Abstract
Background and Objective
Tebipenem pivoxil (TP) is a carbapenem and is applied against pneumonia, otitis media, and sinusitis. This study compared the pharmacokinetics (PK) and safety of a test (T) preparation and reference (R) preparation of TP in healthy Chinese adults.
Methods
This study was a single-center, randomized, open, single-dose (fasting/postprandial) oral administration, two-agent, two-sequence, two-cycle, crossover bioequivalence trial. A total of 60 participants were enrolled (24 fasting and 36 postprandial). All participants were randomly assigned to the TR sequence and RT sequence. Subsequently, they switched T sequences or R sequences 7 days later. PK blood samples were collected according to the protocol, plasma TP concentration was determined by liquid chromatography-mass spectrometry, main PK parameters were calculated based on a non-compartment model, and adverse events were recorded during the test.
Results
In the feeding arm, the geometric mean ratio of maximum concentration (Cmax) was 89.84% (90% confidence interval 84.33–95.70), the geometric mean ratio of area under the plasma concentration–time curve from time 0 to last time of quantifiable concentration (AUC0–t) was 86.80% (83.62–90.10), and the geometric mean ratio of area under the plasma concentration–time curve from time 0 to infinity time of quantifiable concentration (AUC0–∞) was 86.90% (83.73–90.20), which were within the acceptable range of bioequivalence (80–125%). In the fasting arm, the geometric mean ratio of Cmax was 96.07% (89.62–102.99), the geometric mean ratio of AUC0–t was 93.09% (90.47–95.78), and the geometric mean ratio of AUC0–∞ was 93.09% (90.48–95.77), which was within the acceptable range of bioequivalence (80–125%). Hence, the T preparation and R preparation of TP had bioequivalence in the fasting arm and feeding arm of the clinical trial. In addition, all adverse events were mild, and no severe adverse events were noted.
Conclusion
Preparations T and R of TP were bioequivalent in the fasting and postprandial groups in clinical trials, and TP was safe.
Similar content being viewed by others
Both the test preparation and the reference preparation of tebipenem granules showed bioequivalence and good safety in healthy Chinese participants under postprandial/fasting conditions. This study provided data support for the marketing of domestic tebipenem. |
The granulated administration method used in this study can ensure drug exposure in vivo to a greater extent. A reference for drug administration in the granular dosage form in clinical trials was provided. |
1 Introduction
Tebipenem pivoxil (TP) is a carbapenem and is applied commonly against pneumonia, otitis media [1], and sinusitis [2]. TP restrains penicillin-binding proteins and destroys the cell walls of pathogens [3]; therefore, it is less toxic for mammals [4].
The chemical structure of TP is shown in Fig. 1. A structural peculiarity of TP is that thiazole at the C3 side chain forms a prodrug by interacting with C2 carboxylic acids [5], which results in assimilation of oral drugs. The oral absorption of TP is better than that of most β-lactam antibiotics. TP has a broad antibacterial spectrum. It has a stronger antibacterial activity than penicillin or cephalosporins. Escherichia coli, Klebsiella species, Haemophilus species, and Legionella species are more sensitive to TP than imipenem, cefdinir, amoxicillin, and levofloxacin in pediatric patients [6]. Recent in vitro and animal studies have shown TP to be active against Shigella species [7], and one clinical trial found that it could be effective against bacterial pneumonia in children [8].
The binding rate of TP to serum proteins in vitro is 67% [9]. TP can be distributed in the sputum, middle-ear mucosa, maxillary-sinus mucosa, tonsilla palatina (adults) [10], and middle-ear secretions (children) [11]. TP is absorbed from the gastrointestinal tract and has antibacterial activity [12]. The main excretion pathway of TP is urine [13]. Studies have shown that, in people with normal renal function and those with renal impairment (creatinine clearance rate [CCR] < 80 ml/min), after taking TP (250 mg, oral [p.o.] administration), renal clearance and urinary excretion are reduced, the area under the plasma concentration–time curve from time 0 to infinity time of quantifiable concentration (AUC0–∞) and the maximum concentration (Cmax) is increased, and the elimination half-life (t½) is prolonged, together with deteriorated renal function (CCR < 80 ml/min) [14].
Pfizer (New York, NY, USA) were the first to develop TP. Then TP granules were developed by Meiji Seika Kaisha Ltd (Tokyo, Japan) in Aug 2009. But there are no TP granules in China's marketplace. The study of bioequivalence in clinical trials is extremely important for our clinical demand, and Lunan Pharmaceutical Group Corporation carried out such a trial in Zhejiang Provincial People’s Hospital. The bioequivalence of TP acquired from Lunan Pharmaceutical Group (test [T] preparation) and TP (Orapenem®) obtained from Meiji Seika Kaisha Ltd (reference [R] preparation) was assessed in healthy people in the fasting state or fed state. At the same time, the pharmacokinetics (PK) and safety of the T preparation and R preparation were examined.
2 Materials and Methods
2.1 Ethics Authorization
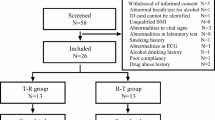
The protocol for this clinical trial was ratified (certificate number 2019YW016) by the Ethics Committee of Zhejiang Provincial People’s Hospital (Hangzhou, China) on 31 July 2019. The feeding arm and fasting arm of this clinical trial were started on 19 September 2019 and 22 October 2019, respectively, at the Phase I Clinical Research Center of Zhejiang Provincial People’s Hospital. All the participants provided written informed consent and were screened. Finally, 60 people (24 in the fasting arm, 36 in the feeding arm) entered the clinical trial, and 59 people completed it (one participant of the feeding arm dropped out because of adverse events [AEs]).
2.2 Study Design
This was a single-center, randomized, open-label, single-dose, oral medication, two-drug crossover trial. TP produced by Lunan Pharmaceutical Group (T preparation) and Orapenem® (R preparation) were compared in terms of bioequivalence. A fasting group and a feeding group were created.
Thirty-six healthy people were required for the feeding group, and 35 of them completed this trial. Twenty-four healthy individuals formed the fasting group and finished this trial. All the participants were assigned randomly to TR sequences and RT sequences equally. Subsequently, they exchanged T sequences or R sequences after 7 days. In the feeding arm, the participants had to eat a high-fat diet (the total calories of the food were about 800~1000 kcal, of which protein provided about 150 kcal, carbohydrates provided about 250 kcal, and fat provided about 500–600 kcal) before taking TP granules.
2.3 Inclusion Criteria
The inclusion criteria were as follows: (1) Chinese people aged ≥ 18 years and ≤ 65 years; (2) body weight ≥ 50 kg (men) and ≥ 45 kg (women); (3) body mass index (BMI) 19.0–26.0 kg/m2; (4) participant fully understood the content, process, and possible AEs of the clinical trial; and (5) participant could complete the clinical trial according to its requirements.
2.4 Exclusion Criteria
The exclusion criteria were as follows: (1) a serious disease that the investigator deemed inappropriate for this clinical trial; (2) a history of serious digestive problems, epilepsy, or vitamin K deficiency; (3) clinically significant abnormal laboratory examinations or specialist tests (e.g., electrocardiography); (4) positivity for hepatitis B virus surface antigen, hepatitis C virus antibodies, Treponema pallidum antibodies, or human immunodeficiency virus (HIV) antibodies; (5) an allergy; (6) use of drugs that could interact with TP or change levels of liver enzymes; (7) special diets (including fruit from the Rutaceae family [e.g., pitaya, mango] or food containing xanthine derivatives [e.g., tea, coffee]) or strenuous exercise (football, basketball, or running > 30 min); (8) tobacco smoking or regular consumption of alcohol; (9) a history of drug abuse/addiction; (10) donation of blood or a bled > 400 ml within 3 months before screening; (11) breastfeeding upon screening or would be breastfeeding after screening; (12) planning to have a child within the time from screening to 3 months after the end of the clinical trial, or unwilling to take contraception; (13) enrolment in another clinical trial and had taken a test drug within 3 months before screening, or participating in another clinical trial; (14) cannot tolerate venipuncture or had difficulty providing venous blood; (15) cannot follow a consistent diet (e.g., people who were lactose intolerant); (16) CCR < 80 ml/min; and (17) poor compliance or other factors deemed unsuitable for this clinical trial according to the lead investigator.
2.5 Estimation of Sample Size
2.5.1 Feeding Arm
Men and women were enrolled according to the Guidelines for Bioequivalence Studies issued by the National Medical Products Administration of China. High variation of TP is not mentioned in the Guiding Principles for Bioequivalence Research of Tebipenem issued by the US Food and Drug Administration (FDA). In the bioequivalence study of TP, the intraindividual coefficient of variation (CV) of Cmax was 32.8%, the intraindividual CV of area under the plasma concentration–time curve from time 0 to last time of quantifiable concentration (AUC0–t) was 20.0%, and the intraindividual CV of AUC0–∞ was 20.0%. If the significance level of the T preparation was set as 0.05/0.0491, the power would be 92.44/97.88 and the T/R would be 80–125%, and the cutoff would be set as 0.8–1.25. As a result, the minimum sample size would be 28. With the expulsion rate taken into account as 20%, the final enrolment number planned was 36.
2.5.2 Fasting Arm
High variation of TP is not mentioned in the Guiding Principles for Bioequivalence Research of Tebipenem issued by the US FDA. In the bioequivalence study of TP, the intraindividual CV of Cmax was 34.1%, the intraindividual CV of AUC0–t was 18.9%, and the intraindividual CV of AUC0–∞ was 18.9%. If the significance level of the T preparation was set as 0.05, the power would be 0.80 and T/R would be 80–125%, and the cutoff would be set as 0.8–1.25. As a result, the minimum sample size would be 24.
2.6 Detection Method
The analytical method was liquid chromatography–mass spectrometry (LC-MS). The internal standard was biapenem. The chromatographic column was Chr. C-100 CAPCELLPAKCR 1:4 2.0 mmI. D×50 mm 5 μml OT:CR-10S/N:U16IE01088 Shiseido. The mobile phase was A (formic acid:saline:ultrapure water at 1:10:2000, v/v/v) and B (methyl alcohol:acetonitrile at 4:1, v/v). The flow rate was 0.4000 ml/min, and the sample size was 5 μl. The detector was API4000 (SCIEX, Framingham, MA, USA). The LC-MS system consisted mainly of the Shimadzu LC-20AD system. The sample pretreatment method was protein precipitation. The linear range of tebipenem concentration was 5–5000 ng/ml, and the lower limit of quantitation was 5 ng/ml.
2.7 PK Assessments
Plasma samples were collected from all participants to measure TP levels at 0, 5, 10, 15, 30, and 45 min, as well as at 1, 1.33, 1.67, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, and 10 h after TP administration. Samples of whole blood were placed in an ice bath before centrifugation (1700 × g for 10 min at 2–8 ℃) to collect plasma and stored at −70 ± 10 ℃ for analyses.
2.8 PK Parameters
The drug level in blood was based on the non-compartmental model and calculated by SAS 9.3 (www.sas.com/, America). Cmax, AUC0–t, AUC0–∞, time to reach maximum concentration (Tmax), t½, elimination rate constant (λz), and AUC_% Extra (formula: [ (AUC0–∞ − AUC0–t) /AUC0–∞]×100%) were measured.
2.9 Safety Assessments
Safety assessments were based on clinical symptoms, laboratory measurements, AEs, physical examination, 12-lead electrocardiography, and vital signs. The AEs (clinical manifestations, severity, duration, treatment measures, outcome) were recorded to ascertain the relationship between AEs and TP.
2.10 TP Administration
2.10.1 Feeding Arm
All participants ate a high-fat meal 30 min before taking TP. They finished eating their meal within 30 min. The T preparation or R preparation was taken punctually.
2.10.2 Granules of TP Administration Route
TP granules (0.5 g, including 50 mg [potency] TP) were dissolved in a glass containing 240 ml of water. The participants took TP granules three times. First, the TP granules were dissolved in about 80 ml of water. Then a muddler was used to stir the granules in suspension. Participants took the TP immediately. Second, the residual TP was rinsed using a muddler and about 80 ml of water. Participants took the TP immediately. Third, residual TP on the muddler and glass was rinsed using remanent water, and the muddler was used for stirring. The participants took the TP immediately. The entire drug-taking process had to be completed in 1 min and the participants were not allowed to chew. The participants were not allowed to drink water 1 h before or after taking TP.
2.10.3 Fasting Arm
At about 8 a.m., the participants took TP as a T preparation or R preparation. The administration was as described in the feeding arm.
3 Results
3.1 Characteristics of Participants at Baseline
3.1.1 Feeding Arm
Thirty-six people (30 men and six women) participated, and 35 individuals completed this part of the clinical trial. The mean age of the study cohort was 26 ± 5 years. The bodyweight range was 51.4–78.4 kg. The BMI range was 19.4–25.5 kg/m2. The height range was 152.0–181.5 cm.
3.1.2 Fasting Arm
Twenty-four individuals (21 men and three women) completed this part of the clinical trial. The mean age of the study cohort was 26 ± 5 years. The bodyweight range was 54.3–84.7 kg. The BMI range was 19.6–25.4 kg/m2. The height range was 157.0–182.5 cm.
3.2 PK and Statistical Analyses
The individuals were separated into different sequences on the basis of random results (TR or RT). Blood samples were collected at 5, 10, 15, 30, and 45 min, as well as at 1, 1.33, 1.67, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, and 10 h after taking TP.
3.3 PK Characteristics
3.3.1 C max
In the fasting arm, the Cmax of the T preparation and R preparation of TP was 3050 ± 499 ng/ml and 3189 ± 600 ng/ml, respectively. The ratio of the logarithmic geometric mean of Cmax was 96.07%. The 90% confidence interval (CI) was 89.62–102.99, and power was 99.68%.
In the feeding arm, the Cmax of the T preparation and R preparation of TP was 1016 ± 347 ng/ml and 1134 ± 372 ng/ml, respectively. The ratio of the logarithmic geometric mean of Cmax was 89.84%. The 90% CI was 84.33–95.70, and power was 92.44%.
3.3.2 T max
In the fasting arm, the Tmax of the T preparation and R preparation of TP was 0.40 ± 0.16 h (median 0.50 h; geometric mean 0.40 h) and 0.35 ± 0.13 h (median 0.38 h; geometric mean 0.35 h), respectively. In the feeding arm, the Tmax of the T preparation and R preparation of TP was 1.02 ± 0.75 h (median 1.33 h; geometric mean 1.02 h) and 1.19 ± 0.88 h (median 1.50 h; geometric mean 1.19 h), respectively.
There was no significant difference in the Tmax of TP between the T preparation and R preparation according to the non-parametric test.
3.3.3 AUC0–t
In the fasting arm, the AUC0–t of the T preparation and R preparation of TP was 2831 ± 560 h*ng/ml and 3039 ± 575 h*ng/ml, respectively. The ratio of the logarithmic geometric mean of AUC0–t was 93.09%. The 90% CI was 90.47–95.78, and power was 100%.
In the feeding arm, the AUC0–t of the T preparation and R preparation of TP was 2289 ± 518 h*ng/ml and 2633 ± 527h*ng/ml, respectively. The ratio of the logarithmic geometric mean of AUC0–t was 86.80%. The 90% CI was 83.62–90.10, and power was 97.88%.
3.3.4 AUC0–∞
In the fasting arm, the AUC0–∞ of the T preparation and R preparation of TP was 2843 ± 563 h*ng/ml and 3052 ± 576 h*ng/ml, respectively. The ratio of the logarithmic geometric mean of AUC0–∞ was 93.09%. The 90% CI was 90.48–95.77, and power was 100%.
In the feeding arm, the AUC0–∞ of the T preparation and R preparation of TP was 2302 ± 518 h*ng/ml and 2645 ± 529 h*ng/ml, respectively. The ratio of the logarithmic geometric mean of AUC0–∞ was 86.90%. The 90% CI was 83.73–90.20, and power was 98.18%.
3.3.5 Summary of Primary PK Parameters
The primary PK parameters (AUC0−t, AUC0−∞, t½, Cmax) of our study are shown in Tables 1 and 2. Mean plasma concentration–time curves for TP are shown in Fig. 2.
Mean plasma concentration–time curves for TP. A Mean plasma concentration–time curve for TP after a single oral dose in the fasting state. B Mean plasma concentration–time curve for TP after a single oral dose in the fasting state (semi-logarithmic graph). C Mean plasma concentration–time curve for TP after a single oral dose after meals. D Mean plasma concentration–time curve for TP after a single oral dose after meals (semi-logarithmic graph). Note: Error bars represent the standard deviation. R reference, T test, TP tebipenem pivoxil
3.4 Statistical Analyses
3.4.1 Fasting arm
A total of 1824 plasma samples (912 test samples and 912 backup samples) were collected for the fasting arm. The ratio of the logarithmic geometric mean of the Cmax, AUC0–t, and AUC0–∞ of the T preparation and R preparation of TP was 96.07%, 93.09%, and 93.09%, respectively. The 90% CI for Cmax, AUC0–t, and AUC0–∞ was 89.62–102.99, 90.47–95.78, and 90.48–95.77, respectively. The CV of Cmax, AUC0–t, and AUC0–∞ was 14.09%, 5.75%, and 5.75%, and power was 99.68%, 100%, and 100%, respectively.
3.4.2 Feeding arm
A total of 2698 plasma samples (1349 test samples and 1349 backup samples) were collected for the feeding arm. The ratio of the logarithmic geometric mean of the Cmax, AUC0–t, and AUC0–∞ of the T preparation and R preparation of TP was 89.84%, 86.80%, and 86.90%, respectively. The 90% CI for Cmax, AUC0–t, and AUC0–∞ was 84.33–95.70, 83.62–90.10, and 83.73–90.20, respectively. The CV of Cmax, AUC0–t, and AUC0–∞ was 15.75%, 9.25%, and 9.23%, and power was 92.44%, 97.88%, and 98.18%, respectively.
3.5 Safety Analyses
In the feeding arm, ten AEs occurred in four participants. In patient C001 (who had a T preparation of TP), alterations in levels of alanine transaminase, aspartate transaminase, and glutamyl transpeptidase were noted on 1 October 2019 (last day of the feeding arm of our clinical trial). These alterations had a relationship with the T preparation of TP according to the lead investigator. In patient C027 (T preparation of TP), an increase in the level of low-density lipoprotein-cholesterol was noted on 1 October 2019, and it had a relationship with the T preparation of TP. Patient C031 (R preparation of TP) experienced urticaria on 25 September 2019 (day 1 of cycle 1), followed by increases in levels of bilirubin, direct bilirubin, and indirect bilirubin on 29 September 2019, and these AEs had a relationship with the R preparation of TP. Patient C017 (R preparation of TP) experienced an increased urinary level of glucose, but a relationship with TP was not suspected. A total of 32 out of 36 trial participants did not suffer AEs, and serious adverse events (SAEs) were not documented in any participant.
In the fasting arm, ten AEs occurred in five participants. In patient K003 (R preparation of TP), an increase in urinary leukocytes and a decrease in hemoglobin level were noted, but a relationship with the R preparation of TP was not suspected. Patient K007 (R preparation of TP) experienced an increase in levels of direct bilirubin, indirect bilirubin, and bilirubin, and these AEs had a relationship with the R preparation of TP. Patient K011 (R preparation of TP) experienced a left-ventricular high voltage and sinus arrhythmia, but a relationship with the R preparation of TP was not suspected. Patient K018 (R preparation of TP) had increased levels of direct bilirubin and bilirubin, and a relationship with the R preparation of TP was suspected. Patient K019 (T preparation of TP) experienced proteinuria, and this AE had a relationship with the T preparation of TP. Nineteen of 24 participants did not suffer an AE, and no participants suffered an SAE.
These results showed that the T preparation and R preparation of TP were safe.
4 Discussion
TP granules have been used to treat pneumonia, otitis media [1], and sinusitis [2]. Some scholars have suggested that TP should be used only if other antibiotics have proved non-efficacious [2, 2]. It was approved for sale in Japan in Aug 2009. In China, there is an urgent need for the development of domestic TP granules to address domestic clinical needs. The bioequivalence of the T preparation (produced by Lunan Pharmaceutical Group) and R preparation (Orapenem®) holds, and the preparations were well tolerated and safe. There were non-conspicuous differences in laboratory parameters, vital signs, and electrocardiography, and no SAEs, deaths, or withdrawal-related AEs were documented.
The study design referred to technical guidelines for the study of human bioequivalence of chemical generic drugs, with PK parameters as the end point evaluation index. Although TP granules are children's drugs [16], the study needed healthy and non-uniform sex adult volunteers. A CCR of < 80 could not be adopted in our bioequivalence trial; the literature of Nakashima et al. showed that CCR is important for TP granules absorption and metabolism [14]. In addition, it was found in a previous clinical trial that, compared with a single oral dose of tebipenem granules (200 potency), tebipenem combined with famotidine and antacids (dried aluminum hydroxide gel, magnesium hydroxide), which increase gastric pH value, reduced plasma Cmax by about 40–60% and AUC0–∞ by 20–30%. The excretion rate in urine is reduced by about 20%, and the Tmax is extended by 10–30 min [17]. Compared with tebipenem alone, the Cmax and AUC0–∞ of plasma tebipenem were increased, t½ was prolonged, renal clearance was decreased, and urinary excretion rate of tebipenem was decreased when tebipenem was combined with probenecid [18]. In view of the above data, these drugs are listed as contraindicated drugs in this trial.
Nakashima and colleagues [13] investigated the PK characteristics of TP (250 mg, p.o.) using a fasting arm and feeding arm in healthy adult men. In that study, the Cmax of the feeding arm was lowered to about 60% that of the fasting arm, but AUC0–∞ was approximately identical. Their data are, in general, consistent with our clinical trial (Tables 1 and 2). Food intake prolonged the Tmax of TP in plasma, which is consistent with the trend reported in the literature of Nakashima and colleagues [13]. The clinical trials by Nakashima and colleagues compared the influence of food on the cumulative urinary excretion rate of TP; they found that food had almost no effect upon it. However, data on TP cumulative urinary excretion were not collected in our clinical trial. Therefore, the study may have a limitation in that the feeding arm compared differences in PK parameters in blood between the T preparation and R preparation after consumption of a high-fat diet, but the differences in the cumulative urinary excretion rate of TP were not compared. In the subsequent trials, the test of cumulative urine excretion rate of tebipenem could be taken into consideration .
The safety of TP has been demonstrated in a clinical trial in Japan [19]. In that clinical trial, 101 of 440 hospitalized children (23%) developed AEs. The main AE was diarrhea (19.5%). In another safety review, 23 of 432 patients (5.3%) had abnormal fluctuations in clinical laboratory parameters, with increased platelet counts as the primary AE. In this clinical trial, there were no adverse reactions (such as diarrhea, increased platelet count) that were common in previous clinical trials. The most common adverse reactions were abnormal liver function (four cases, 6.7%), such as elevated levels of alanine aminotransferase, aspartate aminotransferase, glutamyl transpeptidase, direct bilirubin, indirect bilirubin, and bilirubin. The differences in the types and rates of AEs may be related to the small size of the included population and sample size.
The unique administration method of granules should also be considered in this study. Granules cannot be swallowed like tablets, nor can they form a homogeneous liquid. An incorrect administration method may have led to inaccurate individual doses, resulting in the bioequivalence of T preparation and R preparation being invalid. The method of administration in this study can be used as a reference for granules administration.
5 Conclusions
In the fasting arm and feeding arm of this clinical trial, the T preparation and R preparation of TP were bioequivalent. No SAEs occurred in participants. TP appears to be a safe product.
References
Baba S, Yamanaka N, Suzuki K, et al. Clinical efficacy, safety and PK-PD analysis of Tebipenem pivoxil in a phase II clinical trial in otolaryngological infections. Jpn J Antibiot. 2009;62(2):155.
Kataoka H, Kasahara H, Sasagawa Y, Matsumoto M, Shimada S. Evaluation of safety and efficacy of tebipenem pivoxil granules for pediatric in pneumonia, otitis media and sinusitis. Jpn J Antibiot. 2016;69(1):53–76.
Jain A, Utley L, Parr TR, Zabawa T, Pucci MJ. Tebipenem, the first oral carbapenem antibiotic. Expert Rev Anti Infect Ther. 2018;16(7):513–22.
Sugano T, Yamada K, Baba N, et al. Mechanism for tebipenem antimicrobial activity against Streptococcus pneumoniae and Haemophilus influenzae. Jpn J Chemother. 2009;57:15–29.
Chao T, Li C, Liu S, et al. Crystal structure of tebipenem pivoxil. Acta Crystallogr A. 2018;74(9):1215–7.
Mcentee L, Johnson A, Farrington N, et al. Pharmacodynamics of tebipenem: new options for oral treatment of multi-drug resistant gram-negative infections. Antimicrob Agents Chemother. 2019;63(8):e00603-e619.
Fernández Álvaro E, Voong Vinh P, de Cozar C, et al. The repurposing of tebipenem pivoxil as alternative therapy for severe gastrointestinal infections caused by extensively drug-resistant Shigella spp. Elife 2022;11:e69798. https://doi.org/10.7554/eLife.69798.
Kuroki H, Tateno N, Ikeda H, Saito N. Investigation of pneumonia-causing pathogenic organisms in children and the usefulness of tebipenem pivoxil for their treatment. J Infect Chemother. 2010;16(4):280–7.
Kijima K, Morita J, Suzuki K, et al. Pharmacokinetics of tebipenem pivoxil, a novel oral carbapenem antibiotic, in experimental animals. Jpn J Antibiot. 2009;62(3):214–40.
Baba S, Kasahara H, Morita J, Aizawa K, Sunakawa K. Tissue and aural discharge distribution of tebipenem pivoxil. Jpn J Antibiot. 2009;62(2):127–35.
Sugita R. Good transfer of tebipenem into middle ear effusion conduces to the favorable clinical outcomes of tebipenem pivoxil in pediatric patients with acute otitis media. J Infect Chemother. 2013;19(3):465–71.
Kato K, Shirasaka Y, Kuraoka E, et al. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: involvement of human OATP family in apical membrane transport. Mol Pharm. 2010;7(5):1747–56.
Nakashima M, Morita J, Takata T, et al. Effect of diet on the pharmacokinetics of tebipenem pivoxil fine granules in healthy male volunteers. Jpn J Antibiot. 2009;62(2):136–42.
Nakashima M, Morita J, Aizawa K. Pharmacokinetics and safety of tebipenem pivoxil tablets in healthy volunteers and in patients with reduced renal function. 日本化学療法学会雜誌 Jpn J Chemother. 2009;57;109.
Kishii K, Chiba N, Morozumi M, et al. Antibiotic susceptibility and resistance gene analysis of Haemophilus influenza in clinical tebipenem-pivoxil studies in pediatric patients using PCR method. 日本化学療法学会雜誌 Jpn J Chemother. 2009;57(67):75.
Totsuka K, Aizawa K, Morita J, et al. PK-PD analysis of tebipenem pivoxil in clinical trials for pediatric patients. 日本化学療法学会雜誌 Jpn J Chemother. 2009;57:186–91.
Nakashima M, Morita J, Aizawa K. Pharmacokinetics and safety of oral carbapenem antibiotic tebipenem pivoxil tablets in healthy male volunteers. 日本化学療法学会雜誌 Jpn J Chemother. 2009;57:99.
Nakashima M, Morita J, Aizawa K. Pharmacokinetics and safety of oral carbapenem antibiotic tebipenem pivoxil tablets in healthy male volunteers. 日本化学療法学会雜誌 Jpn J Chemother. 2009;57:103.
Nakashima M, Morita J, Aizawa K. Pharmacokinetics and safety of oral carbapenem antibiotic tebipenem pivoxil tablets in healthy male volunteers. 日本化学療法学会雜誌 Jpn J Chemother. 2009;57:82–9.
Acknowledgments
All authors performed data analysis and interpretation, as well as manuscript review and approval. This study was supported by Meiji Seika Kaisha Ltd (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study passed the Medical Ethics Committee of Zhejiang Provincial People's Hospital review on 31 July 2019, and approval was obtained (no. 2019YW016). Written informed consents were obtained from all participants.
Funding
This study was financially supported by Zhejiang Provincial Program for the Cultivation of New Health Talents (to Yiwen Zhang), Zhejiang Provincial TCM Science and Technology Plan Project (2021ZZ001 and 2022ZB017), and the Medical Science and Technology Project of Zhejiang Provincial (2021KY040 and 2022KY069).
Competing Interests
Rui Hao, Yiming Shao, Sisi Lin, Yi Wu, Li Bian, and Yiwen Zhang declare no conflicts of interest.
Consent for Publication
Not applicable.
Code Availability
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, the license permits any non-commercial use, sharing, adaptation, distribution, and reproduction in any format or medium.
Authors’ Contributions
This study was designed by YZ. RH contributed most to this work, and all authors read and approved the manuscript.
Availability of Data and Materials
The data supporting the conclusions of this article are included within the article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hao, R., Shao, Y., Lin, S. et al. Bioequivalence Study of Tebipenem Pivoxil in Healthy Chinese Adults. Drugs R D 24, 89–96 (2024). https://doi.org/10.1007/s40268-024-00454-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-024-00454-w