medication counseling, training, support, and virtual reminders to improve medication-taking behavior [1]. This definition encompasses a variety of patient-directed interventions, from educational programs aimed at improving disease management, regardless of the patient’s treatment, to specific programs that aim to provide education to patients and a follow-up on patients receiving a particular medicine. The European Medicines Agency (EMA) has adopted a more narrow definition for PSPs, recognizing only organized systems in which a Marketing Authorization Holder (MAH) receives and collects information relating to the use of its medicinal products [2].
Independently of the PSP definition, the majority of these programs fall into one or more of the following three categories: (1) supporting patients and helping them take their medications as prescribed; (2) helping patients to understand their condition and providing advice on managing the disease (e.g., lifestyle, exercise, and diet); and (3) providing a service or financial assistance or reimbursement support to patients (also known as patient assistance programs) [3]. The common goal of these programs is to enhance treatment adherence, improve clinical outcomes, elevate the patient experience with treatment, and/or increase their quality of life.
As PSPs increasingly play an important role in assisting patients with chronic conditions, the number of programs has increased significantly in recent years [1]. Marketing Authorization Holders have launched worldwide PSPs for newly authorized products and numerous observational studies designed to evaluate their impact on healthcare are being reported. A meta-analysis showed that PSPs improve treatment adherence and persistence in the therapeutic area of inflammatory and immunologic diseases [4]. A systematic literature review also described the positive impact of PSPs on adherence, clinical, and humanistic outcomes in chronic diseases [1]. Nonetheless, that review also found that study designs and outcomes reported in the literature were highly heterogeneous owing to different factors such as the purpose of the PSP, type of services and actions provided, disease and medication therapy management, or stakeholders’ involvement [1]. In addition, no guidelines and recommendations from experts and/or authorities exist to guide the development of PSPs, further promoting the heterogeneity between PSPs and, consequently, of the studies that evaluate them. This review aims to characterize the observational studies and pragmatic trials conducted to evaluate PSPs in Europe, with emphasis placed on study results and outcomes.
2 Methods
2.1 Data Source and Strategic Approach
A targeted systematic literature review of international databases (MEDLINE/PubMed, Medicina en español [MEDES], and Índice Bibliográfico Español en Ciencias de la Salud [IBECS]), supplemented by a broad search of gray literature, was performed to identify observational studies and pragmatic trials that have evaluated the impact of PSPs in Europe over the last 10 years. The search was conducted under the “Preferred Reporting Items for Systematic Reviews and Meta-analysis: The PRISMA Statement” and Cochrane guidelines by using search filters and standardized terms [5, 6] (Table S1 of the Electronic Supplementary Material [ESM]).
2.2 Publication Selection and Data Extraction Procedures
Two independent reviewers (LPC and MC) screened publications retrieved based on the title and/or abstract. Full texts of retrieved publications were peer reviewed and ascertained for final eligibility if they meet inclusion/exclusion criteria according to the PICOTS framework [7] (Table 1). Discrepancies were resolved by consensus or by involving a third team member (LL).
A first screening applying a broad approach was used to identify PSP-related articles based on the definition of PSP provided by Ganguli et al. [1]: “PSPs are enhanced self-management support programs that include interventions such as individualized medication counseling, training, support, and virtual reminders to improve medication-taking behavior. The aim is to help patients better manage their disease and complex medications regimes, improve medication adherence, and reduce complications and related costs”. A second screening according to the EMA definition for PSP [2]: “an organized system where a marketing authorization holder receives and collects information relating to the use of its medicinal products” was applied to (1) adopt the definition of PSPs in Europe, according to the EMA Good Vigilance Practice Module VI, in order to remain within a common overarching regulatory framework for the conduct and evaluation of PSPs, (2) reduce the heterogeneity among the intervention evaluated, (3) provide additional and complementary information to that provided by Ganguli et al., and (4) explore the role of MAHs beyond the medication itself. Hence, the second screening analyzed PSPs involving a specific medication, disease, and MAHs. All data were extracted by two independent reviewers (LPC and MC). A standardized data extraction form and data extraction guidelines were used. The characteristics of the reviewed studies were further described based on the study’s outcomes (patient-reported outcomes [adherence, satisfaction, and quality of life], clinical, or economic). In addition, each study’s outcome was assessed based on the achievement of pre-defined objectives and classified as ‘objective achieved,’ ‘objective not achieved,’ ‘inconclusive,’ or ‘not defined a priori’. The quality of publications was assessed using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement 22-item checklist [8]. The quality assessment is provided in Table S2 of the ESM.
3 Results
As shown in the PRISMA flow diagram (Fig. 1), a total of 5228 studies were identified in the databases that were consulted. After duplicate removal, the title and abstract of the resulting 5216 publication records were screened for information relevant to the analysis, resulting in the exclusion of 5110 records. Of the 106 remaining publications, 57 were discarded after full-text reading because of non-compliance with eligibility criteria. In the first broader approach, where any type of intervention on patients was allowed (Ganguli et al. definition [1]), 49 publications were selected. These publications included observational studies that evaluated a wide variety of PSPs. However, 29 of them did not meet the EMA definition and were not selected after the second screening round [2] because they were not linked to a specific drug or medical device (55%; n = 16), and were not funded or initiated by a pharmaceutical company (45%; n = 13) [Fig. 1 and Table S3 of the ESM). Hence, 20 studies focused on PSPs sponsored by a pharmaceutical company and related to a specific medical drug were finally included in the review.
3.1 Characteristics of the Reviewed Studies
Tables 2 and 3 summarize the main characteristics of the 20 studies regarding PSPs that met the EMA definition. All studies were classified as observational (no pragmatic trials identified), designed as prospective (50%, n = 10) [9,10,11,12,13,14,15,16,17,18], retrospective (25%, n = 5) [19,20,21,22,23], cross-sectional (20%, n = 4) [24,25,26,27] (three of which were surveys), or patient-case report studies (5%, n = 1) [28].
Only 45% of the studies (n = 9) conducted a comparative analysis to determine the benefits or effectiveness of PSPs; 35% (n = 7) compared a no-PSP control arm (standard care) to a PSP (intervention group) and another 10% (n = 2) performed before-and-after intervention comparisons.
Most of the PSPs evaluated in the studies reviewed targeted patients with diabetes mellitus [16,17,18, 22, 23, 27] multiple sclerosis [10, 11, 20, 21, 24], rheumatoid arthritis [12, 13], osteoporosis [14, 19], psoriasis [25, 26], non-Hodgkin lymphoma [28], colorectal and breast cancer [9], and various types of diseases [15] that met MAH’s indications for a specific treatment (Tables 2 and 3).
The studies included in the review evaluated eight specific PSPs, most of which (87.5%; n = 7) provided educational programs on treatment and related diseases along with monitoring of patients, via telephone calls (n = 5; 62.5%), face-to-face visits (n = 3; 37.5%), or e-mail contacts (n = 2; 10%). In addition, two PSPs (25%) provided home delivery of medications and/or medical devices (i.e., needles). Nurses were the healthcare professionals most frequently involved in PSPs (see details in Table S4 of the ESM).
3.2 Results from the Reviewed Studies
To evaluate the impact of the PSPs, different outcomes were assessed. Most of the studies (70 %; n = 14 [9,10,11,12, 14, 15, 19,20,21,22, 24, 25, 27, 28]) used patient-reported outcome measures: nine of them to record adherence and persistence (n = 2 evaluated patients’ reported PSP persistence and n = 7 evaluated patients’ reported treatment adherence), eight to assess patient satisfaction, and three to collect patients’ health-related quality of life [HRQoL] (n = 3); 55% (n = 11 [10, 12, 15,16,17,18, 22, 23, 25,26,27]) reported on clinical outcomes achieved by patients participating in the PSP including safety outcomes (n = 1), and 25% (n = 5; [10, 12, 13, 15, 25]) assessed the economic impact of PSPs to determine the impact on health resource use and associated costs (Table 2).
Different instruments were used to evaluate the impact of the PSPs. Satisfaction with PSPs was assessed using either a 10-point Likert scale [22, 24, 27] or an ad hoc questionnaire developed for the study [14, 28]. In two studies, treatment satisfaction was evaluated using the Treatment Satisfaction Questionnaire for Medication [12, 15]. One study did not specify the methodology [25].
Improvements in patient adherence to medication were estimated as (1) percentage of doses taken/doses prescribed at different timepoints of the follow-up [9, 10], (2) percentage of patients who were recorded as having persisted with therapy during the study period [11, 19], and (3) percentage of patients who self-reported adherence (ad-hoc questionnaire or Morisky Medication Adherence Scale 4 questions) [15, 20, 21]. Two studies assessed PSP persistence by reporting on the percentage of patients remaining in the program during the study follow-up [14, 27]. Several HRQoL questionnaires, both generic (EQ-5D, SF-12, SF-36) [15, 24] and disease specific (MusiQoL, SIBDQ, DLQI, ASQoL) [10, 15], were used to determine the impact of PSPs on patients’ HRQoL.
Two approaches were used in the studies reviewed to assess the impact of the PSP on costs. First, five studies evaluated the variations in impairment in the workplace; three of them used the Work Productivity and Activity Impairment Questionnaire [12, 13, 15], two used an ad hoc questionnaire on work capacity [10, 25], and one collected the amount of sick leave [15]. Second, three studies collected and analyzed the use of healthcare resources (hospitalizations and specialist visits, among others) [10, 13, 15].
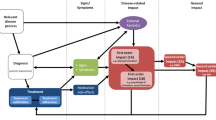
According to the study results, proposed objectives were achieved in 75% of the studies (n = 15), demonstrating an increase in patients reported adherence and persistence [10,11,12, 14, 19], an improvement in patient satisfaction and/or quality of life [10, 11, 14, 22, 24, 25, 28], an improvement in clinical outcomes [10, 12, 16,17,18, 22, 23, 25, 26], and reductions in the costs associated with the use of healthcare resources [10, 12, 13, 25] (Fig. 2). Regarding the remaining publications (n = 5), 10% (n = 2) were inconclusive as it was not possible to assess whether participation in the PSP had induced an improvement in patients outcomes [9, 27], in another 10% (n = 2), the objective to achieve with the PSP was not clearly defined a priori [20, 21], and 5% (n = 1 publication) were an observational study report classified as others because no results had yet been published [15].
Results showed that overall, patients who participated in a PSP were very satisfied with the intervention. Most of them provided satisfaction scores varying from 8.5 to 9 (on a scale of 0–10) and would recommend its use to other patients (data not shown). Moreover, study results suggested that PSPs contributed to increase patients’ adherence to medicines (adherence range from 61.8% to 98%), to improve clinical measures (such as glycemic control in diabetes mellitus), and to reduce healthcare-associated costs (Fig. 2, Table 3).
4 Discussion
Patient support programs are becoming increasingly important as a service that provides support to the patient beyond the medication itself. The objectives of the PSPs vary according to the characteristics of the disease or the type of medication. Patient support programs can provide information and counseling to the patient for better disease management, include training or virtual reminders to improve patient adherence to medication, or provide patient education to identify potential side effects that need to be monitored.
The diversity of the characteristics of the PSPs evaluated, and the heterogeneity of the characteristics of the observational studies reflect the complexity of these disease-specific programs that are built ad hoc for specific diseases, treatments, and patients. The PSPs evaluated in this review were mostly based on supportive or educational interventions to help patients to understand and manage their symptoms. They comprised educational modules to inform and raise awareness [11,12,13, 15, 24,25,26, 28], patient monitoring services [11,12,13, 15, 24,25,26, 28], home delivery of medications [12,13,14,15, 19, 25, 26], and/or nurse home-visit services for treatment administration [11, 14, 19, 24].
The results of the review provide an overview of the benefits of PSPs in terms of improved adherence, HRQoL gains, better clinical outcomes, and a reduction in the use of healthcare resources and associated costs. Overall, 75% of the studies claimed that the expected objectives were achieved after implementing the PSPs, in particular those involving clinical (80%) and economic outcomes (80%) and patient satisfaction (75%). These percentages are somehow higher than those reported by Ganguli et al. [1], where 64.1% of studies reported at least one significant positive clinical outcome and 64% reported a significantly positive humanistic outcome. This difference may be attributed either to the difference in PSP definitions or to a different way of assessing the achievement of the objective between the two studies. However, the heterogeneity in the studies appraised with respect to data collection timing (i.e., retrospective, prospective), comparison approaches (not existing vs existing), and the wide variety of instruments for the collection of evidence (scored surveys, ad hoc questionnaires, specific definitions, qualitative and quantitative measurements) make comparison between studies difficult. Moreover, because of a lack of a control arm in half of the reviewed studies, no conclusion can be drawn from the intervention groups that were compared to standard care. The inclusion of a control arm in future studies conducted to assess the impact of a PSP is highly recommended.
The heterogeneity observed in this review highlights the need to harmonize the definition, methods, and measurement of the effectiveness of PSPs. The application of new digital technologies and big data analysis will open up new opportunities to optimize and accelerate this process [29]. Validated initiatives such as the International Patient Decision Aids Standards, intended to help in the development of patient decision aids, the STROBE [8], which provides recommendations to improve the quality of reporting of observational studies or the systematic use of the PRISMA [5] as a quality standard for systematic literature reviews, may contribute to increase consistency, quality, and methodological rigor.
In addition to the lack of standards, the review also shows that the scarcity of patient safety data from PSPs prevents insight being gained into adverse events from a primary source [2], and therefore, the opportunity to ensure better risk management that would benefit the well-being of patients is lost [30]. Only one of the articles mentioned and discussed the adverse events reported during the study [10]. Additionally, none of the studies included in the review assessed a healthcare professional’s perspective of the PSP. Therefore, an essential opinion needed to gain a complete multidisciplinary perspective [31] contributing to the suitability of methods and its future evolution is missing. Hence, potential improvements to be considered in the design of future studies to evaluate PSPs concern the introduction of patient safety information and more involvement by healthcare professionals.
Therefore, there is a need to develop guidelines and recommendations to improve the framework for the evaluation of PSPs in Europe. This review may serve as a basis for providing recommendations for designing and implementing future PSP evaluation approaches that successfully meet the needs of patients, healthcare professionals, and regulatory authorities.
This literature review has several limitations. Some of these limitations relied on the search methods such as the databases searched because we did not include Embase or the search strategy, as we limited it to studies published in English or Spanish from 2010 onwards or conducted only in European countries. However, limiting our review to studies conducted in Europe is consistent with the study’s approach, has allowed us far greater homogeneity among the PSPs evaluated, and has ensured that these PSPs are part of a common overarching regulatory framework (i.e., EMA).
Other limitations are related to the review studies’ heterogeneity and the lack of control arms in half of them. Consequently, no conclusion could be drawn to determine if the interventions were superior to standard care.
5 Conclusions
The growing number of PSP-related publications in Europe during the last 10 years shows a growing interest in these self-management programs. As these initiatives may be promoted by different stakeholders other than the pharmaceutical industry, points to awareness regarding its relevance at different levels of action. Patient support programs may have an important role to play in improving the effectiveness of treatment, clinical outcomes, and patient satisfaction, especially in chronic diseases. However, in order to reach their full potential, the development of guidelines and recommendations to harmonize the definition of PSPs, standardize methods, systematically measure their impact, and develop and use new digital technologies should be a priority for the different stakeholders involved in their design and implementation.
References
Ganguli A, Clewell J, Shillington AC. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review. Patient Prefer Adherence. 2016;10:711–25. https://doi.org/10.2147/PPA.S101175.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP). Module VI: collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2). Vol. Revision 2. 2017.
Pharmaceutical Industry Associations. Workshop on Patient Support and Market Research Programmes 2011. Available from: https://www.ema.europa.eu/en/documents/presentation/presentation-workshop-patient-support-programmes-market-research-programmes-spectrum-programmes_en.pdf. [Accessed 5 May 2021].
Burudpakdee C, Khan ZM, Gala S, Nanavaty M, Kaura S. Impact of patient programs on adherence and persistence in inflammatory and immunologic diseases: a meta-analysis. Patient Prefer Adherence. 2015;9:435–48. https://doi.org/10.2147/PPA.S77053.
PRISMA guidelines. 2020. Available from: http://www.prisma-statement.org/ [Accessed 5 May 2021].
Higgins JPT, Green S. Manual Cochrane de revisiones sistemáticas de intervenciones. Available from: www.cochrane-handbook.org. [Accessed 5 May 2021].
US FDA. Using the PICOTS framework to strengthen evidence gathered in clinical trials: guidance from the AHRQ’s Evidence-based Practice Centers Program. Available from: https://www.fda.gov/media/109448/download. [Accessed 20 Apr 2021].
Vandenbroucke JP, Von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Gac Sanit. 2009;23:158. https://doi.org/10.1016/j.gaceta.2008.12.001.
Simons S, Ringsdorf S, Braun M, Mey UJ, Schwindt PF, Ko YD, et al. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer. 2011;19:1009–18. https://doi.org/10.1007/s00520-010-0927-5.
Rieckmann P, Schwab M, Pohlau D, Penner IK, Wagner T, Schel E, et al. Adherence to subcutaneous IFN beta-1a in multiple sclerosis: final analysis of the non-Interventional study READOUTsmart using the dosing log and readout function of RebiSmart. Adv Ther. 2019;36:175–86. https://doi.org/10.1007/s12325-018-0839-1.
Pozzilli C, Schweikert B, Ecari U, Oentrich W. Supportive strategies to improve adherence to IFN beta-1b in multiple sclerosis: results of the betaPlus observational cohort study. J Neurol Sci. 2011;307:120–6. https://doi.org/10.1016/j.jns.2011.04.026.
Van den Bosch F, Ostor A, Wassenberg S, Chen N, Wang C, Garg V, et al. Impact of participation in the adalimumab (Humira) patient support program on rheumatoid arthritis treatment course: results from the PASSION study. Rheumatol Ther. 2017;4:85–96. https://doi.org/10.1007/s40744-017-0061-7.
Ostor AGV, Yang M, Chamberlain C, Skup M. Estimating the economic value of a patient support program in rheumatoid arthritis in the United Kingdom. Annual European Congress of Rheumatology, EULAR; 2018;AB1232:1713.1. doi: https://doi.org/10.1136/annrheumdis-2018-eular.5685.
Nogues X, Luz Rentero M, Rodríguez AL. Use of an educational support program to assist patients receiving injectable osteoporosis treatment: experience with teriparatide. Curr Med Res Opin. 2014;30:287–96. https://doi.org/10.1185/03007995.2013.851659.
NCT 02750800. Post-marketing observational study to evaluate the incremental impact of AbbVie’s patient support program on patient reported outcomes and health resource utilization in inflammatory arthritis, psoriasis and inflammatory bowel diseases in Hungary (VALUE). Available from: https://www.clinicaltrials.gov/ct2/show/NCT02750800. [Accessed 20 Apr 2021].
López Giménez C, Checa Díaz P. Análisis observacional de un programa estructurado de soporte a pacientes con DM2 y en tratamiento con insulina glargina Congreso Sociedad Española de Endocrinología y Nutrición (SEEN) 2019. P81. Available from: https://www.elsevier.es/es-revista-endocrinologia-diabetes-nutricion-13-congresos-congreso-seen-2019-latam-congress-101-sesion-diabetes-mellitus-5494-comunicacion-analisis-observacional-de-un-programa-64803. Accessed 20 Apr 2021.
González Carretero J, Parras Rejano J, González Cecilio J, Fernández López S, S PC. Evaluación de un programa de soporte telefónico personalizado de ayuda al paciente diabético tipo 2 insulinizado con Glargina U300. P-232. Congreso Sociedad Española de Diabetes (SED) 2019. p. 144. Available from: https://www.elsevier.es/es-revista-endocrinologia-diabetes-nutricion-13-congresos-xxx-congreso-nacional-sociedad-espanola-98-sesion-tratamiento-dm2-5059-comunicacion-evaluacion-de-un-programa-de-59316. Accessed 20 Apr 2021.
Bellido Guerrero D, Bellido Castañeda V, Morales C, García-Almeida J, Muñoz Garach A, Fernández Morera J, et al. Effect of telephone coaching support on glycemic control in type 2 diabetes treated with glargine U300 insulin. P-717. Congress of American Diabetes Association (ADA). 2019. Available from: https://diabetesjournals.org/diabetes/article/68/Supplement_1/717-P/56339/717-P-Effect-of-Telephone-Coaching-Support-on. Accessed 20 Apr 2021.
van Maren MA WC, Driessen JHM, Visser JV, de Vries F, van de Wijdeven K, Gevers S, Lems WF, Emmelot-Vonk MH, van den Bergh JPW. Two-year persistence with teriparatide improved significantly after introduction of an educational and motivational support program. Osteoporos Int 2019;30(9):1837–44. https://doi.org/10.1007/s00198-019-05052-0.
Morgan K, Lott N, Lyons M. Year 1 performance of adveva® a patient support programme (PSP) for patients taking MAVENCLAD® (Cladribine tablets). National clinical homecare association (NCHA). 2020.
Lyons M, Lott N, Morgan K. Year 1 performance of adveva®, a patient support programme (PSP) for patients taking MAVENCLAD® (cladribine tablets) for highly active relapsing remitting nultiple sclerosis (RRMS) in United Kingdom (UK) European committee for treatment and research in multiple sclerosis (ECTRIMS) 2019:P-1593.
Bellido Castañeda V, Bellido Guerrero D, Morales C, García-Almeida J, Muñoz Garach A, Fernández Moreda J, et al. Análisis observacional retrospectivo de los resultados del T-Coach®, el programa de soporte a pacientes tratados con insulina glargina 300U en España. P-170 Congreso Sociedad Española de Diabetes (SED). p. 119. 2019. Available from: https://www.elsevier.es/es-revista-endocrinologia-diabetes-nutricion-13-congresos-xxx-congreso-nacional-sociedad-espanola-98-sesion-monitorizacion-de-la-diabetes-y-5056-comunicacion-analisis-observacional-retrospectivo-de-los-59261. Accessed 20 Apr 2021.
Afán de la Ribera S, Sierra Poyatos J, Cárdenas Salas J LB, Silva Rodríguez M, Suárez Vásconez C, Vázquez Martínez C. Experiencia clínica con el programa de teleasistencia T-Coach en DM-2 en tratamiento con insulina basal. P-141.Congreso Sociedad Española de Endocrinología y Nutrición (SEEN). 2019. Available from: https://www.elsevier.es/es-revista-endocrinologia-diabetes-nutricion-13-congresos-congreso-seen-2019-latam-congress-101-sesion-diabetes-mellitus-5494-comunicacion-experiencia-clinica-con-el-programa-64827. Accessed 20 Apr 2021.
Kohlmann T, Wang C, Lipinski J, Hadker N, Caffrey E, Epstein M, et al. The impact of a patient support program for multiple sclerosis on patient satisfaction and subjective health status. J Neurosci Nurs. 2013;45(3):E3–14. https://doi.org/10.1097/JNN.0b013e31828a4161.
Ryan C, Middleton C, Lucas J, Hetherington J, Calimlim B. Assessing the impact of patient support programs in adalimumab-treated adults with psoriasis in Europe. J American Academy Dermatology. 2019:P-9826.
Ryan C, Calimlim B, Lucas J, Skup M, Lobosco S. Assessing the impact of patient support programs on patient outcomes in adalimumab-treated patients with psoriasis in Europe. J American Academy Dermatology. 2018:P-6451.
González B, Lorenzo C, Morales C, Santos M. Impacto del programa de soporte telefónico a pacientes con diabetes mellitus tipo 2 tratados con insulina glargina U300 (Plataforma TCOACH). Congreso Sociedad Española de Diabetes (SED) 2019.p. 83.
Murthy V, Weaving S, Paneesha S. Imbruvica® (ibrutinib) patient support programme for chronic lymphocytic leukaemia and mantle cell lymphoma. Br J Nurs. 2017;20(10):S20–5. https://doi.org/10.12968/bjon.2017.26.10.S20.
Thomas C., Wallace P., Ronte H., Ford J. Support programmes driving competitive advantage and commercial success. 2020. Available from: https://www2.deloitte.com/content/dam/Deloitte/uk/Documents/life-sciences-health-care/deloitte-uk-patient-support-programmes.pdf7Patient. Accessed 20 Apr 2021.
Frost and Sullivan. 2018. Available from: https://go.frost.com/NA_PR_MFernandez_K200_Feb18. Accessed 20 Apr 2021.
Feys P, Giovannoni G, Dijsselbloem N, Centonze D, Eelen P, Lykke Andersen S. The importance of a multi-disciplinary perspective and patient activation programmes in MS management. Mult Scler. 2016;22(2 Suppl):34–46. https://doi.org/10.1177/1352458516650741.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Eli Lilly funded the development of the study and the writing of the article.
Conflicts of Interest/Competing Interests
JAS, EA, and SDC are employees of Eli Lilly. MC, LPC, and LL work for an independent research entity that received funding to conduct the study and to write the article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current review are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
JAS: conceptualization; methodology; supervision; roles/writing, original draft; writing, review, and editing; EA: conceptualization; data curation; formal analysis; methodology; roles/writing, original draft; writing, review, and editing; SDC: conceptualization; methodology; supervision; roles/writing, original draft; writing, review, and editing; MC: conceptualization; methodology; supervision; roles/writing, original draft; writing, review, and editing; LPC: data curation; formal analysis; methodology; roles/writing, original draft; writing, review, and editing; LL: conceptualization; methodology; supervision; roles/writing, original draft; writing, review, and editing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sacristán, J.A., Artime, E., Díaz-Cerezo, S. et al. The Impact of Patient Support Programs in Europe: A Systematic Literature Review. Patient 15, 641–654 (2022). https://doi.org/10.1007/s40271-022-00582-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-022-00582-y