Abstract
Background and Objective
Tramadol has been reported to cause hyponatremia but the evidence is conflicting. The risk of hyponatremia resulting from combination oral tramadol/acetaminophen (TA) therapy is thus unknown. This study examined whether, compared with acetaminophen (AA), TA use is associated with an increased risk of hyponatremia.
Methods
Hospital data compatible with the Observational Medical Outcomes Partnership–Common Data Model (OMOP–CDM; version 5.3) for 30,999 patients taking TA or AA from 2011 through 2020 were analyzed. New-onset hyponatremia was defined as a serum sodium level < 135 mEq/L within 10 days after drug initiation. The incidence rate ratio was calculated based on crude and 1:1 propensity-score-matched models. Subgroup analyses compared patients taking TA extended-release (TA–ER) and TA immediate-release (TA–IR) formulations.
Results
Among the 30,999 patients, 12,122 (39.1%) were aged > 65 years and 16,654 (53.7%) were male. Hyponatremia within 10 days developed in 1613 (8.4%) of the 19,149 patients in the TA group; the incidence rate was higher than in the AA group (4.2%; 493 out of 11,850 cases). In the propensity-score-matched model, the incidence rate of hyponatremia in the TA group was 6.8 per 1000 person-days (PD), which was 1.57-fold (1.31, 1.89) higher than that in the AA group (4.3 per 1000 PD). In both the crude and propensity-score-matched models, the incidence rate of hyponatremia was significantly higher in the TA–ER than TA–IR subgroup.
Conclusion
In this real-world study, hyponatremia was more frequently observed in the TA than AA group, and in the TA–ER than TA–IR subgroup. Therefore, it is imperative to prescribe tramadol cautiously and closely monitor electrolyte levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We evaluated the risk of hyponatremia associated with the tramadol/acetaminophen (TA) combination oral pill using hospital data compatible with OMOP–CDM. | |
Compared with acetaminophen, TA was associated with a higher risk of hyponatremia. | |
We also observed a higher risk of hyponatremia among users of TA extended-release formulations compared with those using TA immediate-release formulations. |
1 Introduction
Tramadol/acetaminophen (TA) is an orally administered combination pill indicated for the symptomatic treatment of moderate-to-severe pain [1]. Tramadol is a centrally acting weak mu-opioid agonist with a nonopioid mechanism of action; it inhibits the reuptake of norepinephrine and serotonin [2]. Acetaminophen (AA) exerts central analgesic effects mediated by the activation of descending serotonergic pathways [3]. Given the synergistic effects of TA in the control of acute or chronic pain, its use is increasing worldwide [4, 5].
Hyponatremia, defined as a plasma sodium concentration < 135 mEq/L, is a common but meaningful event [6] associated with an increased risk of in-hospital falls [7, 8], fracture [9], and in-hospital mortality [10, 11]. Recently, there have been conflicting reports regarding the development of hyponatremia after the initiation of tramadol. Case reports of incident hyponatremia after tramadol initiation [12,13,14,15] included one involving profound hyponatremia (serum sodium level of 110 mEq/L) [13]. A Swedish population-based case control study reported a 2.4-fold higher risk of incident hyponatremia in hospital-admitted tramadol users [16]. However, in a recent study from France, the risk of hyponatremia disappeared after the exclusion of patients concomitantly taking hyponatremic drugs [17]. Furthermore, no association has been reported between acetaminophen and the occurrence of hyponatremia. Thus, among TA single-pill combination users, the risk of hyponatremia is unclear.
Two different TA single-pill combination formulations are currently available. In the TA immediate-release (TA–IR) formulation, the dosing interval is every 6 h, whereas in the TA extended-release (TA–ER) formulation it is every 12 h. The area under the curve values of both formulations and their efficacy in managing moderate to severe pain are comparable, whereas the TA–ER formulation exhibited significantly less fluctuation in plasma concentrations compared with the TA–IR formulation [18, 19]. Whether TA–ER and TA–IR differ in terms of their association with hyponatremia has not been evaluated.
With the increasing use of tramadol or TA single-pill combinations worldwide [4, 5, 20, 21], and given the clinical importance of hyponatremia in hospitalized patients, the association between hyponatremia and TA merits clarification. Therefore, this study examined whether TA use increased the incidence of hyponatremia compared with AA, and whether the incidence differed depending on the type of TA formulation. We extracted real-world data from the electronic medical records of our hospital.
2 Methods
2.1 Data Source
The hospital data in this study were compatible with the Observational Medical Outcomes Partnership–Common Data Model (OMOP–CDM; version 5.3.1) constructed by Pusan National University Hospital Consortium as part of a medical data-driven hospital-support project supported by the Korea Health Information Service (KHIS) and funded by the Ministry of Health and Welfare, Republic of Korea. The database comprises in- and out-patient electronic medical record data from the period January 2011 to December 2020, adapted for the ATLAS analytical tool (OHDSI Collaboratives). Approval to perform anonymous analyses of routinely collected clinical data was obtained, with a waiver of informed consent, from the Pusan National University institutional review board (IRB) committee (PNUH IRB 2210-007-119).
2.2 Study Design and Population
This was a single-center, retrospective study comparing TA single-pill combination therapy with AA for hyponatremia. Patients aged > 18 years who were newly started on TA or AA for > 3 days during their hospital stay were included. Patients with hyponatremia (serum sodium < 135 mEq/L) within 30 days prior to the start of medication were excluded.
2.3 Outcome
The primary outcome was the incidence of hyponatremia within 10 days of TA or AA initiation. The secondary outcome was the incidence of moderate hyponatremia (serum sodium < 130 mEq/L). Both outcomes were further analyzed in the TA–ER and TA–IR subgroups.
2.4 Cohort Definition
The target cohort was defined as patients newly started on TA (for > 3 consecutive days) during hospital admission (fixed-dose TA of 75/650 mg, 37.5/375 mg, or 18.75/162.5 mg as ER or IR formulations; eTable 1). Patients with a recorded serum sodium level < 135 mEq/L during the 30 days prior to cohort entry were excluded, along with patients exposed to AA during the 14 days before and after cohort entry. Cohort “exit” was fixed at 10 days after cohort entry. The comparative cohort had the same characteristics as the target cohort except that AA, at a dose of 650, 500, or 160 mg, replaced TA. Details on the target and comparative cohorts are provided in Fig. 1. The primary and secondary outcome cohorts comprised patients with serum sodium levels < 135 and < 130 mEq/L, respectively, within 10 days of initial drug exposure. The target cohort was further divided into TA–ER and TA–IR subgroups.
2.5 Statistical Analyses
The incidence rate of hyponatremia per 1000 person-days (PD) for each group was determined, along with and the incidence rate ratio (IRR) between the target and comparator cohorts. After calculation of the crude IRR, 1:1 propensity score matching was performed with adjustment for a total of 1531 co-variables, including sex, age group, condition, and drug use within 30 days prior to cohort entry, using L1-regularized large-scale logistic regression models. All statistical tests were two sided, and p-values < 0.05 were considered statistically significant. All analyses were performed using ATLAS and R statistical software (version 4.1.2; R Development Core Team, Vienna, Austria).
3 Results
3.1 Baseline Characteristics
From January 2011 to December 2020, a total of 30,999 patients met the study criteria, including 19,149 in the TA group and 11,850 in the AA group. Among the entire cohort, 12,122 (39.1%) patients were aged > 65 years and 16,654 (53.7%) were male. In total, 3441 (11.1%) patients had an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, 7164 (23.1%) were taking proton pump inhibitors [22], 932 (3.0%) were taking thiazide, and 100 (0.3%) were on desmopressin. Details of the comorbidities and concurrent medications of the study patients are provided in Table 1.
3.2 Incidence of Hyponatremia (Serum Sodium < 135 mEq/L)
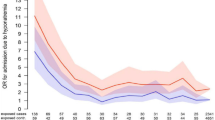
In the TA group, 1613 patients developed hyponatremia (serum sodium < 135 mEq/L) during 199,269 PD (8.09 per 1000 PD), while 493 AA group patients developed hyponatremia during 126,011 PD (3.91 per 1000 PD). The IRR was significantly higher in the TA group [IRR = 2.61; 95% confidence interval (CI) 1.18–2.29, p < 0.001]. In the 1:1 propensity-score-matched model, the incidence rate of hyponatremia was still higher in the TA group (6.81 per 1000 PD) than the AA group (4.33 per 1000 PD) (Table 2), corresponding to an IRR of 1.57 (95% CI, 1.31–1.89, p < 0.001) (Fig. 2). The result remained consistent in patients without concurrent use of hyponatremia-causing medications (eTable 2), and the distribution of the standardized difference of the means for a single covariate before and after adjustment for the propensity score is plotted in eFig. 1.
3.3 Incidence of Moderate Hyponatremia (Serum Sodium < 130 mEq/L)
Of the 2106 patients with hyponatremia (serum sodium < 135 mEq/L), 197 (9.3%) had moderate hyponatremia (serum sodium < 130 mEq/L). The incidence rate of serum sodium < 130 mEq/L in the TA group was 0.68 per 1000 PD; this was significantly higher than in the AA group (0.43 per 1000 PD) (Table 2), corresponding to an IRR of 1.56 (95% CI 1.15–2.13, p = 0.005; Fig. 2). The 1:1 propensity-score-matched model showed the same trend of a higher incidence of moderate hyponatremia in the TA group (IRR = 1.81; 95% CI 1.07–3.09, p = 0.028) than AA group (Fig. 2).
3.4 Incidence of Hyponatremia by TA Formulation
There were 2364 and 3337 TA–ER and TA–IR formulation users, respectively, after 1:1 propensity score matching. The incidence of hyponatremia was 8.62 per 1000 PD in the TA–ER subgroup and 5.682 per 1000 PD in the TA–IR subgroup (Table 3). The respective IRRs were significantly higher than that of the AA comparator (Fig. 2). The incidence of hyponatremia was higher in the TA–ER than TA–IR subgroup (IRR = 1.239; 95% CI 1.080, 1.422) (Fig. 2, eTable 2).
Moderate hyponatremia (serum sodium < 130 mEq/L) developed in 24 (11.4%) of the 211 TA–ER patients and 22 (11.0%) of the 200 TA–IR patients with serum sodium < 135 mEq/L. The IRR of the TA–ER subgroup was significantly higher (2.001; 95% CI 1.001–4.001, p = 0.049) than that of the AA comparator. For TA–IR formulation users, the incidence of moderate hyponatremia was similar to that of the AA comparator (IRR = 1.47; 95% CI 0.76–2.83, p = 0.26) (Fig. 2).
4 Discussion
In our study population, the incidence of hyponatremia was higher in the TA than AA group. Most cases of hyponatremia in the TA group were mild, with moderate hyponatremia (serum sodium < 130 mEq/L) accounting for only 10% of all hyponatremia events. The incidence of hyponatremia differed by TA formulation and was higher in the TA–ER than TA–IR subgroup. Notably, moderate hyponatremia was infrequent in the latter group. This study provides new evidence regarding tramadol-associated hyponatremia in hospitalized patients.
Whether tramadol induces hyponatremia has long been debated. Several case reports suggested an association, and two large population-based cohort studies reported an increased risk of hyponatremia requiring hospitalization in tramadol users [16, 23]. However, another pharmacovigilance study concluded that tramadol alone does not induce hyponatremia, and that other concomitant hyponatremic drugs might explain previous results [17]. The present study was designed to address the discrepancies among these studies. First, TA and AA cohorts were assembled and propensity-score matched in terms of age, sex, condition, and concurrent drugs, including proton pump inhibitors, thiazide, desmopressin, tricyclic antidepressants, and selective serotonin reuptake inhibitors, which are known to induce hyponatremia. Furthermore, we categorized patients based on the usage of concurrent hyponatremia-causing medications and observed an elevated incidence of hyponatremia in the TA group, even in patients not taking any concurrent medications known to cause hyponatremia. Second, hyponatremia events were detected by tracking the serum sodium level for 10 days after drug (TA, AA) initiation. To avoid duplication, only the earliest event was included in the analysis. Hyponatremia that occurred > 10 days after drug initiation was considered to be unrelated, since in previous case reports most of the hyponatremic events occurred within 2–9 days of medication initiation [13, 15, 23]. This criterion resulted in greater accuracy than in previous studies, which detected hyponatremia based on safety reports [17] or ICD-10 codes [16, 23] and therefore might have missed unreported or unrecognized mild hyponatremia.
A possible mechanism of tramadol-associated hyponatremia is the syndrome of inappropriate antidiuretic hormone (SIADH) secretion. Tramadol’s agonistic action on morphogenic receptors can increase antidiuretic hormone (ADH) release, and the enhancement of serotonin release by tramadol can stimulate ADH secretion [14, 23, 24]. Increased ADH levels act on the collecting ducts of the kidney and inappropriately increase free water uptake, leading to dilutional hyponatremia [6].
Mild hyponatremia (serum sodium level of 130–135 mEq/L) is often missed by clinicians, although it is associated with an increased risk of mortality and morbidity [7, 11]. Therefore, awareness of the risk of TA-associated hyponatremia is clinically important.
In a previous case report, severe hyponatremia (serum sodium level of 119 mEq/L) was among the consequences of intentional tramadol overdose [25], but whether a dose-dependent relationship exists between tramadol and the degree of hyponatremia is unclear. In our study, we were unable to calculate the accumulated TA dose due to the characteristics of the data system; further study is therefore required.
The 2022 Centers for Disease Control (CDC) guideline for prescribing opioids for chronic pain recommends that clinicians initially prescribe IR rather than ER formulations for chronic pain, to avoid possible complications [26, 27]. In our comparison of the incidence of hyponatremia between the TA–ER and TA–IR formulations, a higher incidence was observed in the former subgroup. As some of the TA–ER formulations used by patients in this study contained a higher dose of tramadol (75 mg) than the TA–IR formulations, whether the drug dose or release mode accounted for the difference could not be determined, although the higher or more prolonged stimulation of ADH secretion induced by the ER than IR formulation may have played a role. A prospective study in which the dosing interval and cumulative dose of each formulation are controlled is thus required.
We assessed the risk of hyponatremia using crude and propensity-score-matched models; however, residual confounding may still have been present. Among the potential residual confounding factors, pain is a known cause of SIADH [28]. To partially mitigate this confounding, we used patients receiving other pain killers (AA) as controls. This approach represents a major strength of our study.
Conclusively, the results of this large population-based real-world study suggest that, compared with AA, the use of TA is associated with a higher risk of hyponatremia within 10 days of drug initiation. In the TA group, hyponatremia was more frequent among patients taking ER than IR formulations. Given the potential life threatening consequences of hyponatremia in hospitalized patients, tramadol should be prescribed cautiously and electrolyte levels must be closely monitored.
References
Schnitzer T. The new analgesic combination tramadol/acetaminophen. Eur J Anaesthesiol Suppl. 2003;28:13–7.
Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395–400.
Anderson BJ. Paracetamol (acetaminophen): mechanisms of action. Paediatr Anaesth. 2008;18(10):915–21.
Kim K, Lee H, Shin JY. Explosive increase in tramadol use in Korea 2003–2013: analysis of patient trends based on the Korea National Health Insurance Database. J Psychoactive Drugs. 2020;52(2):153–61.
Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014;66(10):1489–95.
Buffington MA, Abreo K. Hyponatremia: a review. J Intensive Care Med. 2016;31(4):223–36.
Boyer S, Gayot C, Bimou C, Mergans T, Kajeu P, Castelli M, et al. Prevalence of mild hyponatremia and its association with falls in older adults admitted to an emergency geriatric medicine unit (the MUPA unit). BMC Geriatr. 2019;19(1):265.
Dokmak A, Madias NE. Hyponatremia and in-hospital falls and fractures in older adults. J Am Geriatr Soc. 2019;67(8):1752–3.
Upala S, Sanguankeo A. Association between hyponatremia, osteoporosis, and fracture: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(4):1880–6.
Teo CB, Gan MY, Tay RYK, Loh WJ, Loh NW. Association of preoperative hyponatremia with surgical outcomes: a systematic review and meta-analysis of 32 observational studies. J Clin Endocrinol Metab. 2022.
Funk GC, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36(2):304–11.
Garakani A. Hyponatremia associated with tramadol in a patient with alcohol use disorder and anxiety taking desvenlafaxine. Prim Care Companion CNS Disord. 2018;20(6):27341.
Udy A, Deacy N, Barnes D, Sigston P. Tramadol-induced hyponatraemia following unicompartmental knee replacement surgery. Anaesthesia. 2005;60(8):814–6.
Yong TY, Khow KSF. Hyponatraemia associated with tramadol use: a case report. Curr Drug Saf. 2018;13(3):217–20.
Le Berre JP, Desrame J, Lecoules S, Coutant G, Bechade D, Algayres JP. Hyponatremia due to tramadol. Rev Med Interne. 2007;28(12):888–9.
Falhammar H, Calissendorff J, Skov J, Nathanson D, Lindh JD, Mannheimer B. Tramadol- and codeine-induced severe hyponatremia: a Swedish population-based case-control study. Eur J Intern Med. 2019;69:20–4.
de Canecaude C, Rousseau V, Chebane L, Lafaurie M, Durrieu G, Montastruc JL. Can tramadol really induce hyponatraemia? A pharmacovigilance study. Br J Clin Pharmacol. 2021;87(2):683–6.
Park YB, Ha CW, Cho SD, Lee MC, Lee JH, Seo SS, et al. A randomized study to compare the efficacy and safety of extended-release and immediate-release tramadol HCl/acetaminophen in patients with acute pain following total knee replacement. Curr Med Res Opin. 2015;31(1):75–84.
Yi S, Chung YJ, Kim TE, Shin HS, Yoon SH, Cho JY, et al. Pharmacokinetics of extended-release versus conventional tramadol/acetaminophen fixed-dose combination tablets: an open-label, 2-treatment, multiple-dose, randomized-sequence crossover study in healthy Korean male volunteers. Clin Ther. 2011;33(6):728–37.
Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76.
Zin CS, Nazar NI, Rahman NS, Alias NE, Ahmad WR, Rani NS, et al. Trends and patterns of analgesic prescribing in Malaysian public hospitals from 2010 to 2016: tramadol predominately used. J Pain Res. 2018;11:1959–66.
Buon M, Gaillard C, Martin J, Fedrizzi S, Mosquet B, Coquerel A, et al. Risk of proton pump inhibitor-induced mild hyponatremia in older adults. J Am Geriatr Soc. 2013;61(11):2052–4.
Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-25 e5.
Sarret D, Le Berre JP, Zemraoui N. Tramadol-induced hyponatremia. Am J Kidney Dis. 2008;52(5):1026 (author reply 7).
Lota AS, Dubrey SW, Wills P. Profound hyponatraemia following a tramadol overdose. QJM. 2012;105(4):397–8.
Frieden TR, Houry D. Reducing the risks of relief—the CDC opioid-prescribing guideline. N Engl J Med. 2016;374(16):1501–4.
Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95.
Mentrasti G, Scortichini L, Torniai M, Giampieri R, Morgese F, Rinaldi S, et al. Syndrome of inappropriate antidiuretic hormone secretion (SIADH): optimal management. Ther Clin Risk Manag. 2020;16:663–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Medical Institute of Technology Policy research project (CMIT PRP 2022-04), Pusan National University Hospital.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval and informed consent:
The Pusan National University IRB Committee (PNUH IRB 2210-007-119) approved the study protocol, and the requirement for informed consent was waived by the review board.
Consent for publication
Not applicable.
Code availability
Not applicable.
Data availability
Materials described in the manuscript, including relevant raw data, will be made freely available to researchers for non-commercial purposes while ensuring the confidentiality of the participants. The analytic R code can be obtained from the author at jinmi@pusan.ac.kr.
Author contributions
RH conceptualized and designed the study, secured funding, and wrote the manuscript. YJL contributed to the study design. JK and YH conducted the statistical analysis and interpreted the data. KH and BC assisted with the construction of the CDM and provided technical support. TRO and IYK reviewed the manuscript and approved the final version for submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, Y.J., Kim, J., Han, Y. et al. Risk of Hyponatremia after Tramadol/Acetaminophen Single-Pill Combination Therapy: A Real-World Study Based on the OMOP–CDM Database. Drugs R D 23, 289–296 (2023). https://doi.org/10.1007/s40268-023-00436-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00436-4