Abstract
Background
Multicentric Castleman’s disease (MCD) is a rare lymphoproliferative disorder driven by dysregulated interleukin-6 production. MCD has a poor prognosis, and treatment is generally noncurative and aimed at symptom relief. Siltuximab is a novel, monoclonal interleukin-6 antibody recently shown to be effective in a registration clinical trial. MCD symptoms, such as fatigue, pain, and weakness, are most appropriately quantified using patient-reported outcome (PRO) measures. We assessed the effect of siltuximab on patient perception of symptoms, functional status, and wellbeing using PRO instruments.
Methods
We analyzed results of a randomized, double-blind trial comparing siltuximab 11 mg/kg every 3 weeks with placebo to treat MCD. Subjects (N = 79) completed the recently developed MCD–Symptom Scale (MCD–SS), the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT–Fatigue) scale, and the Short Form (SF)-36 at predetermined time points throughout the treatment period. Scores were compared at baseline and over time between the treatment arms and PRO instruments.
Results
At baseline, the mean number of symptoms reported was 9.2 (standard deviation 3.76) out of 16 total, as measured by the MCD–SS. Fatigue was a key symptom across all PRO instruments. Siltuximab-treated subjects reported early improvements in symptoms compared with subjects in the placebo arm on both the MCD–SS and FACIT–Fatigue scale. Statistically significant improvements in five SF-36 domains were observed in siltuximab-treated patients, namely role physical, role emotional, vitality, bodily pain, and mental health.
Conclusions
Patients with MCD commonly report impairments in functioning, wellbeing, and fatigue at baseline. Siltuximab-treated patients reported significant improvements in these outcomes after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Established patient-reported outcome (PRO) instruments may not measure the full spectrum of symptoms encountered by patients with rare diseases. |
Multicentric Castleman’s disease (MCD) patients often describe a wide range of subjective symptoms, highlighting the impact of this disease on quality of life. |
Previously, MCD was treated with nonspecific anti-proliferative therapies, which often failed to resolve interleukin-6-driven symptoms, such as fatigue, sweats, and malaise. |
Siltuximab treatment enabled symptom improvement. Specifically, alleviation of fatigue was accompanied by other measures of improved functioning. |
1 Introduction
Castleman’s disease is a rare lymphoproliferative disorder that was first described in the mid-1950s in patients with unicentric disease (single lymph node involvement) [1, 2] and in subsequent decades in patients with multicentric disease (multiple lymph nodes affected) [3, 4].
Multicentric Castleman’s disease (MCD) involves multiple sites throughout the body, and is accompanied by debilitating symptoms [5]. Interleukin (IL)-6 plays a central role in the pathophysiology of MCD [6]. Secretion of the cytokine in human immunodeficiency virus (HIV)-positive cases is typically driven by human herpes virus-8 (HHV-8), which encodes viral IL-6. The mechanisms underlying HIV-negative MCD pathogenesis have not been conclusively elucidated. It has been proposed that a subclass of HIV-negative and HHV-8-negative MCD patients, with unknown etiology and pathophysiology, be referred to as displaying idiopathic disease (iMCD) [7].
Signs and symptoms in MCD are multisystemic, and may include lymphadenopathy, hepatosplenomegaly, ascites, pleural effusion, edema, fever, pain, sweats, pruritus, sensory neuropathy, anorexia, weight loss, fatigue, and weakness [5, 6, 8, 9]. In many patients, MCD is a debilitating disease and, in severe cases, multiorgan failure and death can occur.
As an orphan disease with no accepted standard of care, treatment of MCD is typically aimed at symptom management. Surgical resection, while often curative in unicentric Castleman’s disease, is unfeasible given the systemic nature of MCD [5]. Due to the progressive nature of MCD, a variety of therapies commonly used for lymphoproliferative malignancies (either as single agents or as combination regimens) have been used, but results have only been provided as case reports and small series [9, 10]. Siltuximab, a novel anti-IL-6 monoclonal antibody, was recently tested in patients with HIV-negative, HHV-8-negative MCD in a 2:1 randomized, placebo-controlled trial (N = 79) [11]. Patients treated with siltuximab showed a significantly greater durable tumor and symptom response compared with placebo (34 % versus 0 %; p = 0.0012). On the basis of this study, siltuximab was recently approved in the United States and the European Union, and is indicated for the treatment of patients with MCD who are HIV- and HHV-8–negative [12, 13]. In a single-arm study, blockade of the IL-6-signaling cascade with tocilizumab, an antibody against the IL-6 receptor, proved effective [14]. Tocilizumab has been approved for treatment of MCD in Japan [15].
The subjective symptomatology of MCD, such as fatigue and weakness, is difficult to measure using standard laboratory tests or clinical tools. Patient-reported outcome (PRO) measures best capture the presence and severity of these symptoms. Although there are well-established PRO instruments that measure aspects of functional status and wellbeing relevant to MCD, most do not capture the full spectrum of symptoms encountered by MCD patients. Therefore, we engaged in the development of a symptom questionnaire specific to MCD.
Based on the US Food and Drug Administration’s guidance on best practices for the development and selection of PRO instruments for use in clinical trial settings [16], the Multicentric Castleman’s Disease–Symptom Scale (MCD–SS) was developed to assess a patient’s perception of severity of MCD-related symptoms. The instrument design was based on literature reviews, concept elicitation and cognitive debriefing interviews conducted in patients with a confirmed diagnosis of MCD, and feedback from clinical topic experts. Details of this development process and testing process are reported in the Electronic Supplementary Material and elsewhere [17].
The objective of the present study was to assess the patient’s perception of MCD symptoms, functional status, and wellbeing relative to treatment in the context of the aforementioned siltuximab randomized trial [11].
2 Methods
2.1 Study Design
The data reported here are from a registration study evaluating the efficacy and safety of siltuximab in MCD, which included a secondary objective of demonstrating efficacy through improvement in fatigue, physical function, and other disease-related symptoms. Detailed methods were reported elsewhere [11]. The study was a randomized, double-blind trial comparing siltuximab plus best supportive care with placebo plus best supportive care (ClinicalTrials.gov Identifier: NCT01024036). The primary endpoint was durable tumor and symptomatic response for at least 18 weeks for the intention-to-treat population.
All eligible patients were 18 years or older and had measurable and symptomatic MCD, adequate organ function as assessed by laboratory studies, an Eastern Cooperative Oncology Group performance status of <3, and corticosteroid use not exceeding 1 mg/kg/day of prednisone that had remained stable or decreased in the 4 weeks prior to treatment. Patients were excluded if they were HIV-positive or HHV-8-positive, had skin lesions as the sole manifestation of MCD, had a disease that may have interfered with study participation, or had prior exposure to specific IL-6- or IL-6 receptor-targeted therapies. A history of malignancy, other than lymphoma, from which the patient had been disease-free for 3 or more years was acceptable.
Seventy-nine subjects were randomized to the siltuximab (n = 53) or placebo arms (n = 26) in a 2:1 ratio via permuted blocks stratified by corticosteroid use at baseline. Participants were treated intravenously with 11 mg/kg siltuximab or placebo infusions, respectively, every 3 weeks. Patients continued treatment until treatment failure. Both arms also received best supportive care for MCD. These supportive measures included management of effusions (e.g., drainage, diuretics) and infections (e.g., antibiotics, oral or topical antifungals, and antiviral treatment except for ganciclovir), antipyretics, antipruritics, antihistamines, pain medication, transfusions, and treatment of infusion-related reactions.
2.2 PRO Instruments
Three self-administered questionnaires were used to comprehensively assess patient perceptions of symptoms, functional status, and wellbeing. All PROs were collected on paper and were administered to the patients prior to any other procedures. One of these instruments, the MCD-SS, was newly developed to assess a patient’s perception of MCD-related symptom severity and response to therapy [17].
The MCD–SS is a 16-item questionnaire consisting of four items in the Fatigue domain (tiredness, fatigue, lack of energy, feeling weak), two items in the Rash/Itching domain (sores/rash on skin, itch), two items in the Sweats domain (night sweats, daytime sweating), and eight items that are not categorized to a domain (cough, shortness of breath, fever, loss of appetite, numbness or tingling, pain, swollen lymph nodes, swelling or edema). The respondent was asked to recall the previous 24 hours and choose the response best capturing symptom severity on a 6-point verbal rating scale [very mild (score = 1), mild (2), moderate (3), severe (4), or very severe (5), plus a “did not experience” response option scored as 0]. The MCD–SS domain scores were the sum of the individual domain items rescaled to a range of 0–10, with higher scores representing greater symptom severity. An MCD–SS total score was calculated from the Fatigue, Rash/Itching, and Sweats domains and seven of the eight items that were not categorized to a domain. Fever was excluded from total score calculations because it was determined that it was better assessed using actual temperature data rather than by patient reports. Thus, the total score denominator was ten to account for the seven individual items and the three domain scores. Further details are included in the Electronic Supplementary Material.
The Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT–Fatigue) is a 13-item patient-reported measure of fatigue that has been validated in cancer patients and the general population [18]. Subjects respond to each statement based on experiences from the previous week using a 5-point Likert-type scale. Scores can range from 0 to 52, with low total scores representing greater fatigue severity and impact of fatigue on daily activities. Based on results comparing fatigue in cancer patients with the general US population, the general population mean for the FACIT–Fatigue was found to be 43.6 [18]; this was rounded up to 44 as a threshold score in later analyses.
The MCD–SS and FACIT–Fatigue instruments were assessed at Days 1, 8, and 15 of treatment Cycle 1, and on Day 1 of each subsequent cycle.
Subjects also completed the Short Form (SF)-36, a 36-item assessment consisting of eight domains (physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and general mental health) [19]. A physical component summary (PCS) score and mental component summary (MCS) score may also be calculated. Scores for each domain and the two summary scales can range from 0 to 100, with higher scores representing better health. This instrument has been validated in multiple conditions, including hematologic cancers, and normative scores are available [20]. SF-36 was assessed on Cycle 1 Day 1, Cycle 3 Day 1, and Day 1 of every three subsequent cycles.
2.3 Statistical Analysis
Statistical analyses consisted of a combination of prespecified and ad hoc assessments. Prespecified analyses of repeated measures were conducted comparing the areas under the curve (AUC), adjusted for baseline, for each PRO measure over the first 18 cycles of treatment. Estimates were obtained from a mixed-effects model that included fixed-effect variables of treatment group, corticosteroid use (the stratification factor) and cycle, the interaction between treatment group and cycle, and the subject random effects of intercept and slope with an unstructured variance–covariance matrix. Sensitivity analyses were conducted using data from the first 12 cycles. The same analyses were performed with MCD–SS Fatigue domain results in patients with moderate or severe fatigue at baseline, defined as having a response of at least moderate (i.e., “moderate,” “severe,” or “very severe”) on one or more of the four items composing the MCD–SS Fatigue domain.
The best durable response, defined as the best observed score that was sustained for 120 days (approximately 18 weeks or 6 cycles) during the treatment period or until the end of the treatment period, was assessed for the FACIT–Fatigue instrument as an ad hoc analysis. Using a threshold of 44 or greater on the FACIT–Fatigue as a normal level of fatigue [18], the percentage of patients in each treatment group that had an abnormal baseline FACIT–Fatigue score and had a durable response to values above the normal level was assessed and compared using a chi-square test.
The MCD–SS was a newly developed scale for use in assessing symptoms of a rare condition, and data were not available to evaluate a meaningful change outside of the clinical trial. Thus, it was assumed that a distribution-based criterion of 0.5 standard deviation (SD) of the baseline value could be employed as the definition of a responder [21, 22]. In a prespecified analysis, cumulative distribution function curves were plotted by treatment group [16], showing the proportion of subjects who achieved a change from baseline of greater than or equal to the threshold. These curves used each subject’s best change score observed during the treatment period; the durability of this change was not evaluated.
3 Results
3.1 Patients and Baseline Characteristics
A total of 79 patients were recruited for the registration study of siltuximab in MCD, with 53 randomized to the siltuximab treatment group and 26 randomized to the placebo group. All patients received the treatment to which they were randomized. The first patient signed informed consent on February 9, 2010, and the final patient’s last visit for the primary analysis was on February 28, 2013. In general, demographic and baseline characteristics of subjects were similar among treatment groups, except that there were more males in the placebo arm (Table 1). The median age across both treatment groups was 48 years (range 20–78 years), and the median weight for both groups combined was 69 kg (range 42–121 kg). The majority of subjects (66 %) were men, and the most common race in both treatment groups was Asian (38 subjects; 48 %), followed by white (31 subjects; 39 %).
3.2 Baseline PRO Measures
Baseline total and component scores of the three PRO instruments for the siltuximab and placebo groups are shown in Table 1. Cycle 1 Day 1 data were available for the MCD–SS and FACIT–Fatigue for 78 subjects, and for the SF-36 for 76 subjects. One participant did not respond to the MCD–SS item pertaining to swollen lymph nodes; no other data were missing. Scores were similar at baseline between treatment groups for each of the three instruments.
At baseline, the mean (SD) number of symptoms reported per subject on the MCD–SS was 9.2 (3.76) out of 16 total (Fig. 1). The total score was 2.53 (on a scale of 0–10, from very mild to very severe), indicating that symptom severity at the start of the study was generally mild; however, the Fatigue domain had a mean score of 4.23, suggesting higher severity. Approximately 50 % of scores in the Fatigue domain were moderate or higher.
(a) Cycle 1 Day 1 means and box plots of MCD–SS domains and total score. Light shading indicates median to third quartile and dark shading indicates median to first quartile. (b) Cycle 1 Day 1 frequency distributions of MCD–SS individual items. The mean (SD) number of symptoms reported per patient was 9.2 (3.76). MCD–SS ranges from 0 to 10, higher scores represent greater symptom severity. LN lymph node, MCD–SS Multicentric Castleman’s Disease–Symptom Scale, SD standard deviation
3.3 Repeated Measures
Siltuximab-treated subjects reported significant and durable improvements in fatigue compared with subjects in the placebo arm on both the MCD–SS Fatigue and FACIT–Fatigue scale. These improvements in fatigue were observed at the end of Cycle 1 and continued to improve throughout the study. The mixed-effects mean scores for these assessments by cycle are shown in Fig. 2. The mean FACIT–Fatigue score, where lower values indicated greater severity, increased from 32.0 at Cycle 1 Day 1 to 38.6 at Cycle 18 Day 1 in siltuximab-treated subjects compared with a decrease from 31.1 to 26.9 over the same time period in the placebo group (Fig. 2a). The mean MCD–SS Fatigue score, where a higher value indicated greater severity, was 4.19 at Cycle 1 Day 1 compared with 2.58 at Cycle 18 Day 1 in siltuximab-treated subjects, and in the placebo group the score increased from 4.52 to 5.72 over the same time period (Fig. 2b).
Least squares means (SE) from mixed-effects model of (a) FACIT–Fatigue and (b) MCD–SS scores during the blinded treatment period by cycle. FACIT–Fatigue ranges from 0 to 52, lower scores represent greater fatigue severity. MCD–SS ranges from 0 to 10, higher scores represent greater symptom severity. FACIT–Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue scale, MCD–SS Multicentric Castleman’s Disease–Symptom Scale, SE standard error
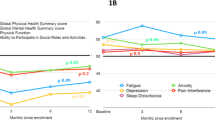
Of the eight domains measured in the SF-36, five showed statistically significant changes with siltuximab, including role physical (p = 0.005), bodily pain (p = 0.0155), vitality (p = 0.003), mental health (p = 0.0103), and role emotional (p = 0.0004; Fig. 3). The following Cycle 1 Day 1/Cycle 18 Day 1 scores are reported for siltuximab- and placebo-treated patients, respectively: role physical, 52.0/63.9 and 56.5/55.4; bodily pain, 65.6/77.0 and 63.0/54.4; vitality, 42.1/57.3 and 45.2/43.9; mental health, 59.7/72.3 and 65.6/65.6; role emotional, 60.8/76.3 and 70.5/57.0.
Overall, during the blinded treatment period, 24 patients (48 %) in the siltuximab group and 8 patients (31 %) in the placebo group achieved a ≥5-point improvement in the SF-36 PCS score. In the siltuximab group, the median time to improvement in the SF-36 PCS score was 420 days. The median time to improvement was not reached in the placebo group [hazard ratio (HR) = 1.421; p = 0.3941]. A ≥5-point improvement in SF-36 MCS score was achieved by 34 (68 %) patients in the siltuximab group and 9 (35 %) patients in the placebo group (p = 0.0074). Median time to improvement of the SF-36 MCS score was significantly faster (104 days) with siltuximab than with placebo (302 days; HR = 2.412; p = 0.0173; Fig. 4).
3.4 Subgroup Analyses
Among subjects with moderate to very severe fatigue at baseline, siltuximab-treated subjects (28 patients) showed significant improvements (i.e., responses indicating significantly decreased severity) compared with placebo (14 patients), as measured by AUC of the MCD–SS Fatigue domain, adjusted for baseline (2.48 compared with 6.99 at Cycle 18 Day 1; p = 0.0311).
At baseline, 19 out of 26 placebo-treated subjects and 43 out of 52 siltuximab-treated subjects reported fatigue scores below the FACIT–Fatigue normal population mean score of 44, demonstrating high levels of MCD-related fatigue. Of these subjects, a larger proportion went on to achieve an improved score of ≥44, with durability for 120 days or more, in the siltuximab-treated group (35 %) compared with the placebo-treated group (11 %; p = 0.0475).
3.5 Cumulative Distribution Functions
Using the assumption that a responder could be defined by a distribution-based criterion of 0.5 SD of the baseline value, the SD of the MCD–SS total score at baseline of 1.56 was divided by 2.0 [21]. This yielded a threshold value of approximately 0.75, which was rounded up to a more conservative threshold of 1.0. Thirteen out of 26 placebo-treated subjects (50.0 %) and 32 out of 51 siltuximab-treated subjects (62.7 %) achieved a change equal to or greater than this threshold. Figure 5 shows a cumulative distribution plot of the proportion of patients achieving a specified level of change or greater in MCD–SS total score across a range of meaningful thresholds for both treatment groups. The chart shows that changes were more pronounced in the siltuximab arm. Exploratory analyses were performed on the time to improvement for two thresholds. The 0.75-point threshold identified by the 0.5 SD approach showed an HR for time to improvement of 1.85 (p = 0.0515). A further analysis, examining the 1.0-point threshold, showed an HR of 1.373 (p = 0.3372).
4 Discussion
Patient-reported symptoms are becoming increasingly utilized in trial and clinical settings due to their ability to measure disease features that cannot be objectively quantified. In its 2009 guidance, the US Food and Drug Administration noted that the “use of a PRO instrument is advised when measuring a concept best known by the patient or best measured from the patient perspective” [16]. Moreover, it has been argued that such patient-reported measures should be a standard part of drug registration trials [23].
MCD is a chronic and debilitating disease, and we incorporated PRO tools in the clinical trial design to evaluate the effect of siltuximab on a patient’s wellbeing. During this randomized, double-blind clinical trial analysis of exploratory endpoints, we found improvements in symptoms, as perceived by participants receiving siltuximab compared with those receiving placebo, through the use of a recently designed PRO instrument for MCD symptom burden (the MCD–SS) as well as previously established instruments. Our assessments demonstrated early and consistent improvement over time in the siltuximab arm compared with patients receiving best supportive care alone, as measured by the MCD–SS, FACIT–Fatigue, and SF-36 instruments.
Using the MCD–SS, FACIT–Fatigue, and SF-36 vitality (the only SF-36 domain that measures fatigue) instruments, we found that fatigue was a key symptom from the patient’s perspective. It is noteworthy that improvements in fatigue among siltuximab-treated subjects not only occurred early in the course of treatment, but were also durable. Furthermore, the ability of siltuximab to confer durable improvements in fatigue was confirmed by the subgroup analysis that focused on the more severely fatigued patients. The importance of fatigue, identified by patient-reported measures, contrasts with the clinician-reported scores that included elements of overall symptom burden, such as fluid retention, neuropathy, and dermatologic symptoms. These may have effectively diluted the impact of fatigue on the clinician-reported symptom score.
Improvements in areas other than fatigue were also noted, including five of the eight SF-36 domains, providing broad support for improving patient functioning and wellbeing with siltuximab. We also found an improvement in mental health, as evidenced by a highly significant change in the SF-36 MCS score in the siltuximab group as compared with best supportive care. This is notable in the context of previous research, which demonstrated an association between depression and IL-6 [24]. Further exploration of the components of the SF-36 that contribute to the apparent improvement in mental health and their associations with inhibition of systemic IL-6 levels may be warranted.
Determining the clinical meaning of patient-reported findings remains a challenge. The MCD–SS has not been used outside of a controlled trial setting. Consequently, score interpretation strategy and application of results to MCD management may not yet be ideal. Our threshold of 1.0 as a meaningful change in MCD–SS was calculated from the SD of assessment scores, showing a distinct difference in meaningful change between siltuximab and control groups. However, further testing of the MCD–SS is required, and its use in non-trial conditions may allow the threshold for meaningful change to take on clinical significance. In clinical situations, FACIT–Fatigue may allow a more rapid assessment of one of the defining features of MCD and its response to therapy, with a more comprehensive assessment provided by the MCD–SS.
We acknowledge limitations of this study, due both to factors related to the study design and inherent limitations of PRO research. The MCD-SS was the first PRO scale developed for the disease, and there may have been other symptoms that were not represented in the instrument that may have contributed to the overall assessment of response. As patients were lost due to disease progression, the PRO assessments for each time point were represented by fewer patients. Because MCD is a rare condition, our sample size was limited. Moreover, MCD is often associated with concurrent disease states. The exclusion of subjects with other health conditions, including HIV, limits the ability to extrapolate our results directly to other subpopulations of patients with MCD.
Future research will likely explicate the utility of siltuximab in improving patient functioning and wellbeing. Analysis of siltuximab in subgroups of MCD patients is warranted, as our results suggest that patients with more burdensome MCD symptoms, such as severe fatigue, may experience greater improvements with siltuximab therapy than patients with less severe symptoms.
5 Conclusion
Given the subjective nature of most symptoms associated with MCD, patient-reported assessments represent an essential component of measuring the impact of disease and treatments. Improvement of symptoms by an effective therapy may be a key aspect of MCD management. Siltuximab, a novel anti-IL-6 antibody, is associated with early and consistent improvement in fatigue, functioning, and wellbeing. Given that MCD is treated primarily through symptom management, these findings support the use of siltuximab for this orphan disease.
References
Castleman B, Towne VW. Case 40011, hyperplasia of mediastinal lymph nodes. N Engl J Med. 1954;230:26–30.
Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9:822–30.
Flendrig JA, Schillings PHM. Benign giant lymphoma: the clinical signs and symptoms. Folia Medica Neerlandica. 1969;12:119–20.
Gaba AR, Stein RS, Sweet DL, et al. Multicentric giant lymph node hyperplasia. Am J Clin Pathol. 1978;69:86–90.
Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129:3–17.
Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–32.
Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123:2924–33.
Dispenzieri A, Gertz MA. Treatment of Castleman’s disease. Curr Treat Options Oncol. 2005;6:255–66.
van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28:3701–8.
Sbenghe MM, Besa E, Mahipal A, et al. HHV-8-associated multicentric Castleman’s disease in HIV-negative patient: a novel therapy for an orphan disease. Oncologist. 2012;17:145–6.
van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15:966–74.
US Food and Drug Administration. FDA approves Sylvant for rare Castleman’s disease. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/UCM394522.htm. Accessed 3 June 2014.
European Commission. Community register of medicinal products for human use: SYLVANT authorisation. http://ec.europa.eu/health/documents/community-register/html/h928.htm. Accessed 3 June 2014.
Matsuyama M, Suzuki T, Tsuboi H, et al. Anti-interleukin-6 receptor antibody (tocilizumab) treatment of multicentric Castleman’s disease. Intern Med. 2007;46:771–4.
Tanaka T, Narazaki M, Ogata A, et al. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26:88–96.
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), et al. Guidance for Industry. Patient-reported outcome measures: use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed 23 Jan 2014.
Casper C, van Agthoven M, Rothman M, et al. The multicentric Castleman’s disease (MCD)-symptom scale (MCD-SS): development and validation of a patient-reported outcome (PRO) measure for an ultra-orphan disease. Value Health. 2014;17:A535.
Cella D, Lai JS, Chang CH, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–38.
Maruish ME, Kosinski M. A guide to the development of certified short form interpretation and reporting capabilities. Lincoln, RI: QualityMetric Incorporated; 2009.
Phillips KM, Pinilla-Ibarz J, Sotomayor E, et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer. 2013;21:1097–103.
McLeod LD, Coon CD, Martin SA, et al. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11:163–9.
Norman GR, Sridhar FG, Guyatt GH, et al. Relation of distribution- and anchor-based approaches in interpretation of changes in health-related quality of life. Med Care. 2001;39:1039–47.
Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–9.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86.
Acknowledgments
We thank the patients who participated in this study, and the investigators and their colleagues who cared for them. The MCD2001 study was funded by Janssen Research and Development. All authors were involved in all stages of manuscript preparation. All authors had access to the data and were responsible for its interpretation. Frits van Rhee, Raymond S. Wong, Alexander Fosså, Angela Dispenzieri, James Cavet, Nikhil Munshi, and Corey Casper were study investigators. Jessica Vermeulen acted as the responsible clinician for the study sponsor. Margaret Rothman, Kai Fai Ho, and Sarah Fleming were responsible for analyzing the PRO data. As corresponding author, Frits van Rhee acts as guarantor of the manuscript. The authors disclose the following support or interests: Frits van Rhee has served on advisory boards for Celgene, Janssen, Millennium, and Sanofi; Margaret Rothman was an employee and stockholder of Janssen Research and Development when the study was conducted; Kai Fai Ho is a paid consultant for Janssen Global Services, LLC, contracted to provide statistical support; Sarah Fleming holds stock and is an employee of Janssen Research and Development; Raymond S. Wong has received research funding and has served on advisory boards for Janssen; Alexander Fosså has received honoraria from Janssen; Angela Dispenzieri has received research funding from Celgene, Janssen, Pfizer, and Millennium; James Cavet has received research funding, and has served on advisory boards (unpaid) and speakers bureau for Janssen; Nikhil Munshi has received honoraria from Celgene, Onyx, Janssen, Sanofi-Aventis, and Oncopep; Jessica Vermeulen holds stock and is an employee of Janssen Research and Development; Corey Casper received consulting fees and research funding from Janssen to evaluate siltuximab. The decision to submit the manuscript was not influenced by the study sponsor and was made by all authors, the majority of whom were study investigators. Medical writing support was provided by Christopher Jones, PhD, of MedErgy, and was funded by Janssen Research & Development.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
van Rhee, F., Rothman, M., Ho, K.F. et al. Patient-reported Outcomes for Multicentric Castleman’s Disease in a Randomized, Placebo-controlled Study of Siltuximab. Patient 8, 207–216 (2015). https://doi.org/10.1007/s40271-015-0120-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-015-0120-5