Abstract
Superficial infections with Streptococcus pyogenes (Strep A), pharyngitis and impetigo can induce acute rheumatic fever, an autoimmune sequela manifesting mostly with arthritis and rheumatic carditis. Valvular heart damage can persist or advance following repeated episodes of acute rheumatic fever, causing rheumatic heart disease. Acute rheumatic fever and rheumatic heart disease disproportionately affect children and young adults in developing countries and disadvantaged communities in developed countries. People living with rheumatic heart disease are at risk of experiencing potentially fatal complications such as heart failure, bacterial endocarditis or stroke. Transthoracic echocardiography plays a central role in diagnosing both rheumatic carditis and rheumatic heart disease. Despite the obvious medical need, no licensed Strep A vaccines are currently available, as their clinical development process faces several challenges, including concerns for cardiac safety. However, the development of Strep A vaccines has been recently relaunched by many vaccine developers. In this context, a reliable and consistent safety evaluation of Strep A vaccine candidates, including the use of transthoracic echocardiography for detecting cardiac adverse events, could greatly contribute to developing a safe and efficacious product in the near future. Here, we propose a framework for the consistent use of transthoracic echocardiography to proactively detect cardiac safety events in clinical trials of Strep A vaccine candidates.
Plain Language Summary
Throat and skin infections caused by certain types of bacteria, named Streptococcus pyogenes, are frequent worldwide; however, in many children from less developed countries and disadvantaged communities, infections with S. pyogenes lead to a condition called acute rheumatic fever, which usually affects the joints and the heart. Damage to the heart valves may evolve to rheumatic heart disease, a permanent condition with often life-threatening complications. Rheumatic heart disease is an important health problem in places and communities where S. pyogenes infections occur frequently. A vaccine against these bacteria would help lower the number of people with valvular heart disease; however, no such vaccine exists yet. Research on vaccines against S. pyogenes was on hold for almost 30 years because of initial concerns that vaccinated children might develop acute rheumatic fever more frequently. Recently, researchers started working again on vaccines against S. pyogenes, but concerns about the safety of such vaccines persist. Doctors can reliably use echocardiography to diagnose cases of rheumatic carditis (as a sign of acute rheumatic fever) and rheumatic heart disease. Here, we propose a simple approach for the consistent use of echocardiography in clinical research of vaccines against S. pyogenes that will allow the detection of any potential heart-related side effects of the vaccine.
Similar content being viewed by others
The development of Group A Streptococcus vaccines has been delayed partly because of safety concerns, including cardiac adverse events. |
Echocardiography is an invaluable tool for evaluating cardiac involvement in acute rheumatic fever episodes and rheumatic heart disease. |
With the approach proposed here, echocardiography could also be used consistently for a cardiac safety evaluation in Group A Streptococcus vaccine clinical trials. |
1 Introduction
Streptococcus pyogenes, or Group A Streptococcus, is a Gram-positive bacterium that colonises the upper respiratory tract or skin, and is responsible for a wide variety of human diseases, including streptococcal pharyngitis, scarlet fever, impetigo, erysipelas and necrotising fasciitis [1]. Children, older adults and immunocompromised individuals are at the highest risk of S. pyogenes infections and their associated complications [2,3,4]. Diseases caused by S. pyogenes are highly prevalent in developing countries, among indigenous populations and in low socioeconomic areas in developed countries [3, 5].
Superficial S. pyogenes infections (pharyngitis and impetigo), while relatively benign, are considered a prerequisite for the development of autoimmune nonsuppurative sequelae, including acute rheumatic fever (ARF) and rheumatic heart disease (RHD) [6, 7]; the latter represents the most severe post-streptococcal sequela and is a major driver of mortality [8,9,10].
Despite the significant global health challenges of S. pyogenes, there are currently no licensed vaccines against this pathogen [11, 12]. The quest to obtain a vaccine against scarlet fever began more than two centuries ago, even before the causative agent of the disease had been unequivocally determined [13, 14]. Nevertheless, the development of a Group A Streptococcus vaccine (Strep A vaccine) came to a halt after the cross-reactivity of antibodies elicited against some streptococcal components with human tissues was progressively recognised since the 1930s [15,16,17], and after safety concerns arose that the vaccines might favor the development of ARF and subsequent cardiac damage in the 1960s [18]. Although the link between the ARF cases observed and the immune response to the vaccine antigen was not demonstrated, the US Food and Drug Administration issued a moratorium on Strep A vaccine development in 1979, which was only lifted almost 30 years later [19]. With interest in Strep A vaccine development being reignited in the last 2 decades, the role of safety monitoring, including of cardiac safety events, remains crucial. Transthoracic echocardiography (TTE) is widely accepted as a sensitive method of detecting cardiac involvement in patients with ARF and subclinical RHD [8, 20]; however, the role of TTE in the screening and monitoring of cardiac pathologies/adverse events in Strep A vaccine clinical trials is not adequately defined. Currently, there are no standardised guidelines for the consistent use of TTE in the clinical development of vaccines against S. pyogenes.
This review aims to provide an overview of the natural history and burden of ARF and RHD, to discuss the current status of TTE in the clinical development process of Strep A vaccines and to propose a framework for its consistent use in surveillance of cardiac adverse events during vaccine clinical trials. A graphical summary of the review (Fig. 1) is also provided.
2 Natural History of Disease Caused by S. pyogenes
Susceptible individuals may develop pharyngitis typically within 5 days after exposure of the upper respiratory tract to S. pyogenes or impetigo within 10 days after exposure of the skin (Fig. 2) [21, 22]. Both types of superficial S. pyogenes infections usually resolve within 1 week [23, 24]. However, some individuals develop ARF typically within 1–5 weeks following an episode of pharyngitis [25, 26] and possibly impetigo, especially in tropical regions [27, 28]. Evidence suggests that repeated superficial infections with S. pyogenes, likely with multiple strains, are required to initiate the autoimmune process leading to ARF [29, 30].
Natural history of disease caused by Streptococcus pyogenes. The syringe icon indicates potential points where vaccines can be used for preventing S. pyogenes infections/acute rheumatic fever (ARF) recurrences/rheumatic heart disease (RHD). The echo icon indicates points where echocardiography (Echo) can be useful for the diagnosis and follow-up of ARF, ARF recurrences and RHD. PSGN post-streptococcal glomerulonephritis
In up to 90% of patients, ARF manifests as an acute febrile illness with joint manifestations; however, in over 50% of adolescents with ARF, carditis also occurs [31]. Rheumatic carditis is a pancarditis characterised by inflammation of the pericardium, myocardium and endocardium, manifesting mostly as valvulitis; its severity can range from subclinical to fulminant carditis that can even result in death [32, 33].
Most ARF episodes, including the clinical and pathological features of rheumatic carditis, observed in the pericardium and myocardium, will fully subside within 6 weeks, and 90% will resolve within 3 months [34]. However, in some individuals, damage to the cardiac valves might persist and lead to scarring [8, 35, 36]. The resulting chronic valvulopathy, named RHD, may develop following the first episode of ARF, or after many years and several recurrent ARF episodes, with each recurrence aggravating the valvular damage [8, 37]. Involvement of the mitral valve is the hallmark of RHD; however, aortic and tricuspid valves are also frequently affected [35, 38]. Clinical manifestations of RHD range from a heart murmur (detected during a routine clinical examination) to atrial fibrillation, infective endocarditis, congestive cardiac failure, embolic stroke and sudden cardiac death [8]. In resource-poor settings, patients may present for the first time with a complication of established RHD rather than ARF, owing to a lack of awareness of ARF symptoms, the subclinical nature of some carditis episodes, a similarity of presenting features with common febrile childhood illnesses or the lack of access to medical care [8].
Currently, the most effective intervention for decreasing recurrence of ARF and preventing RHD involves prophylactic administration of antibiotics (mainly benzathine penicillin) [8, 39]. Although penicillin resistance has not been documented for S. pyogenes, this strategy has failed to control ARF and RHD because of difficulties with adherence and the extensive public health commitment required, which is beyond the capacity of many health systems in low- and m-income countries (LMICs). Additionally, concerns over the emergence of potential antimicrobial resistance following widespread and/or long-term exposure to antibiotics cannot be dismissed.
3 Burden of ARF and RHD
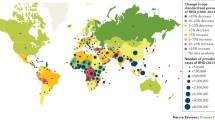
Worldwide, ARF represents the most common cause of acquired heart disease in children [40]. Over the past decades, ARF rates have decreased in middle-income and high-income settings but remain high in LMICs. Estimating the true burden of ARF is challenging, as clinical signs of ARF are being frequently disregarded and reliable notification programmes are lacking in the most affected areas [41]. In a systematic review that included studies published prior to 2011, reported ARF incidence per year varied between 0.1/100,000 persons in Greece and 826/100,000 persons in Sudan; indigenous people from New Zealand and Australia appeared to be disproportionally affected [42]. Most of the RHD burden originates from LMICs (Fig. 3), with around 80% of all RHD cases in children aged 5–14 years coming from “less developed” countries, according to the definition of the United Nations Population Division [9, 40]. A high burden of disease is also seen in impoverished and socioeconomically disadvantaged communities of high-income countries [43]. Rheumatic heart disease is estimated to account for up to 60% of the annual global deaths related to diseases caused by S. pyogenes [40]; in developing countries, mortality rates are likely elevated because of the unavailability of life-saving interventions.
Burden of rheumatic heart disease in low-income and middle-income countries. Data included in the figure were selectively extracted from the World Life Expectancy website (available from: https://www.worldlifeexpectancy.com/cause-of-death/rheumatic-heart-disease/by-country/; https://www.worldlifeexpectancy.com/world-rankings-total-deaths, accessed 19 May, 2022) and refer to the year 2020. Numbers indicate the total number of deaths caused by rheumatic heart disease in each depicted country
Although ARF incidence in a country should be correlated with that country’s RHD prevalence, this is not always the case. For example, a high RHD prevalence in South Africa is associated with a relatively low incidence of ARF (13.4/100,000 persons) [41]; such findings suggest that ARF could be massively underdiagnosed in LMICs [44].
The economic burden of RHD, measured by estimating the resulting economic losses in 107 endemic countries, is huge. The costs of the 222,000 lives lost because of RHD in 2010, mainly originating from South and East Asia, were estimated to range between US$2.2 trillion and US$5.4 trillion. Most of the economic impact of RHD is due to the premature death of children and working-age adults [45].
4 Echocardiography in the Diagnosis of Rheumatic Carditis and RHD
According to the modified Jones diagnostic criteria for ARF, TTE with Doppler should be performed in confirmed or suspected cases of ARF to assess the presence of rheumatic carditis, even in the absence of suggestive auscultatory findings, especially in at-risk populations [46]. The ARF criteria from 1992 only considered symptomatic carditis, which is usually accompanied by a significant pathological murmur, as a major diagnostic criterion [47]. Over time, the importance of subclinical rheumatic carditis, which can go undetected by auscultation, was also recognised, prompting the development of the modified Jones criteria in 2015 [46].
In addition to representing the current gold standard in diagnosing RHD, TTE is also indispensable for identifying subclinical cases of rheumatic carditis [31, 48] by revealing very mild or silent valvular regurgitation [49,50,51,52]. Introduction of TTE screening has revealed a higher prevalence of RHD and more advanced disease in several LMICs than previously identified by clinical examinations [53,54,55]. This highlights the importance of TTE in diagnosing patients suspected to have rheumatic carditis, especially in vulnerable populations. However, to better characterise lesions caused by RHD, TTE examination sometimes needs to be complemented with transesophageal echocardiography [31, 56] or other assessments [31].
The criteria published by the World Heart Federation (WHF) in 2012 aimed to provide a uniform TTE protocol for diagnosing RHD [57]. The sensitivity and specificity of the 2012 WHF criteria have been demonstrated in many countries [58, 59]; however, they were only moderately reproducible even when used by expert cardiologists [60, 61]. The updated 2023 WHF criteria provide further standardisation of disease definitions, as well as new echocardiographic criteria to diagnose rheumatic carditis and RHD, thus facilitating the application of TTE in large-scale screening programmes [62]. The roll out and implementation of these revised guidelines are expected to improve consistency of the RHD diagnostic process, contributing to the optimisation of treatment programmes. Other national and regional guidelines for the diagnosis of ARF in New Zealand, India and Australia have also included TTE/Doppler in the diagnosis and management of ARF and RHD [34, 63, 64].
5 Echocardiography in Strep A Vaccine Clinical Trials
Currently, several Strep A vaccine candidates are under investigation, targeting either the M protein peptide epitopes or other, non-M protein S. pyogenes antigens [11, 65]. Most of these candidates are still in the pre-clinical development phase, with only a few having advanced to phase I and II clinical trials [10].
Because of a risk of Strep A vaccines triggering autoimmune complications, the World Health Organization and the US Food and Drug Administration recommend that researchers seek antigens that have a minimal chance of inducing immunological cross-reactivity, and to prove the safety of candidate vaccines in as many ways as possible. This includes intensive screening of participants for the development of cross-reactive antibodies and TTE to detect subclinical and clinically manifest rheumatic carditis, as a possible adverse event of special interest (AESI) [66]. Despite this call for strict regulations on safety surveillance in Strep A clinical trials, widely applicable strategies for the safety assessment and management of adverse events as well as the harmonisation of trial design and safety protocols are still lacking [12, 67].
Transthoracic echocardiography evaluations have been included in phase I and II clinical trials as part of safety requirements to assess baseline status and to monitor study participants after vaccine administration (i.e. to detect potential subclinical rheumatic carditis) [68,69,70,71,72]. However, the absence of standardised criteria for the consistent use of assessment methods has resulted in variations in the timing and frequency of TTE evaluations performed in trials of Strep A vaccine candidates (Table 1). In addition, only one study [71] provided a detailed description of the TTE examination methodology, which would facilitate the comparison of results across trials and clinical development programmes. While none of the studies reported any relevant TTE findings, in the absence of a clearly described methodology, parameters with varying levels of importance might have been measured, leading to outputs that are confusing or of limited value.
6 Proposal for Harmonisation of TTE Use in the Clinical Development Process of Strep A Vaccines
In the context of developing vaccines against S. pyogenes, cardiac safety evaluation will be focused on detecting cases of rheumatic carditis, rather than RHD, as the time required for the latter condition would exceed the typical duration of a vaccine clinical trial. A stepwise application of criteria currently used to diagnose ARF/rheumatic carditis, with a rationale for each step and the definition of relevant outputs, can provide a solid basis for the consistent use of TTE during Strep A vaccine candidate trials and a timely detection of rheumatic carditis events.
We propose here a streamlined approach aimed to standardise the use of TTE in phase I Strep A vaccine clinical trials, planned to be used in upcoming studies of a Strep A vaccine candidate developed by GSK [73]. If consistently applied, this framework would allow investigators to optimise resource use based on findings of both planned and unplanned visits, while minimising the likelihood of missing any cardiac adverse event.
First, all participants should undergo TTE at two timepoints: at the screening visit, to avoid enrolling and vaccinating participants with pre-existing undiagnosed congenital and acquired cardiac lesions (including persistent or resolving rheumatic carditis, and subclinical RHD), and at 6 months after receiving the last study vaccine dose, to ensure that participants did not develop cardiac abnormalities during the trial. As ARF episodes occur in the first 3 months following exposure to S. pyogenes (Fig. 2), this 6-month timeframe ensures that no cardiac events (including silent subclinical events) potentially caused by the study vaccine are missed.
Second, at each planned vaccination visit (Fig. 4), before administering the study vaccine, investigators should reconfirm that the participant is free from symptoms and signs suggestive of ARF/rheumatic carditis (diagnosed according to the modified Jones criteria [46]) and remains eligible for vaccination. This includes interviewing the participants (using investigator and patient questionnaires similar to those provided in the Electronic Supplementary Material [ESM]) and performing a physical examination (assessing for signs of ARF using the modified Jones criteria [46], Doppler auscultation to identify soft murmurs [74], and electrocardiography). However, even in the absence of findings suggestive of ARF/rheumatic carditis, asymptomatic participants with an incipient S. pyogenes infection should not be vaccinated; in their case, establishing whether a potential subsequent episode of ARF/rheumatic carditis was triggered by naturally occurring S. pyogenes infection or the study vaccine would not be possible. In all participants pre-vaccination, throat swabs should be collected, and the modified Centor score [75] should be determined; samples from participants with a sore throat and a high likelihood of S. pyogenes infection based on modified Centor score >3 should be tested using point-of-care (POC) molecular methods. Point-of-care tests are highly sensitive methods for detecting active S. pyogenes infections, while being more specific than the modified Centor score. Using POC diagnostics to confirm S. pyogenes infection will limit the number of unnecessary withdrawals from the study. Only patients with no findings suggestive of ARF/rheumatic carditis and with a low likelihood of S. pyogenes infection (modified Centor score ≤ 3) or a negative POC test result should be vaccinated.
Proposed process flow for pre-vaccination assessment in Strep A vaccine clinical development programmes. AESI adverse event of special interest, ARF acute rheumatic fever, ECG electrocardiography, POC point-of-care, *see ESM, **see Fig. 5
Third, at any time a participant reports symptoms or signs, and/or has examination findings suggestive of ARF/rheumatic carditis, the steps indicated in the decision tree for monitoring rheumatic carditis as AESI (Fig. 5) should be followed, to ensure the safety of participants and to avoid further vaccination in the case of ARF. The decision tree comprises 3 assessment layers, where positive findings of each layer trigger the progression of the participant to the next one: (1) the participant reports signs or symptoms of ARF/rheumatic carditis as per the investigator/patient questionnaire (ESM); (2) the investigator performs an electrocardiogram to check for a prolonged P-R interval, and Doppler auscultation for heart murmurs of mitral/aortic valves; and (3) TTE, POC test for S. pyogenes and assessment of the modified Jones criteria for ARF are performed to identify the cause of the positive cardiac findings. Anti-streptolysin O antibody titres are expected to rise following vaccination with a streptolysin-containing Strep A vaccine, and therefore could not be used to confirm infection; however, ARF can be diagnosed according to the modified Jones criteria based on anti-DNase B titres (or combined serology testing [76, 77]), throat culture and POC tests. Based on the results of the layer 3 assessments, vaccination of individual participants as well as of the entire trial population (in case an AESI is confirmed) can be either continued or placed on hold. In case the trial is put on hold, all efforts should be made to quickly determine the aetiology of ARF and its relationship to vaccination, to allow a quick recommencement of the study or termination if the vaccine is confirmed to cause rheumatic carditis.
Proposed decision tree for monitoring rheumatic carditis as an adverse event of special interest (AESI) in Group A Streptococcus vaccine clinical development programmes. ARF acute rheumatic fever, DMC Data Monitoring Committee, ECG electrocardiography, pIMDs potential immune-mediated diseases, POC point of care, TTE transthoracic echocardiography, *see Fig. 4, **see ESM, ***determining anti-streptolysin O titres to obtain evidence of Streptococcus pyogenes infection would not be useful in clinical trials of Group A Streptococcus vaccines that contain streptolysin O; nevertheless, infection can be confirmed according to the modified Jones criteria based on anti-DNase B titres or combined serology testing, throat culture and POC tests
The proposed approach has several advantages. It includes an optimised number of TTE examinations that avoids too frequent, resource-consuming evaluations, while being flexible enough to ensure no cardiac safety signals are missed. Starting with the second dose of vaccine, pre-vaccination assessments allow any potential case of vaccine-induced ARF, including rheumatic carditis, to be identified. The timing of these scheduled visits is optimal for this scope, considering that the visits occur no later than 8 weeks after the previous vaccine dose, while ARF/rheumatic carditis develops typically within 1–5 weeks after the triggering contact with S. pyogenes (either through natural infection or potentially through vaccination). The possibility of performing TTE at any time of the study in case there is a suspicion of ARF and rheumatic carditis ensures that symptomatic cases of rheumatic carditis are detected as soon as possible. The key strength of this approach lies in the fact that it is based on existing guidelines and is placed within the context of an existing Strep A vaccine development programme. To our knowledge, this is the first proposal for a simple easy-to-apply algorithm that can be integrated in Strep A vaccine clinical trials, at least in the initial stages of development, to establish the baseline vaccine safety. In large-scale phase III trials, the algorithm could be further simplified to avoid placing an excessive burden on the study resources. Beyond phase I, Strep A vaccine trials would benefit from the implementation of a TTE diagnostic algorithm (as described in the 2023 WHF criteria [62]), which can be applied by non-cardiologists using handheld echocardiographic units.
The approach proposed in this article has the advantage of creating an overlap between safety and efficacy assessments, as the evaluation of 2 clinical trial efficacy endpoints (pharyngitis and impetigo) is integrated into the pathway for investigating rheumatic carditis as an AESI. However, it must be emphasised that this approach is not the result of an expert consensus and has been created to fulfil the need for a safety assessment tool that can be used across different Strep A vaccine clinical development programmes.
7 Implications of Using TTE in Clinical Trials and Future Directions
Ideally, clinical trials of Strep A vaccines should take place in countries where relevant endpoints (e.g. a significant reduction in ARF/RHD incidence following vaccination) can be measured. In such countries, however, the chance of a study participant contracting S. pyogenes disease and subsequently developing ARF and/or rheumatic carditis during the study is also high. A clinical trial design with a well-established safety screening algorithm as the one proposed here can ensure that adverse events following immunisation are correctly detected.
To maximise the utility of TTE and ensure consistency across the different clinical development programmes, a well-defined and easy-to-apply case definition for rheumatic carditis as an AESI/adverse event following immunisation should be formulated and consistently applied. For this, further qualitative research in populations with a high ARF incidence would be needed. In addition, standardising should also be extended to other elements of the TTE surveillance process. The type of equipment used for TTE can influence the diagnostic accuracy of RHD in children [78], so exploring these inter-equipment differences and accounting for them would make the obtained results more reliable. Similarly, some degree of inter-observer variability in how echocardiographic images are interpreted does exist [60, 61, 79]; TTE-specific continuous education and training programmes for sonographers and physicians are needed to improve diagnostic accuracy [79]. In a large clinical trial, using a blinded expert endpoint evaluation committee could ensure consistency in diagnosing key endpoints, including rheumatic carditis and RHD [52].
Integrating TTE in a more complex evaluation frame and combining it with other, less complex tests, as done in the standardised approach proposed here, is key to maximising efficiency while balancing the costs and use of often limited resources. However, as the devices needed to perform TTE become smaller and more autonomous, and interpretation of the findings is performed by artificial intelligence, the challenges of consistently implementing TTE as a cardiac safety assessment tool in Strep A clinical trials, even in LMICs, are removed one by one.
In the future, we can expect to be able to identify individuals at a higher risk of severe cardiac ARF complications [80,81,82]. For example, biomarkers could potentially be used as predictors and measures of rheumatic carditis, perhaps even leading to the replacement of TTE by simple blood tests [83,84,85]. Until then, however, we should fully exploit the potential of TTE in detecting cardiac complications of S. pyogenes disease.
8 Conclusions
Along with other diagnostic methods and tests, TTE remains an invaluable tool in the clinical development process of vaccines against S. pyogenes. Our proposed approach for a simplified, efficient and standardised use of TTE can pave the way for a consensus by experts in the field regarding its consistent application in evaluating the safety of Strep A vaccine candidates.
References
Ferretti JJ, Köhler W. History of streptococcal research. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK333430/. Accessed 4 May 2022.
Marshall HS, Richmond P, Nissen M, et al. Group A streptococcal carriage and seroepidemiology in children up to 10 years of age in Australia. Pediatr Infect Dis J. 2015;34(8):831–8. https://doi.org/10.1097/INF.0000000000000745.
May PJ, Bowen AC, Carapetis JR. The inequitable burden of Group A streptococcal diseases in indigenous Australians. Med J Aust. 2016;205(5):201–3. https://doi.org/10.5694/mja16.00400.
Efstratiou A, Lamagni T. Epidemiology of Streptococcus pyogenes. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK343616/. Accessed 18 May 2022.
Avire NJ, Whiley H, Ross K. A review of Streptococcus pyogenes: public health risk factors, prevention and control. Pathogens. 2021;10(2):248. https://doi.org/10.3390/pathogens10020248.
Sika-Paotonu D, Beaton A, Raghu A, et al. Acute rheumatic fever and rheumatic heart disease. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK425394/. Accessed 18 May 2022.
Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10(8): e0136789. https://doi.org/10.1371/journal.pone.0136789.
Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. https://doi.org/10.1038/nrdp.2015.84.
Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377(8):713–22. https://doi.org/10.1056/NEJMoa1603693.
Rwebembera J, Beaton AZ, de Loizaga SR, et al. The global impact of rheumatic heart disease. Curr Cardiol Rep. 2021;23(11):160. https://doi.org/10.1007/s11886-021-01592-2.
Dale JB, Walker MJ. Update on Group A streptococcal vaccine development. Curr Opin Infect Dis. 2020;33(3):244–50. https://doi.org/10.1097/qco.0000000000000644.
Vekemans J, Gouvea-Reis F, Kim JH, et al. The path to Group A Streptococcus vaccines: World Health Organization research and development technology roadmap and preferred product characteristics. Clin Infect Dis. 2019;69(5):877–83. https://doi.org/10.1093/cid/ciy1143.
Dick GF, Dick GH. Scarlet fever. Am J Public Health (NY). 1924;14(12):1022–8. https://doi.org/10.2105/ajph.14.12.1022.
Peterman MG. Immunization against scarlet fever. Am J Dis Child. 1937;54(1):89–95. https://doi.org/10.1001/archpedi.1937.01980010098009.
Rejholec V, Wagner V. Response by antibodies to tissue antigens in the course of rheumatic fever. Ann Rheum Dis. 1956;15(4):364–72. https://doi.org/10.1136/ard.15.4.364.
Sharma A, Nitsche-Schmitz D. Challenges to developing effective streptococcal vaccines to prevent rheumatic fever and rheumatic heart disease. Vaccine Dev Ther. 2014;4:39–54. https://doi.org/10.2147/VDT.S45037.
Asturias EJ, Excler JL, Ackland J, et al. Safety of Streptococcus pyogenes vaccines: anticipating and overcoming challenges for clinical trials and post-marketing monitoring. Clin Infect Dis. 2023;77(6):917–24. https://doi.org/10.1093/cid/ciad311.
Massell BF, Honikman LH, Amezcua J. Rheumatic fever following streptococcal vaccination: report of three cases. JAMA. 1969;207(6):1115–9.
Food and Drug Administration, HHS. Revocation of status of specific products; Group A Streptococcus. Direct final rule. Fed Regist. 2005;70(231):72197–9.
Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease-an evidence-based guideline. Nat Rev Cardiol. 2012;9(5):297–309. https://doi.org/10.1038/nrcardio.2012.7.
Centers for Disease Control and Prevention. Group A streptococcal (GAS) disease. Pharyngitis (Strep throat). https://www.cdc.gov/groupastrep/diseases-hcp/strep-throat.html. Accessed 24 Apr 2022.
Bryant AE, Stevens DL. Streptococcus pyogenes. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, vol. 2. 8th ed. Philadelphia: Elsevier Saunders; 2015. p. 2285- 99.e4.
Wessels MR. Pharyngitis and scarlet fever. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK333418/. Accessed 5 May 2022.
Stevens DL, Bryant AE. Impetigo, erysipelas and cellulitis. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK333408/. Accessed 4 Apr 2022.
Centers for Disease Control and Prevention. Group A streptococcal (GAS) disease. Acute rheumatic fever. https://www.cdc.gov/groupastrep/diseases-hcp/acute-rheumatic-fever.html. Accessed 8 Apr 2022.
Shulman ST, Bisno AL. Nonsuppurative poststreptococcal sequelae: rheumatic fever and glomerulonephritis. In: Bennett J, Dolin R, Blaser M, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases, vol. 2. 8th ed. Philadelphia: Elsevier; 2015. p. 2300–9.
McDonald MI, Towers RJ, Andrews RM, et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis. 2006;43(6):683–9. https://doi.org/10.1086/506938.
McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect Dis. 2004;4(4):240–5. https://doi.org/10.1016/s1473-3099(04)00975-2.
Raynes JM, Frost HRC, Williamson DA, et al. Serological evidence of immune priming by Group A streptococci in patients with acute rheumatic fever. Front Microbiol. 2016;7:1119. https://doi.org/10.3389/fmicb.2016.01119.
Lorenz N, Ho TKC, McGregor R, et al. Serological profiling of Group A Streptococcus infections in acute rheumatic fever. Clin Infect Dis. 2021;73(12):2322–5. https://doi.org/10.1093/cid/ciab180.
Zühlke LJ, Beaton A, Engel ME, et al. Group A Streptococcus, acute rheumatic fever and rheumatic heart disease: epidemiology and clinical considerations. Curr Treat Options Cardiovasc Med. 2017;19(2):15. https://doi.org/10.1007/s11936-017-0513-y.
Tubridy-Clark M, Carapetis JR. Subclinical carditis in rheumatic fever: a systematic review. Int J Cardiol. 2007;119(1):54–8. https://doi.org/10.1016/j.ijcard.2006.07.046.
Essop MR, Wisenbaugh T, Sareli P. Evidence against a myocardial factor as the cause of left ventricular dilation in active rheumatic carditis. J Am Coll Cardiol. 1993;22(3):826–9. https://doi.org/10.1016/0735-1097(93)90197-9.
RHDAustralia (ARF/RHD writing group) The 2020 Australian guideline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease (3.2 edition, March 2022). https://www.rhdaustralia.org.au/system/files/fileuploads/arf_rhd_guidelines_3.2_edition_march_2022.pdf. Accessed 4 Apr 2022.
Tandon R, Sharma M, Chandrashekhar Y, et al. Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol. 2013;10(3):171–7. https://doi.org/10.1038/nrcardio.2012.197.
World Health Organization. Rheumatic heart disease. 2020. https://www.who.int/news-room/fact-sheets/detail/rheumatic-heart-disease. Accessed 5 May 2022.
Dass C, Kanmanthareddy A. Rheumatic heart disease. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK538286/. Accessed 4 Apr 2022.
Remenyi B, ElGuindy A, Smith SC Jr, et al. Valvular aspects of rheumatic heart disease. Lancet. 2016;387(10025):1335–46. https://doi.org/10.1016/s0140-6736(16)00547-x.
Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev. 2002;2002(3):CD002227. https://doi.org/10.1002/14651858.CD002227.
Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of Group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–94. https://doi.org/10.1016/S1473-3099(05)70267-x.
Sims Sanyahumbi A, Colquhoun S, Wyber R, et al. Global disease burden of group A Streptococcus. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK333415/. Accessed 4 Apr 2022.
Jackson SJ, Steer AC, Campbell H. Systematic review: estimation of global burden of non-suppurative sequelae of upper respiratory tract infection: rheumatic fever and post-streptococcal glomerulonephritis. Trop Med Int Health. 2011;16(1):2–11. https://doi.org/10.1111/j.1365-3156.2010.02670.x.
Kang K, Chau KWT, Howell E, et al. The temporospatial epidemiology of rheumatic heart disease in Far North Queensland, tropical Australia 1997–2017; impact of socioeconomic status on disease burden, severity and access to care. PLoS Negl Trop Dis. 2021;15(1): e0008990. https://doi.org/10.1371/journal.pntd.0008990.
Okello E, Ndagire E, Atala J, et al. Active case finding for rheumatic fever in an endemic country. J Am Heart Assoc. 2020;9(15): e016053. https://doi.org/10.1161/jaha.120.016053.
Watkins D, Daskalakis A. The economic impact of rheumatic heart disease in developing countries. Lancet Glob Health. 2015;3:S37. https://doi.org/10.1016/S2214-109X(15)70156-7.
Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131(20):1806–18. https://doi.org/10.1161/cir.0000000000000205.
Dajani AS, Ayoub E, Bierman FZ, et al. Guidelines for the diagnosis of rheumatic fever: Jones criteria, 1992 update. JAMA. 1992;268(15):2069–73. https://doi.org/10.1001/jama.1992.03490150121036.
Ramakrishnan S. Echocardiography in acute rheumatic fever. Ann Pediatr Cardiol. 2009;2(1):61–4. https://doi.org/10.4103/0974-2069.52812.
Wilson N. Echocardiography and subclinical carditis: guidelines that increase sensitivity for acute rheumatic fever. Cardiol Young. 2008;18(6):565–8. https://doi.org/10.1017/S1047951108003211.
Pekpak E, Atalay S, Karadeniz C, et al. Rheumatic silent carditis: echocardiographic diagnosis and prognosis of long-term follow up. Pediatr Int. 2013;55(6):685–9. https://doi.org/10.1111/ped.12163.
Agarwal PK, Misra M, Sarkari NB, et al. Usefulness of echocardiography in detection of subclinical carditis in acute rheumatic polyarthritis and rheumatic chorea. J Assoc Physicians India. 1998;46(11):937–8.
Beaton A, Okello E, Rwebembera J, et al. Secondary antibiotic prophylaxis for latent rheumatic heart disease. N Engl J Med. 2022;386(3):230–40. https://doi.org/10.1056/NEJMoa2102074.
Ansa VO, Odigwe CO, Agbulu RO, et al. The clinical utility of echocardiography as a cardiological diagnostic tool in poor resource settings. Niger J Clin Pract. 2013;16(1):82–5. https://doi.org/10.4103/1119-3077.106772.
Zahari N, Yeoh SL, Muniandy SR, et al. Pediatric rheumatic heart disease in a middle-income country: a population-based study. J Trop Pediatr. 2022;68(1):fmac05. https://doi.org/10.1093/tropej/fmac005.
Bhaya M, Panwar S, Beniwal R, et al. High prevalence of rheumatic heart disease detected by echocardiography in school children. Echocardiography. 2010;27(4):448–53. https://doi.org/10.1111/j.1540-8175.2009.01055.x.
Saxena A. Echocardiographic diagnosis of chronic rheumatic valvular lesions. Glob Heart. 2013;8(3):203–12. https://doi.org/10.1016/j.gheart.2013.08.007.
Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease: an evidence-based guideline. Nat Rev Cardiol. 2012;9(5):297–309. https://doi.org/10.1038/nrcardio.2012.7.
Clark BC, Krishnan A, McCarter R, et al. Using a low-risk population to estimate the specificity of the World Heart Federation criteria for the diagnosis of rheumatic heart disease. J Am Soc Echocardiogr. 2016;29(3):253–8. https://doi.org/10.1016/j.echo.2015.11.013.
Roberts K, Maguire G, Brown A, et al. Echocardiographic screening for rheumatic heart disease in high and low risk Australian children. Circulation. 2014;129(19):1953–61. https://doi.org/10.1161/CIRCULATIONAHA.113.003495.
Culliford-Semmens N, Nicholson R, Tilton E, et al. The World Heart Federation criteria raise the threshold of diagnosis for mild rheumatic heart disease: three reviewers are better than one. Int J Cardiol. 2019;291:112–8. https://doi.org/10.1016/j.ijcard.2019.02.058.
Scheel A, Mirabel M, Nunes MCP, et al. The inter-rater reliability and individual reviewer performance of the 2012 World Heart Federation guidelines for the echocardiographic diagnosis of latent rheumatic heart disease. Int J Cardiol. 2021;328:146–51. https://doi.org/10.1016/j.ijcard.2020.11.013.
Rwebembera J, Marangou J, Mwita JC, et al. World Heart Federation guidelines for the echocardiographic diagnosis of rheumatic heart disease. Nat Rev Cardiol. 2023. https://doi.org/10.1038/s41569-023-00940-9.
Atatoa-Carr P, Lennon D, Wilson N, et al. Rheumatic fever diagnosis, management, and secondary prevention: a New Zealand guideline. N Z Med J. 2008;121(1271):59–69.
Working Group on Pediatric Acute Rheumatic Fever and Cardiology Chapter of Indian Academy of Pediatrics, Saxena A, Kumar RK, et al. Consensus guidelines on pediatric acute rheumatic fever and rheumatic heart disease. Indian Pediatr. 2008;45(7):565–73.
Azuar A, Jin W, Mukaida S, et al. Recent advances in the development of peptide vaccines and their delivery systems against Group A Streptococcus. Vaccines (Basel). 2019;7(3):58. https://doi.org/10.3390/vaccines7030058.
World Health Organization. Group A streptococcal vaccine development: current status and issues of relevance to less developed countries. 2005. http://apps.who.int/iris/bitstream/handle/10665/69065/WHO_IVB_05.14_eng.pdf?sequence=1. Accessed 28 Mar 2022.
Schödel F, Moreland NJ, Wittes JT, et al. Clinical development strategy for a candidate Group A streptococcal vaccine. Vaccine. 2017;35(16):2007–14. https://doi.org/10.1016/j.vaccine.2017.02.060.
Kotloff KL, Corretti M, Palmer K, et al. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA. 2004;292(6):709–15. https://doi.org/10.1001/jama.292.6.709.
McNeil SA, Halperin SA, Langley JM, et al. Safety and immunogenicity of 26-valent Group A Streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41(8):1114–22. https://doi.org/10.1086/444458.
McNeil S, Halperin S, Langley J, et al. A double-blind, randomized phase II trial of the safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adults. Int Congr Ser. 2006;1289:303–6. https://doi.org/10.1016/j.ics.2005.12.002.
Sekuloski S, Batzloff MR, Griffin P, et al. Evaluation of safety and immunogenicity of a Group A Streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS One. 2018;13(7): e0198658. https://doi.org/10.1371/journal.pone.0198658.
Pastural É, McNeil SA, MacKinnon-Cameron D, et al. Safety and immunogenicity of a 30-valent M protein-based Group A streptococcal vaccine in healthy adult volunteers: a randomized, controlled phase I study. Vaccine. 2020;38(6):1384–92. https://doi.org/10.1016/j.vaccine.2019.12.005.
Di Benedetto R, Mancini F, Carducci M, et al. Rational design of a glycoconjugate vaccine against Group A Streptococcus. Int J Mol Sci. 2020;21(22):8558. https://doi.org/10.3390/ijms21228558.
Mc Loughlin MJ, Mc LS. Cardiac auscultation: preliminary findings of a pilot study using continuous Wave Doppler and comparison with classic auscultation. Int J Cardiol. 2013;167(2):590–1. https://doi.org/10.1016/j.ijcard.2012.09.223.
McIsaac WJ, White D, Tannenbaum D, et al. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158(1):75–83.
Hanson-Manful P, Whitcombe AL, Young PG, et al. The novel Group A Streptococcus antigen SpnA combined with bead-based immunoassay technology improves streptococcal serology for the diagnosis of acute rheumatic fever. J Infect. 2018;76(4):361–8. https://doi.org/10.1016/j.jinf.2017.12.008.
Whitcombe AL, Hanson-Manful P, Jack S, et al. Development and evaluation of a new triplex immunoassay that detects Group A Streptococcus antibodies for the diagnosis of rheumatic fever. J Clin Microbiol. 2020;58(9):e00300-e320. https://doi.org/10.1128/jcm.00300-20.
Beaton A, Aliku T, Okello E, et al. The utility of handheld echocardiography for early diagnosis of rheumatic heart disease. J Am Soc Echocardiogr. 2014;27(1):42–9. https://doi.org/10.1016/j.echo.2013.09.013.
Grayburn PA, Bhella P. Grading severity of mitral regurgitation by echocardiography: science or art? JACC Cardiovasc Imaging. 2010;3(3):244–6. https://doi.org/10.1016/j.jcmg.2009.11.008.
Scalzi V, Hadi HA, Alessandri C, et al. Anti-endothelial cell antibodies in rheumatic heart disease. Clin Exp Immunol. 2010;161(3):570–5. https://doi.org/10.1111/j.1365-2249.2010.04207.x.
Rastogi M, Sarkar S, Makol A, et al. Anti-endothelial cell antibody rich sera from rheumatic heart disease patients induces proinflammatory phenotype and methylation alteration in endothelial cells. Genes Dis. 2018;5(3):275–89. https://doi.org/10.1016/j.gendis.2018.02.002.
Delunardo F, Scalzi V, Capozzi A, et al. Streptococcal-vimentin cross-reactive antibodies induce microvascular cardiac endothelial proinflammatory phenotype in rheumatic heart disease. Clin Exp Immunol. 2013;173(3):419–29. https://doi.org/10.1111/cei.12135.
Ralph AP, Webb R, Moreland NJ, et al. Searching for a technology-driven acute rheumatic fever test: the START study protocol. BMJ Open. 2021;11(9): e053720. https://doi.org/10.1136/bmjopen-2021-053720.
News Medical. Collaborative network wins $8 million grant to identify biomarkers for acute rheumatic fever. https://www.news-medical.net/news/20220919/Collaborative-network-wins-248-million-grant-to-identify-biomarkers-for-acute-rheumatic-fever.aspx. Accessed 25 Oct 2023.
Passos LSA, Jha PK, Becker-Greene D, et al. Prothymosin alpha: a novel contributor to estradiol receptor alpha-mediated CD8(+) T-cell pathogenic responses and recognition of type 1 collagen in rheumatic heart valve disease. Circulation. 2022;145(7):531–48. https://doi.org/10.1161/circulationaha.121.057301.
Acknowledgements
Medical writing (Timea Kiss), design and coordination support were provided by Akkodis Belgium c/o GSK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Trademark statement
StreptAvax is a trademark of ID Biomedical. StreptAnova is a trademark of Dalhousie University.
Funding
This work was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the conduct and analysis of the studies and covered the costs associated with the development and publishing of the present article.
Conflict of interest
Usman Nakakana, Alimamy Serry-Bangura, Bassey Effiom Edem, Pietro Tessitore, Leonardo Di Cesare, Danilo Gomes Moriel, Audino Podda, Iris Sarah De Ryck and Ashwani Kumar Arora are or were employees of GSK at the time of this study. Usman Nakakana, Alimamy Serry-Bangura, Danilo Gomes Moriel, Audino Podda, Iris Sarah De Ryck and Ashwani Kumar Arora hold shares in GSK. GSK is currently developing a 4-component vaccine candidate against S. pyogenes.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the interpretation of the data and a critical review of the paper for important intellectual content. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nakakana, U., Serry-Bangura, A., Edem, B.E. et al. Application of Transthoracic Echocardiography for Cardiac Safety Evaluation in the Clinical Development Process of Vaccines Against Streptococcus pyogenes. Drugs R D 24, 1–12 (2024). https://doi.org/10.1007/s40268-024-00452-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-024-00452-y