Abstract
Background
Dopamine antagonists are the main pharmacological options to treat gastroparesis. The aim of this study was to conduct a systematic literature review (SLR) to evaluate the profile of adverse events (AEs) of dopamine antagonists used in the treatment of children and adults with gastroparesis.
Methods
We searched EMBASE and MEDLINE up to March 25, 2021, for relevant clinical trials and observational studies. We conducted a proportional meta-analysis to estimate the pooled occurrence of AEs (%), with 95% confidence interval (CI), from arm-level data across studies and the comparative occurrence of AEs from placebo-controlled clinical trials (odds ratio [OR] with 95% CI).
Results
We identified 28 studies assessing AEs experienced by patients treated for gastroparesis with domperidone and metoclopramide; 22 studies contributed data to the meta-analyses. Cardiovascular, neurological, and endocrine AEs were commonly observed, with point incidences varying from 1 to > 50%. Clinically important AEs, such as QTc prolongation, occurred in 5% of patients treated with domperidone (95% CI: 3.32–8.62). Restlessness, an extrapyramidal AE, occurred in 15% of patients (95% CI: 7.48–26.61) treated with metoclopramide, with a 7-fold increase compared with patients receiving placebo (OR: 7.72; 95% CI: 1.27–47.05). Variation in terminology to describe extrapyramidal events precluded further pooled analyses. Additional meta-analyses were not feasible due to discrepancies in the assessment and reporting of the AEs.
Conclusions
The evidence confirms concerns of cardiovascular, extrapyramidal, and endocrine AEs in patients with gastroparesis treated with domperidone and metoclopramide. Imprecise AE reporting limits firm interpretation and conclusions.
Registration
PROSPERO international prospective register of systematic reviews (registration number: CRD42021248888).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The limited pharmacological treatment options for gastroparesis—domperidone and metoclopramide—are associated with known safety concerns. |
This review and meta-analysis of clinical trial and real-world evidence demonstrated that serious cardiovascular, endocrine, and extrapyramidal adverse events (AEs) were commonly experienced by patients treated with these drugs. |
Cardiovascular and endocrine AEs, such as QTc prolongation and hyperprolactinemia, were more frequently reported following treatment with domperidone than for metoclopramide, whereas extrapyramidal events, such akathisia and tardive dyskinesia, were reported more frequently following treatment with metoclopramide. |
The profile of AEs induced by domperidone and metoclopramide ranged across 13 organ system categories; however, complete data on the AEs actively measured and observed in patients were lacking. To improve patient care, future research should improve the reporting of the AEs experienced by patients receiving these treatments. |
1 Introduction
Gastroparesis is defined as symptomatic delayed gastric emptying rate in the absence of mechanical obstruction [1, 2]. Symptoms can include early satiety, postprandial fullness, nausea, vomiting, bloating, and upper abdominal pain, with variations in the severity, frequency, and duration of symptoms [1,2,3]. In the United Kingdom (UK) and United States (US), population-based studies have reported gastroparesis age- and sex-adjusted prevalence ranging from 13.8 to 24.2 per 100,000 persons [4, 5].
The underlying pathophysiology of gastroparesis is not completely understood [6]. The etiology of the condition is associated with several exposures capable of altering gastric motility [6]. Diabetes is a recognized cause of gastroparesis, which accounts for approximately 40% of gastroparesis cases; about one-third of gastroparesis cases are idiopathic [2, 5, 7]. Additionally, surgical procedures, such as fundoplication and bariatric surgery, and pharmacological agents, such as opiate analgesics and anticholinergic agents, can be associated with iatrogenic causes of gastroparesis [2].
For patients who develop gastroparesis, the burden of disease ranges from psychosocial comorbidities, such as anxiety disorders and depression, and the associated decrease in quality of life [8, 9], to increase in hospital and emergency department admissions [7, 10, 11]. Gastroparesis also accounts for an individual and populational economic burden. Patients often need long-term, continued care comprising non-pharmacological and pharmacological interventions [2]. In addition, costs related to hospitalizations are relevant and, in the US, healthcare costs related to gastroparesis have increased significantly since 2017 [10, 12].
Treatment options for patients with gastroparesis are relatively sparse, partially due to limited understanding of the disease pathophysiology. Prokinetic agents, such as domperidone and metoclopramide, are the most used pharmacological approach which, combined with dietary modifications, aim at accelerating gastric motility and relieving symptoms related to delayed gastric emptying, such as postprandial fullness and upper abdominal pain [1, 2, 5]. These drugs are non-selective dopamine (D2/D3) receptor antagonists with pharmacological activity on gastric and intestinal motility [13, 14] and have historically been used in the treatment of gastroparesis [15].
Although available over the counter in many countries, metoclopramide is a drug approved for treatment of gastroparesis in the US but not Europe [13, 16], and domperidone can be requested through an expanded access investigational process for gastrointestinal disorders such as gastroparesis in the US [17], while not approved for treatment of gastroparesis in Europe [18]. The restriction in the use of domperidone in the US stems from domperidone-associated risks of cardiac arrhythmias, cardiac arrest, and sudden death, deemed relevant by the Food and Drug Administration (FDA) [17] but inconclusive by the European Medicines Agency (EMA) [19]. Despite regulatory approval, the use of metoclopramide has also recently been restricted to short-term (maximum 5 days) treatment in Europe over concerns of risks of neurological events [16]. Overall, there is a continued need to assess and better understand the risks associated with the use of these dopamine antagonist drugs in the treatment of patients with gastroparesis. Moreover, systematic analyses that include adverse event (AE) data from clinical trials and observational studies assessing patients with gastroparesis treated with dopamine antagonists are lacking. Therefore, the aim of this study was to conduct a systematic literature review (SLR) to evaluate the profile of AEs of available dopamine antagonists for symptomatic treatment of gastroparesis in children and adults.
2 Methods
This SLR was conducted in accordance with the guidelines established in the Cochrane Handbook for Systematic Reviews of Interventions [20], and the study report follows the methodology of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [21]. The study protocol was registered in PROSPERO (registration number: CRD42021248888).
2.1 Eligibility Criteria and Study Selection
We included studies based on the populations, interventions and comparators, outcomes, and study design (PICOS) framework. The included studies comprised clinical trials (randomized, non-randomized, and single-arm) and observational studies (prospective, retrospective, and cross-sectional) published in English that assessed children and adults with gastroparesis who were treated with any dose of dopamine receptor antagonists, including domperidone, bromopride, metoclopramide, NG101 (metopimazine), and CIN-102. Studies reporting on the occurrence of any AE or discontinuations due to AEs after initiating a drug of interest were included.
To ensure homogeneity among the patient population, we excluded studies involving (i) patients with symptomatic nausea and vomiting not related to gastroparesis; (ii) patients with other gastrointestinal disorders and conditions; (iii) patients with functional disorders, such as functional dyspepsia and gastroparesis-like syndrome; and (iv) patients with eating disorders. Studies assessing a population with mixed etiology were included if at least 80% of the patient population presented with gastroparesis.
2.2 Information Sources and Search Strategy
Electronic searches were conducted in Embase®, MEDLINE®, and MEDLINE® In-Process using the OvidSP® platform on March 25, 2021. The searches utilized a combination of controlled vocabulary and keywords related to gastroparesis and dopamine receptor antagonists, specific to each database. Appropriate search filters for study designs and publication types not of interest (e.g., case reports or opinion pieces) were employed, and the searches were limited to the English language. No date limit was applied. The search strategies are provided in Supplementary Table 1 (see electronic supplementary material [ESM]). As a search validation step, the reference lists of SLRs published since 2018 were reviewed for additional eligible publications.
2.3 Screening and Eligibility Assessment
Search results were imported into Endnote Version X9®, and duplicates were removed. Screening was conducted in DistillerSR® version 2.35.10 in two stages. First, the title and abstract of each retrieved publication were screened against the PICOS criteria by two independent reviewers. Second, the full texts of any records deemed potentially relevant were obtained and screened independently by two reviewers. Any disagreements between reviewers about screening decisions were resolved by a third, senior researcher.
2.4 Data Extraction and Data Items
Study, patient, and treatment characteristics, as well as outcomes of interest, were extracted from the included publications into a pre-specified Microsoft Excel® data extraction table by one reviewer, and independently validated by a second, senior reviewer.
Clinical trial data were summarized for patients treated with the dopamine antagonists of interest (domperidone, bromopride, metoclopramide, NG101, and CIN-102) and comparator treatments. Data from observational studies were extracted and summarized specifically for patients treated with domperidone, bromopride, metoclopramide, NG101, or CIN-102, and no comparative data were sought.
We collected outcome data reported as the number of patients (n) who developed AEs when receiving any of the drugs of interest. Additionally, the proportion of patients experiencing AEs in relation to the number of patients receiving treatment were extracted from the primary studies or calculated. The reported AEs were then classified according to organ systems.
2.5 Meta-analysis
A feasibility assessment established whether the characteristics of the studies identified in the SLR (i.e., study design, patient and treatment characteristics, and timepoints) were sufficiently similar to be quantitatively synthesized in a meta-analysis. Briefly, meta-analyses were considered feasible if two or more studies in a given analytic scenario reported on frequency (incidence and prevalence) data on specific AEs. The analytic scenarios considered were arm-level analyses ([i] domperidone or [ii] metoclopramide arm-level data) and treatment-controlled analyses ([iii] domperidone vs placebo, [iv] domperidone vs metoclopramide, [v] metoclopramide mixed formulations vs placebo/active comparator; [vi] metoclopramide nasal formulation vs placebo, and [vii] metoclopramide oral formulation vs nasal formulation). In addition, threats to the validity of available analyses were assessed based on between-study clinical and methodological heterogeneity, and studies contributing to substantial heterogeneity were excluded from the analyses.
When deemed feasible, we conducted a meta-analysis to estimate the pooled risk of AEs according to drug type used in the treatment of gastroparesis. Two types of meta-analyses were undertaken. Firstly, arm-level meta-analyses pooled the proportions of patients (presented as percentage (%) with 95% confidence interval [CI]) with given AEs, discontinuations due to AEs, or total AEs; this type of meta-analysis was conducted for metoclopramide (as mixed formulations analysis of oral and nasal routes of administration) and domperidone. Secondly, placebo-controlled meta-analyses estimated the odds ratios (OR) with 95% CI for metoclopramide (as oral, nasal, and mixed formulations) versus placebo, for the same outcomes (i.e., patients with the given AEs, discontinuations due to AEs, or total AEs). Data for placebo-controlled comparisons for domperidone were not available. The safety populations (i.e., all patients who received the study drugs and were included in the analysis) were used for the analyses of AEs.
Analyses were conducted using a fixed-effect (FE) model to improve power to detect safety signals when only sparse data were available from less than three studies and statistical heterogeneity was low, as recommended by the FDA [22], whereas the random-effects (RE) model was applied to analysis when outcome data were available from more than three studies, and the number of events reported in the studies were different than 0.
When zero events occurred in a treatment arm of a study, a continuity correction of 0.5 was applied for a meta-analysis of proportions, whereas the correction sizes proportional to the arm sizes (e.g., 0.33 and 0.67 if the arm sizes are 100 and 150, respectively) were applied for a meta-analysis of OR, before performing the analyses. Studies with zero events in both arms were excluded from the placebo-controlled OR analysis. Finally, the Peto method was also employed (without continuity correction) as a sensitivity in a meta-analysis of OR if an events rate < 2% was observed and one or more studies had zero events in a treatment arm.
Heterogeneity between studies was assessed using the I2 and Cochran’s Q statistic. Whenever a result demonstrated significant heterogeneity (I2 > 75%; p < 0.05 in Cochran’s Q), reasons for heterogeneity were explored (in scenarios with > 2 studies). The sensitivity analyses investigated characteristics of the studies suspected of contributing to the statistical heterogeneity by removing outlier studies differing with respect to study design, route of administration (metoclopramide only), treatment or follow-up duration.
In this article, we present results of the best-fit analysis.
3 Results
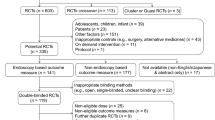
The electronic searches returned 767 records; of those, 625 unique records were screened after removing duplicates (Fig. 1). Following screening, 28 studies were included in the SLR and 22 were deemed feasible to be included in the meta-analysis.
3.1 Study and Patient Characteristics
Among the 28 included studies (Table 1), 14 were clinical trials, including eight with a parallel design [23,24,25,26,27,28,29,30,31], five with a crossover design [32,33,34,35,36], and one reported on a non-randomized single-arm trial [37]. The 14 observational studies comprised six prospective studies [38,39,40,41,42,43], four retrospective studies [44,45,46,47], two cross-sectional studies [48, 49], and two case series [50, 51]. Of the dopamine antagonists of interest, the included studies only assessed domperidone and metoclopramide (15 studies assessed domperidone and 14 metoclopramide among the 28 studies); no data on bromopride, NG101, and CIN-102 were identified. Eight of the clinical trials were placebo-controlled [23, 24, 26, 28,29,30,31,32, 34], one compared domperidone with metoclopramide [27], one compared different routes of metoclopramide administration (oral versus oral) [25], and two compared metoclopramide with carbachol [35] or erythromycin [33]. One trial was a single-arm trial [37], and one reported on a comparison group not of interest (acupuncture) [36].
Most of the studies (23/28; 82%) were conducted in the US. The population recruited in the studies comprised mainly outpatients (7/28; 25%), though most studies did not report the setting where the patients were recruited (20/28; 71%). The patient population included adults only in most of the included studies (27/28; 96%), with a similar mean age pattern among clinical trials and observational studies (mean age of participants ranged from 30 to 57 years among clinical trials [24,25,26, 30, 32,33,34, 36, 37]; and from 39 to 49 years [39, 40, 44, 46,47,48,49, 51] among observational studies). The percentage of female participants ranged from 10 to 94% among the clinical trials [24,25,26,27, 30,31,32,33,34,35], and from 50 to 90% among the observational studies [39,40,41,42, 44,45,46,47,48,49,50, 52]; one clinical trial included only female participants [37].
The patients assessed in the included studies presented with diverse gastroparesis etiology. Among the included studies reporting on patients exclusively with diabetic gastroparesis (13/28; 50%), nine comprised clinical trials [24,25,26,27, 30, 32,33,34, 36] and four were observational studies [42, 43, 45, 50]. One clinical trial included patients exclusively with idiopathic gastroparesis [23] and another two trials evaluated patients exclusively with post-surgical gastroparesis [31, 35].
Among the studies reporting on a mixed population of diabetic, idiopathic, neuropathic, and post-surgical gastroparesis (10/28; 36%), two comprised clinical trials [29, 37] and eight were observational studies [38,39,40,41, 46,47,48,49]. The percentage of patients with diabetic and idiopathic gastroparesis varied across studies from 9 to 40% and 20 to 85%, respectively. One observational study did not report data on the etiology of gastroparesis [44].
Ethnicity, weight, and body mass index (BMI) of participants were sparsely reported. Figures on alcohol and tobacco consumption, and existent comorbidities were also scarce. Supplementary Tables 2 and 3 summarize additional patient characteristics data available from the included studies (see ESM).
3.2 Treatment Characteristics
Domperidone was administered orally in all the included studies, while metoclopramide was administered in diverse routes of administration. Among the clinical trials, metoclopramide was used as oral [24, 25, 27, 29, 31, 33,34,35] and nasal formulations [25, 26]. Among the observational studies, metoclopramide was administered orally in one study [43], subcutaneously in another [39], and intravenously followed by oral administration in two studies [38, 47]. Treatment dosing was generally consistent with the recommended dose for domperidone and metoclopramide (5–10 mg orally three to four times daily), with treatment durations ranging from 3 to 6 weeks. Substantial between-study heterogeneity was observed in the treatment duration, and one outlier study, which was later excluded from the meta-analysis, reported subcutaneous treatment with metoclopramide for 3 days. Other identified outliers consisted of mean treatment with domperidone ranging from 6 to 4 years [45, 46, 48, 50], and treatment with metoclopramide ranging from 3 to 2 years in one study [31]. Details on treatment characteristics are provided in Supplementary Table 4 (see ESM).
3.3 Adverse Events of Domperidone and Metoclopramide
The AEs reported across the included studies were classified into 13 organ system categories of AEs (Table 2). The reporting of AEs was heterogeneric and different studies reported on different organ system categories of events. When considering AEs of special interest (Tables 3, 4), seven studies reported on cardiovascular events, 24 on neurological events, and 12 studies reported on endocrine events. Details on cardiovascular, neurological, and endocrine AEs are provided in Supplementary Table 5 (see ESM). Of note, six studies were identified that reported zero total AEs (i.e., no events observed; in Supplementary Table 6, see ESM) [31, 36, 42, 43, 50, 51].
3.3.1 Cardiovascular Events
Two clinical trials (one reporting on domperidone, one on metoclopramide) [33, 37] and five observational studies (four reporting on domperidone, one on metoclopramide) [40, 44, 46,47,48] reported data on cardiovascular events.
Among the clinical trials, palpitations were the most frequently observed AE with incidences of 15% and 8% in patients treated with domperidone and metoclopramide, respectively. Despite the occurrence of chest pain in 3% of the patients taking domperidone in one clinical trial [37], no AEs of prolongation of the heart rate-corrected QT (QTc) interval was reported. The occurrence of cardiovascular events was not reported in the carbachol, erythromycin, or placebo control arms.
In contrast, the occurrence of prolongation of the QTc interval was commonly reported across the observational studies in percentages ranging from 1 to 27% in patients treated with domperidone [40, 44, 46]. Between-study heterogeneity in the percentages of patients identified with prolongation of the QTc interval appeared to vary according to the definition of QTc prolongation—or the absence of an outcome definition. For instance, in one study [44], the percentage of patients with QTc prolongation rose from 2% when events were defined as QTc interval prolongation > 60 ms over baseline to 25% if QTc interval prolongation was defined as ≥20 ms but < 60 ms from baseline. When defined as prolongation of the QTc interval of > 450 ms for males and > 470 ms for females, this AE occurred in 5% of patients in one study [44], while in studies not reporting the definition of a QTc prolongation outcome, the percentage of patients with QTc prolongation varied from 1 [40] to 27% [46]. Among patients treated with metoclopramide, one study reported that 30% of patients developed prolongation of the QTc interval [48]. These four observational studies reporting prolonged QTc intervals were all conducted in mixed etiology populations.
A meta-analysis was conducted evaluating the pooled proportion of chest pain, palpitations, and QTc prolongation among patients treated with domperidone. Meta-analyses evaluating the occurrence of cardiovascular AEs of metoclopramide and comparing the occurrence of cardiovascular AEs of metoclopramide with placebo were not feasible.
3.3.1.1 Meta-analysis of Cardiovascular Events
Chest pain Two studies involving 149 patients provided data to a meta-analysis evaluating chest pain in patients treated with domperidone. The pooled proportion of chest pain was 2% (95% CI 0.67–6.22; FE) following treatment with domperidone (Fig. 2). There was no evidence of statistical heterogeneity (p = 0.665; I2 = 0%).
Meta-analysis of cardiovascular adverse events among patients treated with domperidone. FE model. Results of sensitivity analysis for QTc prolongation removing an outlier study with longer treatment duration [46]. CI confidence interval, FE fixed effects, QTc heart rate-corrected QT
Palpitations Three studies involving 197 patients provided data to a meta-analysis evaluating palpitations in patients treated with domperidone. The pooled proportion of palpitations was 7% (95% CI: 3.57, 11.96; FE) following treatment with domperidone (Fig. 2). There was evidence of moderate statistical heterogeneity (p = 0.037; I2 = 70%).
QTc prolongation Three studies involving 398 patients provided data to a meta-analysis evaluating QTc prolongation in patients treated with domperidone. Results from the base-case analysis demonstrated high statistical heterogeneity (p < 0.001; I2 = 90.5%), and a sensitivity analysis removed an outlier study with longer treatment duration (mean treatment duration of 8 months [range 3 months to 4 years]) [46]. The sensitivity analysis results involved 361 patients and the pooled proportion of QTc prolongation was 5% (95% CI: 3.32–8.62; two studies, FE) following treatment with domperidone (Fig. 2). There was evidence of moderate statistical heterogeneity (p = 0.054; I2 = 73%) in this analysis.
3.3.2 Neurologic and Extrapyramidal Events
Eleven clinical trials (three reporting on domperidone, eight on metoclopramide) and seven observational studies [38,39,40, 45, 47,48,49] (three reporting on domperidone, four on metoclopramide) reported data on neurologic events, including non-extrapyramidal and extrapyramidal AEs.
Among neurological non-extrapyramidal events, asthenia/weakness, mental acuity, and somnolence were the frequently reported AEs among patients treated with domperidone and metoclopramide in clinical trials, with high observed percentages equal to 24% (asthenia with domperidone treatment), 33% (mental acuity with metoclopramide treatment), and 14% (somnolence with metoclopramide). Other AEs were also evident among the clinical trial data, such as taste perversion occurring in proportions ranging from 3 to 6% with oral and nasal formulations of metoclopramide. Among the observational studies, the most common observed AEs were headache, reported in 8% of patients receiving domperidone, and muscle cramps, described in 4% of patients treated with domperidone. One study [49] reported seizures in 2% of 100 patients assessed after treatment of gastroparesis with metoclopramide.
Among the neurological extrapyramidal AEs reported in the clinical trials, akathisia was described in percentages ranging from 22% among patients treated with domperidone to 36% in patients treated with metoclopramide. Restlessness and tremors were reported among patients treated with metoclopramide, with frequencies ranging from 21% in clinical trials and 11% in observational studies (restlessness) and from 8% in clinical trials to 7% in observational studies (tremors). Tardive dyskinesia was reported in one observational study affecting 2% of patients (2/100) treated with metoclopramide [49].
Treatment duration appeared generally consistent among studies reporting neurological AEs in patients treated with either domperidone or metoclopramide, thus not allowing for such examinations of variability in the reported percentages of these AEs. Similarly, there was no clear pattern regarding the etiology of gastroparesis among clinical trials and observational studies reporting on neurological AEs.
Noticeably, the terminology used in the included studies to describe the extrapyramidal AEs varied across studies. For instance, akathisia, restlessness, uncontrolled movements, restlessness with uncomfortable feeling in arms and legs, and feeling of restless were diverse terms identified in the included studies to describe extrapyramidal AEs. Hand tremor, tremor, and parkinsonism symptoms were also terms inconsistently applied in the studies.
Meta-analyses were conducted evaluating the pooled proportion of neurological AEs among patients treated with domperidone and metoclopramide. Analyses on three neurological non-extrapyramidal AEs reported in patients receiving domperidone were feasible (dizziness, drowsiness, headache), showing that the pooled frequency of these events varied from 2 to 7% (Supplementary Figure 1, see ESM). For the neurological extrapyramidal AEs, meta-analyses were feasible on restlessness among patients treated with metoclopramide, and on the risk of restlessness among patients treated with metoclopramide compared with placebo. A meta-analysis evaluating the occurrence of extrapyramidal AEs of domperidone was not feasible.
3.3.2.1 Meta-analysis of Neurologic and Extrapyramidal Events
Restlessness Three studies involving 55 patients contributed data to a meta-analysis on the occurrence of restlessness among patients treated with mixed formulations of metoclopramide. The results indicated that the pooled proportion of restlessness was 15% (95% CI: 7.48–26.61; FE) in patients treated with metoclopramide. There was no evidence of statistical heterogeneity (p = 0.05; I2 = 0%) (Fig. 3).
Two studies involving 108 patients contributed data to a meta-analysis comparing the occurrence of restlessness among patients treated with mixed formulations of metoclopramide and compared with placebo. Rates of restlessness were higher among patients treated with metoclopramide compared with placebo (OR: 7.72; 95% CI: 1.27–47.05; FE) (Fig. 4). There was no evidence of statistical heterogeneity (p = 0.902; I2 = 0%).
3.3.3 Endocrine Events
Nine clinical trials [23,24,25,26,27, 30, 32, 37] and four observational studies [40, 41, 45, 48] reported data on endocrine AEs. Among these studies, nine reported on patients treated with domperidone and four reported on metoclopramide; no observational studies of patients treated with metoclopramide reported endocrine AEs.
Among the clinical trials, most endocrine AEs reported were prolactin-related AEs such as breast enlargement and tenderness, galactorrhea, and decrease in libido in patients treated with domperidone. The incidence of breast tenderness and galactorrhea was observed in up to 44% of patients receiving domperidone [23] while in participants treated with placebo, the incidence of breast symptoms/tenderness was reported in up to 23% [32]. Two trials reported on prolactin-related symptoms associated with domperidone without detailing the specific symptoms [27, 30]. Rates of endocrine AEs in these trials did not appear to vary according to the etiology of gastroparesis.
Clinical trials of metoclopramide in diabetic gastroparesis reported mostly on the occurrence of hypoglycemia and hyperglycemia AEs. Hypoglycemia was the most often reported AE among patients taking metoclopramide (incidences ranging from 3% to 6%). One clinical trial in diabetic gastroparesis reported the occurrence of hypoglycemia and hyperglycemia in patients taking nasal metoclopramide compared with placebo [26]. In this trial (n = 95), hypoglycemia occurred in 1% and 3% of patients using a 10-mg and 14-mg nasal spray of metoclopramide, respectively; and a proportion of 1% of patients developed hypoglycemia while treated with placebo. Similarly, among patients using metoclopramide 10 mg and 14 mg nasal spray, hyperglycemia was observed in 1% and 3% of patients, respectively, and in 1% of patients receiving placebo. Notably, neither of the two clinical trials of domperidone in diabetic gastroparesis reported these endocrine AEs.
Among the observational studies, endocrine AEs were reported only in studies assessing patients treated with domperidone. In these studies, the occurrence of breast-related AEs such as tenderness, galactorrhea, and gynecomastia ranged from 2 to 18%. One study [41] involving 17 patients described hyperprolactinemia in 100% of the patients treated with domperidone, and a symptom related to hyperprolactinemia, gynecomastia, in 18% of these patients with hyperprolactinemia associated with domperidone.
Meta-analyses were conducted evaluating the pooled proportion of endocrine AEs (breast tenderness, hyperglycemia, and prolactin-related symptoms) among patients treated with domperidone and metoclopramide each. A meta-analysis of the risk of endocrine AEs among patients treated with metoclopramide compared with placebo was not feasible.
3.3.3.1 Meta-analysis of Endocrine Events
Breast tenderness Two studies involving 149 patients provided data to a meta-analysis evaluating breast tenderness in patients treated with domperidone. The pooled proportion of breast tenderness was 3% (95% CI: 1.19–8.18; FE) following treatment with domperidone (Fig. 5). There was evidence of moderate statistical heterogeneity (p = 0.216; I2 = 35%).
Hyperprolactinemia Two studies involving 26 patients provided data to a meta-analysis evaluating the occurrence of hyperprolactinemia in patients treated with domperidone. The pooled proportion of hyperprolactinemia was 96% (95% CI: 77.80–99.48; FE) following treatment with domperidone (Fig. 5). There was no evidence of statistical heterogeneity (p = 0.765; I2 = 0%).
Prolactin-related symptoms Two studies involving 334 patients provided data to a meta-analysis evaluating the occurrence of prolactin-related symptoms in patients treated with domperidone. The pooled proportion of prolactin-related symptoms was 3% (95% CI: 1.74–5.90; FE) among patients treated with domperidone (Fig. 5). There was evidence of moderate statistical heterogeneity (p = 0.168; I2 = 48%).
Hypoglycemia Two studies involving 279 patients contributed data to a meta-analysis on the total number of patients treated with mixed formulations of metoclopramide and experiencing hypoglycemia. The pooled frequency of hypoglycemia was 3% (95% CI: 1.23–5.30; FE) in patients treated with metoclopramide. There was no evidence of statistical heterogeneity (p = 0.532; I2 = 0%) (Fig. 6).
3.3.4 Other Adverse Events
In this article, we focused on AEs of special interest, cardiovascular events, neurological and extrapyramidal events, and endocrine events. Other AEs reported included mainly gastrointestinal, infections and infestations, non-specific, psychiatric, and respiratory events.
Results of the meta-analyses on other AEs that were feasible (Supplementary Figs. 2–10, see ESM) demonstrated that 3% to 8% of patients treated with mixed formulations of metoclopramide were described with gastrointestinal, non-specific, and respiratory AEs. Psychiatric events of anxiety and depression were experienced by up to 21% of patients, and 35% of patients experienced somnolence. Compared with placebo, patients treated with mixed formulations of metoclopramide had reduced risk of experiencing diarrhea (OR 0.34; 95% CI 0.12–0.98; two studies; FE), and comparable risk of experiencing headache (OR 2.23; 95% CI 0.77–6.45; two studies; FE). Additional details on other AEs are summarized in Supplementary Table 7 (see ESM).
3.3.5 Discontinuations Due to Adverse Events
Data on the total number of discontinuations due to AEs were not always available from the study reports, and only one clinical trial reported data on discontinuations due to AEs in comparator arms. Among the clinical trials, six patients (of 48 patients analyzed in one trial; 13%) who received domperidone, 21 patients (of 317 patients analyzed across four trials; 3–15%) treated with metoclopramide, and four patients (of 95 patients analyzed in one trial; 4%) who received placebo discontinued treatment due to any AE. Across the observational studies, 47 patients (of 523 across two studies; 8–12%) who received treatment with domperidone discontinued treatment due to any AE, and no discontinuations due to AEs were reported among patients who received treatment with metoclopramide. Details on treatment discontinuations due to AEs are summarized in Supplementary Table 8. Results of the meta-analysis are presented in Supplementary Figures 11 and 12 (see ESM).
3.3.6 Total Adverse Events
The total number of patients experiencing AEs was reported among 18 studies, nine clinical trials and nine observational studies. Across the clinical trials, the total numbers of patients with AEs were reported for 323 participants who received domperidone [23, 30, 36], 218 participants who received metoclopramide [24, 26, 31], 145 participants assigned to a placebo comparison group [23, 24, 26, 31], and 13 participants who received erythromycin. Across the observational studies, total numbers of patients with AEs were reported for 250 participants who received domperidone [40, 42, 45, 48, 50, 51] and 169 participants who received metoclopramide [43, 47, 49]. The percentage of patients experiencing AEs varied widely across studies, ranging from 0 to 70%. Details on total treatment AEs are summarized in Supplementary Table 9. Results of the meta-analysis are presented in Supplementary Figures 13–15 (see ESM).
4 Discussion
This is the first review to evaluate the evidence of the AEs experienced by patients with gastroparesis undergoing treatment with the dopamine receptor antagonists domperidone and metoclopramide. This study demonstrates that cardiovascular AEs such a QTc prolongation were commonly reported among patients treated with domperidone, and neurological extrapyramidal events occurred markedly among patients treated with metoclopramide. The risk of restlessness with metoclopramide was also notable as, compared with placebo, patients treated with metoclopramide were demonstrated to have 7-fold increased risk of this AE. Despite the discrepancy in the accuracy of the assessment and reporting of the AEs among studies, the evidence confirms concerns of cardiovascular events and neurological extrapyramidal events in patients treated with domperidone or metoclopramide [16, 17, 19, 53,54,55].
After conducting comprehensive searches, evidence from 28 studies on domperidone and metoclopramide was found in the literature. The patterns depicted in this review on the AEs experienced by patients with gastroparesis, treated with domperidone or metoclopramide, demonstrated that the safety profile of these drugs ranges across 13 organ system categories. Noticeably, the studies contributing data to this SLR were diverse in their study design, etiology of gastroparesis and, overall, published over the past 50 years; these studies characterize the investigation of these dopamine receptor antagonists since these drugs were licensed in the 1970s [56].
The included studies also varied regarding the completeness of the reporting of AEs, regardless of the publication period. For instance, it was often unclear whether studies applied measures to determine the occurrence of AE or whether AEs were (i) not observed; (ii) observed but not reported; or (iii) reported only if they were deemed relevant. Furthermore, heterogeneity in the reporting of AEs limited the feasibility of a meta-analysis of several AEs described in observational studies and clinical trials. For example, endocrine AEs reported as “breast discharge,” “galactorrhea, breast tenderness or fullness,” or “expressive galactorrhea and bilateral breast tenderness” did not contribute to any pooled analysis despite probably encompassing similar AEs. This limitation was expected since the reporting of AEs in clinical trials has been shown to be suboptimal [57, 58], and a similar pattern was anticipated to impact the reporting of observational studies despite the lack of objective evidence.
Despite the limitations with the reporting of AEs, events that are considered serious, such as prolonged QTc interval, were reported during the observation/treatment period of the studies. Results of the meta-analysis on cardiovascular AEs among patients treated with domperidone showed that QTc prolongation was reported in up to 5% of patients described with this AE. It is possible that variability in the occurrence of prolongation of the QTc interval was related to the mean duration of treatment, as lower rates were seen in one of the shorter studies and higher rates were seen in one of the longer studies (1% over a mean of 2.4 months vs 27% over a mean of 8 months) [40, 46]. However, this observation is complicated by the fact that the longer study was specifically designed to measure the effect of domperidone on the occurrence of QTc prolongation [46], and it is possible that the intensity of outcome assessment and clinician awareness could also explain some of the variability observed in the frequency of events of QTc prolongation. In contrast, most QTc interval data in observation studies were collected retrospectively; therefore, it is plausible that events of QTc prolongation might not have been proactively assessed in most studies, leading to underreporting of this AE. Interestingly, symptoms of QTc prolongation such as chest pain were frequently reported among patients treated with domperidone; however, it is unclear if the studies reporting chest pain also accurately and actively monitored for QTc prolongation. Due to limitations with data availability, a meta-analysis was not feasible to summarize the incidence of cardiovascular AEs among patients treated with metoclopramide. Nevertheless, patients treated with metoclopramide were also described as developing QTc prolongation.
In line with previous knowledge, neurologic AEs were frequently reported among patients treated with metoclopramide. These AEs were also observed among patients receiving domperidone to treat gastroparesis. It is important to highlight that the incidence of some neurologic AEs, such as akathisia, could have been underestimated by the wide discrepancy in the terminology used in the primary studies. For instance, it is unclear whether terms such as restlessness or uncontrolled movements could be translated into akathisia considering the limited descriptions available in the included studies. This variability in the definitions of the events might be an essential factor challenging the analyses of the impact of neurologic AEs in the population of patients with gastroparesis treated with domperidone or metoclopramide, since other factors such as the etiology of gastroparesis and treatment duration were similar among studies. To avoid biasing the results, the AEs were extracted and summarized as documented in the studies’ report, with no interpretations conducted by reviewers.
Looking specifically at neurologic extrapyramidal AEs, a type of AE that comprises drug-induced movement disorders, including tardive dyskinesia but also other symptoms such as dystonia (symptoms of involuntary muscle contractions), akathisia (symptoms of agitation or restlessness), and parkinsonism (symptoms resembling Parkinson disease, such as tremors and muscle rigidity), these events were frequently described among patients treated with metoclopramide (approximately 14% of these patients were described with restlessness as an AE). Tardive dyskinesia, a black box warning of the use of metoclopramide, occurred in 2% of the patients assessed in one study [49]. Overall, the results consolidate previous knowledge while also adding to the documentation of the potential magnitude of occurrence of these AEs.
In addition to cardiovascular and neurological events, endocrine events comprise another category of AEs of interest among patients with gastroparesis receiving treatment with domperidone or metoclopramide. Prolactin-related AEs and hyperprolactinemia leading to amenorrhea, galactorrhea, and breast tenderness are known endocrine AEs associated with dopamine antagonist receptors [55, 59, 60]. In this review, the heterogeneity in AE reporting challenged the synthesis and analysis of prolactin-related AEs and hyperprolactinemia since some studies reported specific symptoms (e.g., galactorrhea, breast tenderness, amenorrhea, etc.), and others reported the combined occurrence of prolactin-related AEs/hyperprolactinemia. In the meta-analysis, any high heterogeneity detected was resolved by the exclusion of studies considered a priori clinical and methodological outliers. The remaining moderate heterogeneity observed in some of the arm-level meta-analyses could be expected since time and place will vary among prevalence and incidence studies [61].
In the literature, prolactin-related AEs with domperidone treatment are considered rare AEs [16]. In contrast, our meta-analysis showed high pooled frequencies of hyperprolactinemia (96%) and prolactin-related symptoms (3%) among patients treated with domperidone. The diverse reporting of prolactin-related AEs might explain why the occurrence of prolactin-related AEs/hyperprolactinemia depicted in this review varied significantly across studies and displayed discrepancies in comparison with the literature. Of interest, prolactin-related AEs were described even among patients treated for shorter periods (e.g., 4–6 weeks). This also slightly contradicts the literature that describes prolactin-related AEs mainly occurring with chronic use [16] and should be further evaluated in future research.
4.1 Limitations
The AE data summarized in this review represent a mix of clinical trial and real-world evidence from observational studies, which likely overcome common limitations of the assessment of AEs in clinical trials, including short duration, restricted study populations, and lack of statistical power to assess rare events [57, 62,63,64]. Nevertheless, the evidence summarized in this SLR is limited by some aspects related to the data provided in the included clinical trials and observational studies.
As previously observed, the reporting of AEs was inadequate in several aspects, including availability of complete information of the AEs actively measured and fully reported. Therefore, it is unclear if the events summarized in this review comprised all AEs observed in the studies. It is also unclear if events not described across the clinical trials and observational studies were assessed but not observed or simply not surveyed. These challenges and opportunities of selective detection and reporting of AEs imply that one cannot construe whether there is an indication of “absence of harms” (i.e., if no event was reported then none occurred) or of “absence of evidence of harm” (i.e., no event was reported because either its detection or reporting was neglected) [65]. The chosen analytic approach suggested was not to make assumptions regarding the lack of occurrence of AEs in studies not mentioning whether a specific event occurred, that is, these studies were excluded from the analysis for that outcome. This means that events were not imputed (i.e., assumed zero) for an outcome (specific AE or total AEs or discontinuations) in a study unless the study report mentioned that the outcome was assessed, but the event did not occur. This approach was robust to generate results valuable for indicating safety signals of the treatments evaluated in patients with gastroparesis. However, the utility of the actual percentages resulting from the analyses should be considered limited, given the possibility of overestimating event rates when the approach for imputation is extremely strict. In other words, the overall occurrence of the AEs in this meta-analysis may be overestimated since data on all events may be lacking.
Since this SLR applied an exploratory approach to assessing the incidence and prevalence of AEs, a formal risk of bias (RoB) assessment was not conducted. This is because aspects impacting the assessment of the RoB on AEs differ from pre-specified efficacy outcomes [66,67,68], and available RoB tools are designed to assess the RoB based on a pre-specified efficacy outcome in clinical trials or observational studies.
Another limitation is that AEs that might be similar or might represent more than one event (e.g., tachycardia and tachycardia/chest pain) were reported in diverse ways across the studies, limiting the ability to compare the data appropriately. Additionally, the studies in the review differed in terms of treatment duration and follow-up period, with treatment durations ranging from just 3 days to 4 years. Treatment duration might impact the occurrence of AEs, despite studies generally reporting that patients received treatment doses generally consistent with those recommended for domperidone and metoclopramide (5–10 mg orally 3–4 times daily). We considered the limitations of combining studies with short and long treatment duration and follow-up periods; however, the limited number of studies contributing data to the analyses on specific AEs prevented subgroup analyses by treatment duration and follow-up timepoint.
Finally, this SLR focused on specific treatments of interest, including domperidone, bromopride, metoclopramide, NG-101, and CIN-102. Comparative data was extracted and summarized from clinical trials, but not from observational studies, to minimize issues with confounding bias from non-randomized trials. Nevertheless, comparisons between the safety profile of domperidone and metoclopramide with carbachol and erythromycin were available from a limited number of clinical trials (n = 2); and the paucity of the data reported precluded further analyses and interpretation. The figures presented mainly refer to absolute measures and not differences between groups in controlled studies, unless otherwise specified. Therefore, the impact of the patient’s baseline risk is unclear, particularly in observational studies.
5 Conclusion
Despite limitations with the inadequate and heterogenetic reporting of AEs, this review on the safety profile of the dopamine receptor antagonists domperidone and metoclopramide demonstrated that serious and significant cardiovascular, endocrine, and extrapyramidal AEs are commonly experienced by patients treated with these drugs. In addition, cardiovascular and endocrine AEs, such as QTc prolongation and hyperprolactinemia, appear to be more common following treatment with domperidone, while extrapyramidal AEs, such akathisia and tardive dyskinesia, might occur more frequently following treatment with metoclopramide. These results confirm a scenario of concerns with patient safety associated with the use of domperidone and metoclopramide. Nevertheless, the highly imprecise evidence generated in primary studies and the lack of a placebo comparator arm for the majority of AEs examined precludes firm interpretation and conclusions. To improve patient safety and additional evidence, future research should strive to better assess and report the AEs experienced by patients receiving these treatments.
References
Schol J, Wauters L, Dickman R, Drug V, Mulak A, Serra J, et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. United Eur Gastroenterol J. 2021;9(3):287–306.
Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American CoG. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37 (quiz 8).
Parkman HP, Hasler WL, Fisher RS, American GA. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127(5):1592–622.
Jung HK, Choung RS, Locke GRR, Schleck CD, Zinsmeister AR, Szarka LA, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–33.
Ye Y, Jiang B, Manne S, Moses PL, Almansa C, Bennett D, et al. Epidemiology and outcomes of gastroparesis, as documented in general practice records, in the United Kingdom. Gut. 2021;70(4):644–53.
Bekkelund M, Sangnes DA, Gunnar HJ, Aabakken L. Pathophysiology of idiopathic gastroparesis and implications for therapy. Scand J Gastroenterol. 2019;54(1):8–17.
Navas CM, Wadas ED, Zbib NH, Crowell MD, Lacy BE. Gastroparesis and severity of delayed gastric emptying: comparison of patient characteristics, treatments and medication adverse events. Dig Dis Sci. 2021;66(2):526–34.
Hasler WL, Parkman HP, Wilson LA, Pasricha PJ, Koch KL, Abell TL, et al. Psychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesis. Am J Gastroenterol. 2010;105(11):2357–67.
Woodhouse S, Hebbard G, Knowles SR. Psychological controversies in gastroparesis: a systematic review. World J Gastroenterol. 2017;23(7):1298–309.
Wadhwa V, Mehta D, Jobanputra Y, Lopez R, Thota PN, Sanaka MR. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;23(24):4428–36.
Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103(2):313–22.
Chen YJ, Tang W, Ionescu-Ittu R, Ayyagari R, Wu E, Huh SY, et al. Health-care resource use and costs associated with diabetic and idiopathic gastroparesis: a claims analysis of the first 3 years following the diagnosis of gastroparesis. Neurogastroenterol Motil. 2022;30: e14366.
Alaven PL. Product information: REGLAN(R) oral tablets, metoclopramide oral tablets. 2009.
Co-pharma LpM. Product information: Dismotil oral tablets, domperidone oral tablets. 2014.
Zegveld C, Knape H, Smits J, Belopavlovic M, Caron D, Gallant J, et al. Domperidone in the treatment of postoperative vomiting: a double-blind multicenter study. Anesth Analg. 1978;57(6):700–3.
European MAE. Metoclopramide-containing medicines. 2014. Cited 2022.
Food aDAF. How to request domperidone for expanded access use. 2021. Cited 2022.
European MAE. Domperidone-containing medicines. 2014. Cited 2022.
Yuksel K, Tuglular I. Critical review of European Medicines Agency (EMA) assessment report and related literature on domperidone. Int J Clin Pharm. 2019;41(2):387–90.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). http://www.training.cochrane.org/handbook: Cochrane; 2020. Accessed 12 Sep 2022.
Page MJ, McKenzie J, Bossuyt P, Boutron I, Hoffmann. T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. 2020.
Food aDAF. Meta-analyses of randomized controlled clinical trials to evaluate the safety of human drugs or biological products guidance for industry. 2018. Cited.
Davis RH, Clench MH, Mathias JR. Effects of domperidone in patients with chronic unexplained upper gastrointestinal symptoms: a double-blind, placebo-controlled study. Dig Dis Sci. 1988;33(12):1505–11.
McCallum RW, Ricci DA, Rakatansky H, Behar J, Rhodes JB, Salen G, et al. A multicenter placebo-controlled clinical trial of oral metoclopramide in diabetic gastroparesis. Diabetes Care. 1983;6(5):463–7.
Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tablet. Neurogastroenterol Motil. 2014;26(4):521–8.
Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray reduces symptoms of gastroparesis in women, but not men, with diabetes: results of a phase 2b randomized study. Clin Gastroenterol Hepatol. 2015;13(7):1256–63.
Patterson D, Abell T, Rothstein R, Koch K, Barnett J. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94(5):1230–4.
Perkel MS, Moore C, Hersh T, Davidson ED. Metoclopramide therapy in patients with delayed gastric emptying. A randomized, double-blind study. Dig Dis Sci. 1979;24(9):662–6.
Perkel MS, Hersh T, Moore C, Davidson ED. Metoclopramide therapy in fifty-five patients with delayed gastric emptying. Am J Gastroenterol. 1980;74(3):231–6.
Silvers D, Kipnes M, Broadstone V, Patterson D, Quigley EMM, McCallum R, et al. Domperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. Clin Ther. 1998;20(3):438–53.
McClelland RN, Horton JW. Relief of acute, persistent postvagotomy atony by metoclopramide. Ann Surg. 1978;188(4):439–47.
Braun AP. Domperidone in the treatment of symptoms of delayed gastric emptying in diabetic patients. Adv Ther. 1989;6(2):51–62.
Erbas T, Varoglu E, Erbas B, Tastekin G, Akalin S. Comparison of metoclopramide and erythromycin in the treatment of diabetic gastroparesis. Diabetes Care. 1993;16(11):1511–4.
Ricci DA, Saltzman MB, Meyer C. Effect of metoclopramide in diabetic gastroparesis. J Clin Gastroenterol. 1985;7(1):25–32.
Stadaas JO, Aune S. Clinical trial of metoclopramide on postvagotomy gastric stasis. Arch Surg (Chicago, Ill: 1960). 1972;104(5):684–6.
Danielli MN, Schiff E, Ben-Arye E, Singer J, Tsadok PT, Flaut S, et al. Benefits of acupuncture for diabetic gastroparesis: a comparative preliminary study. Acupunct Med. 2014;32(2):139–45.
Heckert J, Parkman HP. Therapeutic response to domperidone in gastroparesis: a prospective study using the GCSI-daily diary. Neurogastroenterol Motil. 2018;30(1): e13246.
Hitch DC, Vanhoutte JJ, Torres-Pinedo RB. Enhanced gastroduodenal motility in children. Am J Dis Child. 1982;136(4):299–302.
McCallum RW, Valenzuela G, Polepalle S, Spyker D. Subcutaneous metoclopramide in the treatment of symptomatic gastroparesis: clinical efficacy and pharmacokinetics. J Pharmacol Exp Ther. 1991;258(1):136–42.
Schey R, Saadi M, Midani D, Roberts AC, Parupalli R, Parkman HP. Domperidone to treat symptoms of gastroparesis: benefits and side effects from a large single-center cohort. Dig Dis Sci. 2016;61(12):3545–51.
Soykan I, Sarosiek I, McCallum RW. The effect of chronic oral domperidone therapy on gastrointestinal symptoms, gastric emptying, and quality of life in patients with gastroparesis. Am J Gastroenterol. 1997;92(6):976–80.
Horowitz M, Harding PE, Chatterton BE. Acute and chronic effects of domperidone on gastric emptying in diabetic autonomic neuropathy. Dig Dis Sci. 1985;30(1):1–9.
Schade RR, Dugas MC, Lhotsky DM. Effect of metoclopramide on gastric liquid emptying in patients with diabetic gastroparesis. Dig Dis Sci. 1985;30(1):10–5.
Field J, Wasilewski M, Bhuta R, Malik Z, Cooper J, Parkman HP, et al. Effect of chronic domperidone use on QT interval: a large single center study. J Clin Gastroenterol. 2019;53(9):648–52.
Kozarek R. Domperidone for symptomatic management of diabetic gastroparesis in metoclopramide treatment failures. Adv Ther. 1990;7(2):61–8.
Ortiz A, Cooper CJ, Alvarez A, Gomez Y, Sarosiek I, McCallum RW. Cardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomiting. Am J Med Sci. 2015;349(5):421–4.
Roe NA, Sakaan S, Swanson H, Twilla JD. Evaluation of prokinetic agents used in the treatment of gastroparesis. J Drug Assess. 2017;6(1):6–9.
Parkman HP, Jacobs MR, Mishra A, Hurdle JA, Sachdeva P, Gaughan JP, et al. Domperidone treatment for gastroparesis: demographic and pharmacogenetic characterization of clinical efficacy and side-effects. Dig Dis Sci. 2011;56(1):115–24.
Parkman HP, Mishra A, Jacobs M, Pathikonda M, Sachdeva P, Gaughan J, et al. Clinical response and side effects of metoclopramide: associations with clinical, demographic, and pharmacogenetic parameters. J Clin Gastroenterol. 2012;46(6):494–503.
Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: Effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84(9):1069–75.
Molino D, Mosca S, Angrisani G, Magliacano V. Symptomatic effects of domperidone in postvagotomy gastric stasis. Curr Ther Res Clin Exp. 1987;41(1):13–6.
Ho CS, Rubin E, Renouf JHP. Metoclopramide in gastrointestinal radiology. Can Assoc Radiol J. 1978;29(1):51–5.
Cowan A, Garg AX, McArthur E, Muanda TF, Weir MA. Cardiovascular safety of metoclopramide compared to domperidone: a population-based cohort study. J Can Assoc Gastroenterol. 2021;4(5):e110–9.
European MAE. Committee for proprietary medicine product (CPMP). Motilium and associated names. https://www.ema.europa.eu/en/documents/referral/summary-information-referral-opinion-following-arbitration-pursuant-article-30-council-directive/83/ec-motilium-associated-names-international-non-proprietary-name-inn-domperidone-background-inf_en.pdf. Accessed 12 Sep 2022.
Food aDAF. REGLAN—metoclopramide tablet. Cited 2021.
Trafford JA, Fisher AM, Marshall S, Douthwaite AH. Metoclopramide ('Maxolon’)—a new anti-emetic. Br J Clin Pract. 1967;21(9):457–60.
Hodkinson A, Kirkham JJ, Tudur-Smith C, Gamble C. Reporting of harms data in RCTs: a systematic review of empirical assessments against the CONSORT harms extension. BMJ Open. 2013;3(9): e003436.
Junqueira DR, Phillips R, Zorzela L, Golder S, Loke Y, Moher D, et al. Time to improve the reporting of harms in randomized controlled trials. J Clin Epidemiol. 2021;10(136):216–20.
(Domperidone). In: IBM Micromedex® DRUGDEX® (electronic version). IBM Watson Health/EBSCO Information Services, Greenwood Village, Colorado; Cambridge, Massachusetts, USA. https://www.dynamed.com. Cited 29 July 2021.
(Metoclopramide). In: IBM Micromedex® DRUGDEX® (electronic version). IBM Watson Health/EBSCO Information Services, Greenwood Village, Colorado; Cambridge, Massachusetts, USA. https://www.dynamed.com. Cited 29 July 2021.
Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21(1):189.
Gewandter JS, Smith SM, McKeown A, Edwards K, Narula A, Pawlowski JR, et al. Reporting of adverse events and statistical details of efficacy estimates in randomized clinical trials of pain in temporomandibular disorders: analgesic, anesthetic, and addiction clinical trial translations, innovations, opportunities, and networks systematic review. J Am Dent Assoc. 2015;146(4):246–54 e6.
Golder S, Loke YK, Wright K, Norman G. Reporting of adverse events in published and unpublished studies of health care interventions: a systematic review. PLoS Med. 2016;13(9):e1002127.
Mayo-Wilson E, Fusco N, Hong H, Li T, Canner JK, Dickersin K. Opportunities for selective reporting of harms in randomized clinical trials: selection criteria for non-systematic adverse events. Trials. 2019;20(1):553.
Loke YK, Mattishent K. If nothing happens, is everything all right? Distinguishing genuine reassurance from a false sense of security. CMAJ. 2015;187(1):15–6.
Peryer G, Golder S, Junqueira DR, et al. Chapter 19: adverse effects. Cochrane handbook for systematic reviews of interventions: cochrane; 2021.
Loke YK, Price D, Herxheimer A. Chapter 14: adverse effects. cochrane handbook for systematic reviews of interventions version 5: cochrane collaboration; 2011.
Viswanathan M, Ansari MT, Berkman ND, Chang S, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. 2012.
Acknowledgements
The authors would like to thank Dr. Andreas Freitag for his involvement in the early stages of designing this SLR. We are also grateful to the team supporting the screening, data collection and data summary, Ananth Kashyap, Renuka Raorane, Solange Gonzalez, Ishaaq Altaf-Haroon, Vishnu Baby Kumar, and Anita Engh, and to Simin Hua for conducting the analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Takeda Development Center Americas, Inc.
Conflict of interest
Dr. Bennett and Dr. Huh are current and former employees, respectively, of Takeda Development Center Americas, Inc., and received stock or stock options at the time of study. Currently, Dr. Huh is with Ironwood Pharmaceuticals. Dr. Junqueira, Ms. Betts, Dr. Fahrbach, and Dr. Neupane provided consultancy support to Takeda Development Center Americas, Inc., as employees of Evidera.
Ethics approval
None required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Code availability
Not applicable.
Author contribution statement
Dr. Junqueira, Dr. Bennett, Dr. Huh, and Ms. Betts led the research conceptualization and methodology. Dr. Junqueira led the data curation, investigation, project administration, and visualization. Dr. Fahrbach and Dr. Neupane led the analysis in collaboration with Dr Junqueira and Ms Betts. Dr. Junqueira wrote the original draft and worked with all co-authors in reviewing and editing the final manuscript. All authors reviewed and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Junqueira, D.R., Bennett, D., Huh, S.Y. et al. Risk of Adverse Events Associated with Domperidone and Metoclopramide in Gastroparesis: Systematic Review and Meta-analysis. Drugs R D 23, 1–20 (2023). https://doi.org/10.1007/s40268-023-00413-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00413-x