Abstract
Introduction
Qualitative exit interviews can supplement clinical trial results by providing a rich and detailed picture of the patient’s experience, while highlighting the treatment benefits that are meaningful to patients. Exit interviews can be particularly useful for providing insight into newer medications when less is known about the patient’s subjective experience of treatment. Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist for type 2 diabetes mellitus. The purpose of this study was to conduct exit interviews with patients following participation in two trials to better understand the impact of tirzepatide from the patients’ point of view.
Methods
Telephone interviews were conducted with patients with type 2 diabetes treated with tirzepatide soon after completing one of two trials (SURPASS-2, SURPASS-3). Interviews, conducted according to a semi-structured interview guide, were recorded, transcribed, and analyzed following a content analysis approach using ATLAS.ti.
Results
A total of 28 patients (64% female; mean age 57.6 years) completed interviews. All participants (100%) reported at least one treatment benefit. Patients provided descriptions of treatment benefits, including improved glycemic control (reported by 96% of the sample), weight loss (93%), decreased appetite (79%), and increased energy (79%), as indicated by qualitative coding. All participants said these treatment-related changes mattered to them. Patients described improvements in quality of life and daily activities associated with these treatment benefits. Despite adverse events reported by some patients (most commonly nausea, reported by 13 patients), all 28 said they would recommend tirzepatide to others, and 27 said they would be willing to continue treatment. Examples of representative quotations are presented for descriptions of treatment benefits, quality-of-life impact, and adverse events.
Discussion
The current results indicate that treatment benefits observed in clinical trials of tirzepatide are important to patients. As demonstrated in quotations from patients, the most enthusiastic descriptions of treatment outcomes focused on the weight loss associated with tirzepatide. The study also highlights the usefulness of exit interviews, which can supplement quantitative trial data by showing how these benefits have a meaningful impact on patients’ quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This exit interview study adds to research supporting tirzepatide for treatment of type 2 diabetes, with detailed descriptions of treatment outcomes coming directly from patients. |
In these interviews, patients’ most enthusiastic descriptions of treatment outcomes focused on the weight loss associated with tirzepatide. |
While published clinical trial outcomes demonstrate efficacy of tirzepatide, these exit interviews indicate that these benefits are important to patients, with a meaningful impact on their quality of life. |
1 Introduction
Qualitative interviews with patients in a clinical trial can provide insight into the patient’s treatment experience [1]. When conducted after the trial’s treatment period is completed, these are typically called ‘exit interviews.’ These interviews have been recommended as a method for determining whether treatment-related changes are truly meaningful to patients [2, 3], and they have been used for this purpose across a range of medical conditions [4,5,6,7,8]. Because exit interviews can explore the same outcomes as those assessed by the clinical trial endpoints, the resulting qualitative data can increase interpretability of the quantitative trial results [9]. Exit interviews can be particularly helpful with providing insight into newer medications when less is known about the patient’s subjective experience of treatment.
Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist administered as a once-weekly injection for treatment of type 2 diabetes mellitus [10]. Tirzepatide has demonstrated efficacy and safety in the SURPASS phase III clinical trial program. In SURPASS-1, tirzepatide was superior to placebo in reduction of HbA1c and body weight [11]. SURPASS-2 was a head-to-head trial versus semaglutide, a once-weekly injectable GLP-1 receptor agonist [12]. Tirzepatide demonstrated superiority over semaglutide in glycemic control and weight reduction, and the two treatments had similar rates of gastrointestinal adverse events. In SURPASS-3, tirzepatide was superior to insulin degludec as an add-on to oral medication, with greater reductions in HbA1c and body weight and a lower risk of hypoglycemia [13]. As in SURPASS-2, tirzepatide demonstrated a similar safety profile to that of GLP-1 receptor agonists, with the most common adverse events being mild to moderate gastrointestinal events.
While these phase III results demonstrate efficacy and safety of tirzepatide, quantitative analysis of clinical endpoints does not provide direct insight into the patient’s experience of treatment with this novel medication. Therefore, the purpose of this study was to conduct exit interviews with patients following their participation in the SURPASS trials to better understand the impact of treatment from the patients’ point of view. This qualitative study was designed to supplement the clinical trial results by providing a rich and more detailed picture of the patient’s experience, while highlighting the treatment benefits that are most meaningful to patients.
2 Methods
2.1 Overview of Study Design
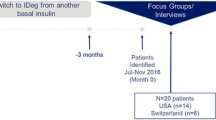
This cross-sectional qualitative exit interview study involved one-on-one telephone-based interviews with 28 patients who had been treated with tirzepatide as part of the SURPASS-2 or SURPASS-3 clinical trials. Previously published articles have reported an overview of the SURPASS trial program [14] as well as the primary results of the SURPASS-2 and SURPASS-3 open-label trials [12, 13]. Participants were recruited from six US clinical sites, located in California, Florida, North Carolina, Oklahoma, and Texas, which had two sites. All interviews were conducted by interviewers who had been trained by the principal investigator and project manager of this qualitative study with regard to the interview guide for this study. Each interviewer had also received prior general training on qualitative research methods. In addition, to maximize quality and consistency across interviews, each interviewer was observed conducting an interview by either the principal investigator or project manager. Each interview lasted approximately 90 minutes and focused on the patient’s experiences with tirzepatide during the SURPASS trial. Interviews were recorded and transcribed, and the transcriptions were coded so that patterns of responses could be identified and quantified. All methods and materials were approved by an Independent Review Board (Ethical and Independent Review Services [E&I], E&I study number 20122-01), and all participants provided written informed consent for participation in the follow-up exit interview study prior to completing any study procedures. Each participant received remuneration of US$150.
2.2 Participants
Adult patients with type 2 diabetes who received treatment with tirzepatide in the SURPASS-2 and SURPASS-3 open-label phase III trials were invited to participate in this study. Detailed study inclusion criteria have been published previously [12, 13]. SURPASS-2 and SURPASS-3 had 40-week and 52-week treatment periods, respectively. In both studies, the treatment period was followed by a 4-week safety period in which patients did not receive tirzepatide. Participants were recruited for the qualitative exit interview as soon as possible after completing the 4-week follow-up safety period.
Although both trials had comparator arms (semaglutide in SURPASS-2 and insulin degludec in SURPASS-3), only patients with tirzepatide were invited to participate in the exit interviews because the purpose of this qualitative study was to gain insight into the experience of patients receiving this new class of medication. Patients who discontinued tirzepatide prior to completing the 40-week or 52-week treatment periods were eligible to participate in the exit interviews to allow for a range of experiences to be examined in this qualitative study. Representatives from the six clinical sites contacted all tirzepatide-treated patients who completed the study at their sites within the enrollment period and met eligibility criteria, except for one patient who was lost to follow-up and was no longer responsive to any contact related to the trial. A target sample size of 20 to 30 participants was specified a priori in the study protocol. Saturation (i.e., the point at which interviews no longer yield important new information) was checked to confirm that the sample size was sufficient.
2.3 Data Collection
2.3.1 Qualitative Interview
Qualitative interviews were conducted following a semi-structured interview guide (provided as electronic supplementary material [ESM]) designed to elicit discussion of patients’ experience with tirzepatide during the SURPASS trial. Patients were asked about the effects of the treatment, treatment-related changes, aspects of treatment they liked/disliked, the impact of treatment on daily activities and quality of life, the emotional impact of treatment, and the tirzepatide injection device.
2.3.2 Patient-Completed Questionnaires
After the qualitative interview, participants completed the EQ-5D-5L, a self-administered, generic, preference-weighted measure designed to assess health status [15]. The EQ-5D-5L was scored as suggested by Pickard and colleagues [16] for US samples. Higher scores for the index score and the visual analogue scale (VAS) indicate better overall health status. The EQ-5D-5L was administered to provide an indication of the general health status of the sample.
Participants also completed a sociodemographic and clinical form including items on age, gender, living situation, employment, education level, racial/ethnic background, and general health-related questions. Printed copies of both questionnaires were mailed to participants before the interview, and participants completed the questionnaires when instructed by the interviewer. Responses were then dictated to the interviewer over the telephone.
2.3.3 Site-Completed Questionnaire
Staff members at the six clinical sites completed a clinical information form for each enrolled participant to report the type 2 diabetes diagnosis date, current medications for type 2 diabetes, most recent HbA1c value, and the patient’s height and weight for calculation of body mass index (BMI).
2.4 Analysis Procedures
2.4.1 Quantitative Analysis
Responses on the patient-completed and site-completed forms were summarized with descriptive statistics (means and standard deviations [SDs] for continuous variables; frequencies and percentages for categorical variables). No statistical comparisons were conducted.
2.4.2 Qualitative Analysis
The transcribed interview dialogue was analyzed following a content analysis approach [17] using ATLAS.ti (version 8), a software program designed for analysis of qualitative data. A coding dictionary was developed based on the themes and concepts that emerged during the discussions. The coding dictionary standardizes the coding process by providing a list of all potential codes with the definition of each code and instructions to coders for how each code should be applied and combined with other codes. Words and phrases from the transcripts were selected based on the coding dictionary and grouped into key themes, attributes, concepts, and relationships.
All coders had received training in qualitative analysis theory and practice, in addition to study-specific training on the coding dictionary. Three staff members independently coded the first interview transcript. A post-coding comparison and reconciliation occurred, and all codes were compared, discussed, and reconciled wherever differences emerged. After agreement was reached and the analysis team was confident that the coders were applying the codes in a consistent manner, the three coders independently coded the remaining transcripts. A quality review by senior staff members was performed. After the coding was finalized, the coded data were used to develop a saturation grid where concepts that emerged were listed along the y-axis, and the interview participants were listed along the x-axis. The saturation grid documents each concept emerging in each interview and the number of respondents who report each concept and category.
3 Results
3.1 Sample Characteristics
A total of 28 patients participated in the qualitative interview study between September and November 2020. One participant discontinued treatment early (after 105 days in the SURPASS-2 trial). All other participants completed the full treatment, which was 40 weeks in SURPASS-2 and 52 weeks in SURPASS-3. The average age of participants was 57.6 years (Table 1). Most participants were female (n = 18; 64%), and the sample was predominantly White (n = 16; 57%) or Black or African American (n = 7; 25%). All participants (n = 28; 100%) reported being treated with metformin prior to study entry. Over half of the participants (n = 17; 61%) reported no prior experience with injectable medications.
Almost all patients were enrolled in the SURPASS-2 trial (n = 27; 96%), whereas only one patient was enrolled in SURPASS-3. Exit interview participants were treated with tirzepatide for an average of 272.1 days. The exit interviews occurred an average of 65.6 days after the end of the 4-week follow-up safety period. Most participants were taking metformin (n = 23; 82%) at the time of the exit interview, and the participants’ average HbA1c was 6.6%.
The average EQ-5D-5L scores were similar to those for previous clinical trial samples of patients with type 2 diabetes [18]. In each of the five dimensions assessed by this questionnaire (mobility, self-care, usual activities, pain/discomfort, anxiety/depression), there were some patients who reported having slight-to-moderate problems. The areas where patients were most likely to report problems were pain/discomfort (slight: 46% of the sample; moderate: 18%) and mobility (slight: 36%; moderate: 14%). The mean VAS was 81.43 (SD 11.54), and the mean EQ-5D-5L index score was 0.83 (SD 0.14).
3.2 Saturation
Saturation was tracked as part of the analysis to confirm that the sample size was sufficient for the study purpose. All treatment benefits listed in Table 2 were mentioned by the 20th interview. Furthermore, no new adverse events were mentioned after the 22nd interview. Based on these data, it appears that saturation was reached, and the current sample size of 28 participants can be considered adequate for the current study purpose.
3.3 Treatment Benefits Associated with Tirzepatide
During the interview, participants were asked if they experienced any changes due to treatment (i.e., “Did you notice any changes due to the treatment?”). All participants (100%) reported at least one treatment benefit. Treatment benefits reported by the participants are summarized in Table 2, and examples of representative quotations for the four most commonly reported outcomes (i.e., blood glucose, weight, appetite, energy) are presented in Table 3.
As presented in Table 2, > 90% of patients reported positive changes in blood glucose levels. When discussing the improvements in blood glucose levels, participants reported blood glucose staying in a lower range, better blood glucose control, less fluctuation in blood glucose, less worry about high blood glucose, and lower HbA1c. Nearly all participants (93%) also reported positive experiences related to weight loss during treatment with tirzepatide. Many noticed clothes fitting more loosely and reported that they needed to buy new clothes. Participants also expressed feeling ‘confident,’ ‘optimistic,’ and ‘excited’ because of their weight loss. Twenty-two (79%) participants reported positive changes related to appetite suppression, being able to control cravings, and reduced binge eating. The majority of patients (n = 22; 79%) also reported positive changes in energy. Participants said they had more energy, did not feel tired or sluggish, and were able to go outside and exercise more.
Less frequently reported areas of treatment benefit included sleep (29%) and blood pressure (21%). Participants described improved sleep due to reduction in sleep apnea (“It helped me decrease my issues with my sleep apnea” [M, 52 y]), foot pain (“I'm sleeping better… my feet would drive me nuts before. I'd be in pain somewhere, somehow, some way” [M, 64 y]), and night-time urination (“I was able to sleep through most of the night without getting up” [M, 57 y]). Six participants noted that their blood pressure decreased during the tirzepatide trial (e.g., “I felt healthier. With my weight loss, the blood pressure came down. That was important to me because high blood pressure runs in the family and getting that lower really eased my mind about the side effects of high blood pressure” [F, 56 y]).
3.4 Impact of Tirzepatide on Quality of Life and Daily Activities
Participants were asked about the impact of treatment in the SURPASS trials on their ability to participate in daily activities, exercise, leisure activities, social activities, and work, as well as the impact of treatment on the time they had available for these activities (Table 4) (e.g., “Has the study medication had an impact on your ability to perform activities?” “Has the study medication had an impact on the time you have available for these activities?”). Impact on quality of life and daily activities was coded as positive or negative. Almost all (n = 26; 93%) participants reported a positive impact of tirzepatide on quality of life and daily activities.
Eighteen participants reported a positive impact of tirzepatide in areas coded as ‘daily activities,’ often resulting from increased energy and weight loss (see quotations in Table 5). Two participants (7%) reported a negative impact on their ability to participate in these daily activities due to adverse events of the treatment.
Eighteen participants (64%) reported increased ability to exercise during treatment with tirzepatide. Most of these participants perceived the improvement in their ability to exercise to be associated with weight loss and increased energy. Twelve participants (43%) reported a positive impact of tirzepatide on their ability to work. Most of these participants associated the improvement in their ability to work with their increased energy level resulting from treatment. Two participants (7%) reported a negative impact of tirzepatide on their ability to work. One of these reported being concerned about diarrhea at work, and the other reported experiencing some loss of strength that had an impact on work capacity. Thirteen participants (46%) reported a positive impact of tirzepatide on their ability to participate in leisure activities, and four participants (14%) reported that tirzepatide had a positive effect on their ability to participate in social activities.
3.5 Importance of Treatment-Related Changes
Participants were asked if the changes experienced during the trial mattered to them. All participants (n = 28; 100%) reported that the changes did matter. For example, one participant said “Yes. Because the quality of life changed a lot. I could eat. I can walk. No more pain in my knee. I didn’t have to control what I eat that much. I could sleep. The change was enormous” (F, 61 y). Another said “Oh yeah. Absolutely… The main thing is that I just feel more confident about managing diabetes, that it can be done and I'm more aware. It just kinda woke me up, you know? And definitely not something that stresses me out and depresses me anymore” (F, 55 y).
3.6 Willingness to Continue Treatment with Tirzepatide
Participants were asked about their willingness to continue treatment with tirzepatide, and nearly all participants (n = 27; 96%) reported that they would be willing to continue treatment with tirzepatide (e.g., “I would take it. I would definitely take it” [F, 63 y]). Only one participant (4%) was unsure about continuing treatment due to concern about a plateau in weight loss.
All participants (n = 28; 100%) said they would recommend tirzepatide to others. For example, one participant said “I would definitely recommend it,” (F, 51 y) and another reported having already spoken with friends about the medication: “I always talk about this medicine with everybody that is diabetic…Because the impact was, oh my god, phenomenal” (F, 61 y). Another participant emphasized her willingness to share information about tirzepatide by saying “Oh, definitely. Put me on. I'll do a commercial. I'm serious. My friends just told me, ‘You look amazing’" (F, 66 y).
3.7 Injection Device
Of the 28 participants, 27 were asked about the tirzepatide injection device. All 27 reported that the device was simple or easy to use. For example, one participant said “It was very simple to use. I'd tap it, unlock it, clean your site, inject. It was the easiest thing I ever had to use... All in one, twist and done” (F, 56 y). Another said “[It was] pre-loaded, and all you had to do was unlock it and push the button. I mean it can't be any simpler than that…There wasn't any guesswork about it at all. You didn't have to draw up a certain amount of medication or anything. It was all there for you” (F, 67 y). Only two participants reported challenges associated with the injection device. Both of these participants had difficulty with the cap. One participant was worried about breaking the needle when removing the cap, and the other had difficulty remembering to remove the cap.
3.8 Adverse Events
When asked whether they had experienced adverse events during the trial, 24 participants reported at least one adverse event. The most common adverse event was nausea, reported by 13 participants. This adverse event varied in severity. For example, one participant described mild brief nausea: “Sometimes in the mornings when I would first get up, I would feel nauseous. I never threw up, but I just would feel nauseous. And I kept ginger ale here and I would just sit and sip on it for just a little bit and then the nauseousness would go away” (F, 73 y). Another participant reported more persistent nausea: “I would take it, I would be nauseous. Would take it on a Saturday. By Saturday night…I didn’t feel well. In bed all day Sunday usually. Didn’t feel great on Monday. Started feeling a little better on Tuesday. I felt better on Wednesday, Thursday, finally had an appetite on Friday, and then I took the shot again on Saturday” (F, 55 y). Six of the 13 participants who reported nausea said it occurred shortly after each weekly injection and faded before the next administration. Four of the 13 said the nausea resolved after the first few weeks of treatment (e.g., “The first couple of weeks, I had a little bit of nausea and a little bit of diarrhea, the first day or two after the shot, but after those I had no sign, nothing” [M, 65 y]). Seven participants (n = 7) reported vomiting associated with the tirzepatide injection, but this also tended to occur early during the treatment (e.g., “The first couple of weeks I took the medication, I was getting sick. I'd be throwing up. And that lasted for like two or three weeks. And then after that, it tapered off. It got better” [F, 56 y]).
Eight participants reported other gastrointestinal adverse events including diarrhea (n = 5, “I would have diarrhea once a week” [M, 64 y]), abdominal pain/cramping (n = 3, “My abdomen hurt all the time for six weeks. It's like I'm catching cramps left and right” [M, 52 y]), belching (n = 3, “It just made me burp a lot, and the smell of the burp of the medication was just horrible” [F, 35 y]), and constipation (n = 2, “Constipation was not fun either… There are a few remedies to fix the side effects, to correct them and one of them was to take laxatives or stool softener every day” [M, 52 y]).
Thirteen participants reported non-gastrointestinal adverse events, but these were usually described as mild. Participants described side effects related to the injection site, including bruising (n = 3, “Every once in a while…it would bruise a little bit. But that was rare” [M, 56 y]), swelling (n = 3, “I only had one type of swelling, and after they told me how to massage it, I didn't have it anymore, so it was very simple” [F, 64 y]), redness (n = 2, “Minor redness…It was minor. It wasn’t anything major” [F, 36 y]), itchiness (n = 2, “It just itched a lot when I did the needle, but I rubbed alcohol and stuff on it” [F, 66 y]), and pain (n = 1, “Sometimes when you've angled the pen, if you're not angled in a good position, you could get a bruise, and it hurts” [F, 63 y]). Participants also reported rash or hives (n = 3, “And, broke out in hives from the injection site all the way up to my arm and possibly across my back” [M, 64 y]) and fatigue (n = 2, “I got real slow, like lazy. I don't know what it was, but I was tired or whatever” [F, 66 y]).
Only one participant discontinued tirzepatide treatment due to adverse events (vomiting, rash), but this participant explained that she would like to use the medication again because she liked the weight loss: “I would try it again…I liked the way I felt because I [was] losing weight” (F, 64 y).
4 Discussion
These qualitative findings add to clinical trial results [12, 13] by providing detailed descriptions of the benefits of tirzepatide from the perspective of patients treated for type 2 diabetes. While the published clinical trial outcomes demonstrate clinical efficacy, the current qualitative results indicate that these changes are important to patients, with a meaningful impact on their quality of life. Almost all patients (96%) who completed the exit interviews reported improved glycemic control, and their detailed descriptions (Table 3) suggest that they perceive this treatment benefit to be meaningful with significant impact on quality of life (Table 5), allowing them to live with less worry and a greater activity level. Furthermore, when asked if the treatment-related changes mattered to them, all patients said “yes,” while reporting benefits for health-related quality of life (e.g., “I feel more confident about managing diabetes” [F, 55 y], “I could be more active” [M, 64 y], “I have more energy” [These words were spoken by two participants; [F, 55 y and F, 66 y], “Quality of life changed a lot” [F, 61 y]).
In these exit interviews, the patients’ most enthusiastic descriptions of treatment outcomes focused on the weight loss associated with tirzepatide. Over 90% of participants reported losing weight during the trial, and quotations (Table 3) clearly indicate that patients were pleased with these results (e.g., “the weight loss was fabulous” [F, 55 y], “It brought my weight down about 10–15 pounds. It’s a wonderful drug” [M, 64 y]). Many of the statements in Table 5 highlight that patients believed the weight loss had a broad impact in multiple aspects of quality of life and daily activities, such as exercise, leisure activities, being more active, spending time with children, yardwork, and household chores. While clinical trial results have documented the weight loss associated with tirzepatide [11,12,13], the qualitative exit interview data provides a rich picture of the impact of this weight loss on patients’ lives.
These qualitative data also show that improvements in glycemic control, body weight, and energy can have an emotional impact. The sample quotations presented in Tables 3 and 5 include a wide range of emotional language. Patients reported less ‘worrying,’ not feeling ‘fearful,’ and feeling ‘happy’ about the weight loss. There were also examples of patients reporting increased optimism (“My outlook on life was even better” [F, 66 y], “There was hope” [F, 63 y]). Despite the emotional effects of these clinical benefits, clinical trials of treatment for type 2 diabetes do not generally include measures of emotional impact. Current results suggest it may be useful to develop a patient-reported outcome measure assessing emotional impact of treatment for type 2 diabetes. Such a measure could be administered as an outcome in clinical trials to better understand the impact of treatment benefits from the patient’s perspective.
Participants also described their experience with adverse events during the trials. As in the larger clinical trial samples [12, 13], the most common side effects were gastrointestinal, most commonly nausea, vomiting, and diarrhea. Incidence of adverse events reported in this sample (N = 28) differed from those in the full clinical trial samples (N = 1409 treated with tirzepatide in SURPASS-2 [12]; N = 1077 treated with tirzepatide in SURPASS-3 [13]). Because this exit interview study was conducted in a small subset of the trial samples, the frequency of adverse events is not comparable to those from the full sample and should be interpreted with caution. Still, qualitative exit interview data provide insight into how and when patients tend to experience these adverse events. For example, patients reported that the side effects tended to occur most frequently early in the trial and during the days immediately following the weekly injection. Despite the fact that these adverse events were relatively common, qualitative results suggest that patients believe the benefits of tirzepatide outweigh the risk of adverse events. All patients (100%) reported that they would recommend tirzepatide to others, and 96% said they would be willing to continue treatment.
Some of the qualitative results raise questions about the appropriate tone for a scientific publication reporting exit interview results. Many of the patients spoke in colorful positive terms about the weight loss they experienced during the trial, and some of these enthusiastic quotes are presented in Table 3. Many of these quotes include language and tone not typically included in peer-reviewed scientific journals. However, the authors decided to include these quotes so that the patients' perspective could be represented accurately in their own words. Detailed quotations in which patients describe adverse events have also been presented to provide a balanced picture of patients’ experience with this new treatment class. In future exit interview studies, researchers will need to consider how to represent the patient perspective when patients’ language diverges from the typical tone of scientific publications.
Like most exit interview studies, this research was conducted in a small subset of 27 patients who had completed the SURPASS-2 trial (N = 1879 [12]) and one patient who had completed SURPASS-3 (N = 1444 [13]). It is possible to compare the current sample to the larger trial populations with regard to key demographic and clinical variables. For example, this sample’s mean age of 57.6 years was similar to the mean age of the SURPASS-2 sample (56.6 years at baseline, roughly a year before these exit interviews). Compared with the full SURPASS-2 sample, the current sample had a higher percentage of women (64.3% vs 53.0%) and a lower percentage of White participants (57.1% vs 82.6%). The current sample seems similar to the full SURPASS-2 sample in key clinical characteristics. For example, baseline HbA1c of the trial sample was 8.28%, with mean baseline-to-endpoint decreases of 2.01 to 2.30%, depending on dose. The mean post-trial HbA1c of the current sample was 6.6%. The mean BMI of the current sample (33.0 kg/m2) also seems typical of patients following tirzepatide treatment in the SURPASS-2 trial (i.e., 34.2 kg/m2 at baseline, followed by mean reductions of 7.8–12.4 kg from baseline to endpoint). Furthermore, the positive perceptions of treatment outcomes reported by patients during the exit interviews are consistent with the significant improvements in glycemic control and body weight among the larger trial population. In sum, the current sample appears reasonably similar to the overall SURPASS-2 trial population, although there are some differences in gender and race.
A limitation of this study is that the interviews were conducted only with tirzepatide-treated patients, not with trial participants who received one of the comparator treatments (semaglutide in SURPASS-2 and insulin degludec in SURPASS-3). While the qualitative results were consistently favorable for tirzepatide, it is not known whether patients treated with the comparators would have had similarly positive impressions of their treatments. Future research involving exit interviews with patients from subsequent SURPASS trials may examine whether between-treatment differences in clinical results are reflected in patients’ qualitative descriptions.
Another limitation is that data availability did not allow for consideration of differences associated with the dose of tirzepatide. Patients were randomly assigned to receive one of three weekly doses of tirzepatide (5 mg, 10 mg, or 15 mg). Although both treatment benefits and adverse events may be dose dependent, information on dosing was not available to the team conducting the exit interview study. Therefore, the current results did not provide insight into whether specific doses of tirzepatide are most likely to be associated with the treatment benefits that patients perceived to be meaningful.
There may also be a limitation associated with recall bias. On average, these exit interviews were conducted 65.6 days after patients had completed the trial, and their descriptions of treatment outcomes could have been different if interviews had been conducted immediately upon exiting the trial. It is not known whether their perceptions would have been more positive or negative with a shorter duration between the trial and the exit interviews.
5 Conclusion
Overall, this exit interview study adds to the body of literature supporting tirzepatide for treatment of type 2 diabetes, with detailed descriptions of treatment outcomes coming directly from patients. The study also highlights the usefulness of exit interviews, which can be an important source of information across a wide range of diseases and treatments. While clinical trials demonstrate treatment benefit quantitatively in large samples, qualitative exit interviews can supplement trial data by showing how these benefits have a meaningful impact on patients’ lives.
References
Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CBER). Patient-focused drug development: methods to identify what is important to patients guidance for industry, Food and Drug Administration Staff, and Other Stakeholders—DRAFT guidance. October 2019, US Department of Health and Human Services FDA: Silver Spring, MD. p. 1–45.
Food and Drug Administration (FDA). Patient-focused drug development guidance public workshop—incorporating clinical outcome assessments into endpoints for regulatory decision-making. Workshop Date: December 6, 2019: Silver Spring, MD. p. 1–50.
Food and Drug Administration (FDA). Patient-focused drug development guidance public workshop—methods to identify what is important to patients and select, develop or modify fit-for-purpose clinical outcomes assessments. Workshop Date: October 15–16, 2018: Silver Spring, MD. p. 1–57.
Anthony L, et al. Understanding the patient experience with carcinoid syndrome: exit interviews from a randomized, placebo-controlled study of telotristat ethyl. Clin Ther. 2017;39(11):2158–68.
Ervin C, et al. Insights into patients’ experience with type 1 diabetes: exit interviews from phase III studies of sotagliflozin. Clin Ther. 2019;41(11):2219-2230 e6.
Ervin CM, et al. Exploring the diabetic gastroparesis patient experience: patient exit interviews. Adv Ther. 2017;34(12):2680–92.
Gelhorn HL, et al. Patient-reported symptom experiences in patients with carcinoid syndrome after participation in a study of telotristat etiprate: a qualitative interview approach. Clin Ther. 2016;38(4):759–68.
Lewis S, et al. Analysis of clinical trial exit interview data in patients with treatment-resistant depression. Patient. 2019;12(5):527–37.
Staunton H, et al. An overview of using qualitative techniques to explore and define estimates of clinically important change on clinical outcome assessments. J Patient Rep Outcomes. 2019;3(1):16.
Frias JP, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–93.
Rosenstock J, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–55.
Frias JP, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15.
Ludvik B, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583–98.
Min T, Bain SC. The role of tirzepatide, dual gip and glp-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 2021;12(1):143–57.
EuroQol Research Foundation. EQ-5D-5L user guide. 2019 August 9, 2021]; Available from: https://euroqol.org/publications/user-guides.
Pickard AS, et al. United states valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–41.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Janssen MF, et al. The use of the EQ-5D preference-based health status measure in adults with Type 2 diabetes mellitus. Diabet Med. 2011;28(4):395–413.
Acknowledgements
This study was funded by Eli Lilly and Company. The authors would like to thank Jennifer Weller for assistance with site recruitment; Timothy Howell for assistance with literature review; Jessica Jordan for assistance with protocol development; Haylee Andrews, Sonya Stanczyk, and Marissa Walsh for assistance with data collection and coding; Robyn Cyr for statistical programming; and Amara Tiebout for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly and Company (Grant no. EVA-28349).
Conflict of interest
Authors LSM and KDS are employed by Evidera, a company that received funding from Eli Lilly and Company for time spent conducting this research. Authors LFL, HP, and KSB are employed by and own stock in Eli Lilly.
Ethics approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent to participate
Informed consent was obtained from all study participants.
Consent for publication
Individuals provided consent to participate in the study and have their anonymized data published.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Data collection and analysis were directed by LM, KS, and KB. The first draft of the manuscript was written by LM and KS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matza, L.S., Stewart, K.D., Landó, L.F. et al. Exit Interviews Examining the Patient Experience in Clinical Trials of Tirzepatide for Treatment of Type 2 Diabetes. Patient 15, 367–377 (2022). https://doi.org/10.1007/s40271-022-00578-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-022-00578-8