Abstract
Background and Objective
Anemia caused by iron depletion is common in patients with hemodialysis-dependent stage 5 chronic kidney disease (CKD-5HD) patients. To maintain the iron levels, external administration of iron is essential. Ferric pyrophosphate citrate (FPC) is a novel, water-soluble complex iron salt. The present study was conducted to evaluate the pharmacokinetic (PK) parameters and safety of FPC in adult healthy Chinese subjects and patients with CKD-5HD.
Methods
Two open-label, single-center studies were conducted in healthy subjects and patients with CKD-5HD. Healthy subjects received a single intravenous dose of 6.5 mg FPC solution, while CKD-5HD patients were randomized to two different sequences of FPC administration at two sequential hemodialysis (HD) treatments (dose 1 and dose 2). Patients received 27.2 mg of FPC at a dialysate concentration of 95 μg/L for 4 h or a single 6.5 mg dose of FPC administered intravenously via the pre-dialyzer blood circuit. The primary objective was to determine the PK parameters of total serum iron (Fetot), while the secondary objective was the safety of the FPC solution. PK parameters were calculated using Phoenix WinNonlin 8.1 and other parameters were analyzed using SAS 9.4 software. Comparison between HD dose 2 and HD dose 1 was performed using the Wilcoxon rank-sum test and analysis of variance (ANOVA).
Results
A total of 14 healthy subjects with a mean age of 30.8 ± 5.92 years and 12 HD patients with a mean age of 54.3 ± 16.47 years were included. In healthy subjects, the peak serum concentration was reached at the end of infusion of FPC, with an adjusted mean maximum concentration (Cmax,) of 33.46 ± 4.83 μmol/L at a mean time to reach Cmax (Tmax) of 4.09 ± 0.19 h. In patients with CKD-5HD, the adjusted mean Cmax of HD dose 2 was 25.37 ± 4.30 μmol/L at a Tmax, of 3.09 ± 0.32 h, whereas the Cmax, of HD dose 1 was 24.59 ± 4.77 μmol/L at a Tmax, of 3.96 ± 0.26 h. The Fetot concentration-time curves were observed to be similar for both administration methods (HD doses 1 and 2), while the PK parameters differed significantly for Tmax (p = 0.001; baseline correction) and area under the concentration-time curve from time zero to time t (AUCt) [p = 0.031 for cycle variance; without baseline correction] between HD doses 1 and 2. The geometric mean ratios (HD dose 1/HD dose 2) for Cmax and AUCt were within the 85–125% range (Cmax 96.56%; AUCt 96.07%). A total of three and two incidences of adverse events were reported in healthy subjects and patients with CKD-5HD, respectively.
Conclusion
FPC showed a good PK and safety profile and hence can be used as maintenance therapy for patients with CKD-5HD by choosing a better method of administration based on clinical feasibility and requirement.
Clinical Trial Registration
CTR20181113 and CTR20181119.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It has been established that anemia caused by iron depletion is seen in patients with hemodialysis-dependent stage 5 chronic kidney disease. Hence, external administration of iron is an essential goal. |
FPC is complex iron salt that is an iron replacement treatment to maintain hemoglobin for such patients. |
The pharmacokinetics and safety profile supports its use as maintenance therapy. |
1 Introduction
Iron deficiency anemia is a frequently encountered clinical condition in patients with hemodialysis-dependent stage 5 chronic kidney disease (CKD-5HD) [1]. An iron deficiency could be categorized as absolute iron deficiency wherein there is an inadequacy in stored iron, or functional iron deficiency characterized by adequate stored iron but lack of iron available for absorption into erythroid precursors [2]. Both forms of iron deficiency are seen in CKD-5HD patients. While absolute iron deficiency is due to an increased rate of blood loss during dialysis, gastrointestinal bleeding and platelet dysfunction, functional iron deficiency is mediated by the elevated levels of hepcidin that could interfere with the sequestration of iron from the reticuloendothelial system for the process of erythropoiesis [1].
To counter absolute and functional iron deficiency, patients with CKD may require oral or intravenous iron supplementation depending on their need for hemodialysis [3]. While the therapeutic clinical practice guidelines recommend intravenous iron supplementation in patients with CKD-5HD, intravenous iron could potentially lead to inflammation, culminating in cardiovascular disease and immune deficiency [4, 5]. Furthermore, all the currently available intravenous iron supplements consist of a core of ferric hydroxide within a carbohydrate shell designed for the slow release of iron [6]. However, despite the slow release of intravenous iron supplementation, smaller quantities of non-transferrin bound iron could lead to the abovementioned cascade of adverse events (AEs), which has been confirmed by the identification of biomarkers of oxidative stress in plasma in previous clinical trials [7, 8].
In order to overcome the potential issues with intravenous iron supplementation, ferric pyrophosphate citrate (FPC; Triferic®), a complex iron salt, was approved by the US FDA in January 2015 for the iron replacement treatment of patients with CKD-5HD [9]. FPC is administered along with the dialysate, providing a source of iron administered over a course of hours that alleviates the adverse effects of intravenous iron supplementation [9]. Furthermore, owing to the strong bond between iron, pyrophosphate and citrate, the availability of free iron is limited, which further minimizes the oxidative damage [10].
In China, as per a previous study, the age-adjusted prevalence of CKD-5HD is 122.19 per million and approximately 20% of patients with end-stage renal disease requiring dialysis treatment were anemic [11, 12]. Despite the high prevalence of anemia in patients with CKD-5HD in China, only 39–46% of patients achieved target hemoglobin levels of 10–12 g/dL, highlighting the existence of an unmet therapeutic need in China [13]. Hence, FPC may provide a therapeutic option for treating anemia in Chinese patients with CKD-5HD. The CRUISE 1 and 2 and PRIME studies demonstrated the efficacy of FPC administered with dialysate in maintaining hemoglobin levels from baseline to end of treatment in a Caucasian population [14, 15]. However, due to perceived genetic differences in an ethnic Chinese population, the pharmacokinetics (PK) of FPC need to be established in the Chinese population. Therefore, the current study was performed to evaluate the PK and PD of FPC in healthy Chinese subjects and Chinese patients with CKD-5HD.
2 Methods
2.1 Study Design
Two open-label, single-center studies were designed to evaluate the PK and safety of FPC in Chinese adult subjects with and without (healthy subjects) CKD-5HD. Evaluation of the PK and safety of FPC in healthy subjects was performed in a single-arm study approved by the Ethics Committee of Beijing Hospital (registered number: CTR20181113; ethic approval number: 2018BJYYEC-188-02) and subjects were recruited in January 2019. The PK and safety assessment of patients with CKD-5HD was a two-sequence, randomized study approved by the Medical Ethics Committee of Peking University People’s Hospital (registered number: CTR20181119; ethic approval number: 2018HA059-002) and patients were recruited from 2018 to 2019. Both studies were carried out in accordance with the ethical principles of the Helsinki Declaration, and according to the guidelines of the Chinese National Medicinal Products Administration (NMPA) for clinical trial application. All patients provided written informed consent before participating in this study.
2.2 Subjects
For the PK and safety assessment study of Chinese subjects without CKD-5HD, healthy adult subjects (19–55 years of age) with a body weight ≥ 50.0 kg and a body mass index (BMI) ≥ 19 to ≤ 28 kg/m2, with transferrin saturation (TSAT) of 20–45% during screening and total iron binding capacity (TIBC) of ≥ 25 μg/dL (40 μM), as well as those who agreed to discontinue all iron preparations 14 days before the baseline period, were included. Female subjects were premenopausal, non-pregnant, and non-lactating. Subjects with hemoglobin concentration (Hgb) < 120 g/L (male) or < 110 g/L (female) during screening, those with comorbid or recurrent diseases or those who developed acute disease within 14 days before the screening period, subjects who were intolerant or allergic to iron preparations, those who received blood or blood components (plasma or platelets) within 30 days before baseline period, and those who had participated in any other clinical trial 3 months prior to enrollment were excluded from the study. The PK and safety assessment study of healthy subjects included a screening period of 28 days followed by an iron-restricted diet for 2 days (day 1 to day 2), estimation of baseline parameters (day 3), and treatment period (day 4 to day 5).
For the PK and safety assessment study of Chinese patients with CKD-5HD, patients aged 18–80 years who had undergone long-term HD for CKD for at least 3 months and were expected to undergo maintenance hemodialysis (HD) for at least three times a week, and with ferritin, TSAT and Hgb concentrations of ≥ 200 μg/L, 15–45%, and ≥ 9.0 g/dL at screening, respectively, as well as those who were receiving or could receive dialysis anticoagulant therapy with a single dose of unfractionated heparin or low molecular weight heparin before dialysis or through intermittent intravenous heparin bolus injection through a dialyzer blood circuit, and those who agreed to discontinue all iron supplements (oral and intravenous) 14 days before the start of treatment and throughout the study period were included. In the case of female patients, only non-pregnant and non-lactating patients were included. Furthermore, all included patients must have had a minimum of one dialysis adequacy measurement, defined as a single pool Kt/V [(dialyzer clearance rate of urea × dialysis time)/patient’s body water content] ≥ 1.2, or KI Dt/V [(dialyzer clearance rate measured by ion dialysate × dialysis time)/patient’s body water content) ≥ 1.2 within 120 days before recruitment into the study. Patients who had received a blood transfusion within 14 days of the start of treatment or those with known active bleeding in any part of the body except the arteriovenous fistula or artificial vascular access, patients who were scheduled for renal transplantation during the study period, and those with any other inflammatory diseases, cirrhosis, infections needing antimicrobial therapy, abnormal alanine transaminase (ALT; ≥ 2-fold) and/or aspartate transaminase (AST) levels were excluded from the study. The PK and safety assessment study of Chinese subjects with CKD-5HD involved a screening period of 28 days followed by 3 days of treatment (1–3 days) and 4 days of follow-up (4–7 days).
2.3 Randomization and Treatment
For the single-arm PK and safety assessment study of healthy volunteers, a total of 16 subjects were included. All patients were administered 180 mL of FPC solution containing 6.5 mg of iron intravenously for 4 h. For the PK and safety assessment study of CKD-5HD patients, a total of 12 patients were randomized to either of the two sequences of FPC administration. Patients were randomized using SAS 9.4 software in a 1:1 ratio (SAS Institute Inc., Cary, NC, USA). In sequence 1, 27.2 mg of FPC was added to 10 L of bicarbonate concentrate and administered through the dialysate for 4 h on day 1 of HD treatment (HD dose 1) followed by a single 6.5 mg dose of FPC administered intravenously via the pre-dialyzer blood circuit for 3 h on the third day (HD dose 2). In sequence 2, HD dose 2 on day 1 was followed by HD dose 1 on day 3 (Table 1). The final concentration of iron in the dialysate was 95 µg/L (1.7 µM). The study designs are presented in Electronic Supplementary Fig. 1.
2.4 Sample Collection
Blood samples for the evaluation of PK in healthy subjects were drawn 0 h before administration, and 1, 2, 3, 4, 4.5, 5, 6, 8, 10, 12 and 18 h after administration on the fourth day, and total serum iron (Fetot) was determined. Based on the pattern of metabolism of iron, baseline Fetot values were taken on the third day by collecting blood samples at different time points (0, 1, 2, 3, 4, 4.5, 5, 6, 8, 10, 12, and 18 h) starting at 08.00 in the morning (0 h). On the morning of day 5 of the study, subjects were discharged from the hospital. In the case of patients with CKD-5HD, the blood samples were drawn at 0 h before administration and 1, 2, 3, 3.5, 4, 4.5, 5, 6, 8, 10, and 12 after administration of each dose of FPC (HD dose 1 and HD dose 2).
2.5 Bioanalytical Methods
Serum iron was detected indirectly using a validated autoanalyzer method. Under acidic conditions, the transferrin-iron complex releases iron (ferric ion), which is reduced to ferrous ion by ascorbic acid, and this ferrous ion reacts with ferrozine to form a colored complex; the intensity of color formed is directly proportional to the concentration of iron. Serum unsaturated iron binding capacity was measured by photometry. The principle involved was that in alkaline buffer solution, ferrous ion reacts with transferrin to form transferrin iron complex and free excess ferrous ions. The color intensity is directly proportional to the concentration of unbound excess ferrous ions and indirectly proportional to the binding force of unsaturated iron. For the detection of serum ferritin, particle enhanced immunoturbidimetric assay was used, in which the concentration of agglutinate precipitate formed by interaction of serum ferritin and latex particles coated with anti-ferritin antibody was measured at 570/800 nm by turbidimetry. Serum transferrin was also measured by the same method.
2.6 Pharmacokinetic Analysis
PK analysis was performed on the absolute Fetot value and baseline-corrected Fetot value. The absolute value of Fetot was the Fetot value measured after administration of FPC, while the baseline-corrected Fetot value was the absolute Fetot value measured after administration minus the baseline value of Fetot. The non-compartmental method was used to calculate the PK parameters. The PK parameters evaluated were Cmax, Tmax, AUCt, AUCend, AUC∞, λZ, t½Z, CLZ, VZ and AUC%Extrap. The definitions of the PK indexes are provided in Online Resource 1. PK parameters were evaluated in the PK dataset consisting of all patients who received the study drug and in whom data on at least one blood concentration were available during the trial.
2.7 Safety Analysis
Safety was assessed in terms of reported and observed AEs and serious AEs (SAEs), while safety monitoring indicators included physical examination, vital signs (blood pressure, pulse rate and body temperature), 12-lead electrocardiogram (ECG), chest X-ray, abdominal ultrasound, and laboratory examinations (blood, urine, stool routine examination and blood biochemistry). AEs were coded according to Medical Dictionary for Regulatory Activities (MedDRA) 22.0, and safety was monitored in the safety dataset consisting of all patients who received the study drug and had safety monitoring data.
2.8 Statistical Analysis
According to the ‘technical guidelines for clinical pharmacokinetics of chemical drugs’, the sample size of a single-dose PK study should be 8–12 subjects. Considering the dropout rate, the sample size planned for our study was about 16 cases. Continuous variables were presented as mean ± standard deviation (SD) and median (quartile, minimum and maximum). The count and grade data were described by frequency and percentage. Individual and average concentration-time curves and semi-logarithmic concentration-time curves were constructed. For subjects whose concentration before administration was more than 5% of the Cmax, descriptive statistical analysis was not performed for each parameter. The PK parameters were evaluated using the linear mixed-effects model in which the PK parameters were log-transformed. In the case of CKD-5HD patients, the administration mode, sequence and period were fixed effects and the individual patients were random effects. Paired Wilcoxon rank-sum test and analysis of variance (ANOVA) was used to compare the PK parameters between HD dose 1 and HD dose 2 in CKD-5HD patients. The limits for the geometric mean ratios (GMRs) considered was 85–125%. PK parameters were calculated using Phoenix WinNonlin 8.1 (Pharsight Certara, Princeton, NJ, USA), while others were analyzed using SAS 9.4 software.
3 Results
3.1 Participants and Baseline Demographics
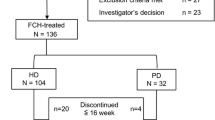
A total of 133 healthy Chinese subjects and 23 Chinese patients with CKD-5HD were screened for assessing the PK and safety of FPC, among whom 14 healthy subjects and 12 patients with CKD-5HD were included in our study. The mean age of healthy subjects and patients with CKD-5HD was 30.8 ± 5.92 and 54.3 ± 16.47 years, respectively, and the mean BMI was 23.93 ± 2.18 kg/m2 and 23.47 ± 4.18 kg/m2, respectively, in healthy subjects and patients with CKD-5HD. The demographic and baseline characteristics of the healthy subjects and patients with CKD-5HD are summarized in Table 2.
3.2 Pharmacokinetics
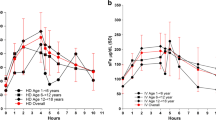
In the 14 healthy subjects, the baseline mean serum iron concentration did not fluctuate as a whole but reached the peak level at about 5 h, after which the concentration started to decrease, reaching the lowest value at 12 h and recovering at 18 h (a small diurnal variation was observed in the baseline mean serum iron concentration) (Fig. 1). After a single intravenous infusion of FPC, the average Fetot concentration reached the peak at the end of the infusion, and, until 12 h after administration, the average Fetot concentration at each time point was higher than the baseline average serum iron concentration. After baseline correction, the mean serum iron concentration also reached the maximum at the end of the infusion and trended towards zero at 18 h after administration. The key PK parameters after intravenous infusion of FPC post baseline correction in healthy subjects are summarized in Table 3. The mean Cmax was 33.46 ± 4.83 μmol/L at a mean Tmax of 4.09 ± 0.19 h and mean AUCt of 201.98 ± 46.73 h*μmol/L. No significant abnormality was observed in the indexes of Fetot, TSAT, TIBC, unsaturated iron binding capacity, and ferritin during the screening period and on the fifth day post administration of intravenous FPC. On the fourth day after FPC administration, the TSAT levels peaked at 4 h and then decreased. TSAT maximum was < 100%, within the acceptable safety range. No obvious change in ferritin levels and TIBC were observed on days 3 and 4. Unsaturated iron binding capacity was observed to decrease until 4 h on day 4 and then reached baseline levels by 18 h (Fig. 2).
In patients with CKD-5HD, during the screening period, there was no obvious abnormality in the indexes of Fetot, TSAT, TIBC, unsaturated iron binding capacity, and ferritin. Similarly, no significant abnormality was observed in all the indexes, even on the first and third days of the study. Post administration of HD dose 1 or HD dose 2, TSAT levels peaked until the end of infusion and then declined, while an opposite trend was observed in unsaturated iron binding capacity (Fig. 3). After baseline correction and post HD dose 2, the peak serum concentration (Cmax) was 25.37 ± 4.30 μmol/L at a Tmax of 3.09 ± 0.32 h and AUCt of 125.24 ± 51.27 h*μmol/L. Furthermore, the maximum serum concentration of post HD dose 1 (Cmax 24.59 ± 4.77 μmol/L) reached 3.96 ± 0.26 h, with AUCt of 122.39 ± 57.90 h*μmol/L. The key PK parameters after HD dose 1 and HD dose 2 are summarized in Table 3 and the statistical comparisons of the primary PK parameters in patients with CKD-5HD are summarized in Electronic Supplementary Table 1.
The Fetot concentration-time curves were observed to be similar for both administration methods (HD dose 1 and 2) (Fig. 4); however, comparison of the PK parameters of the two different administration methods revealed significant differences in Tmax (p = 0.001; baseline correction) and AUCt (p = 0.031 for cycle variance; without baseline correction) by the paired Wilcoxon rank-sum test and ANOVA, respectively. The GMR (HD dose 1/HD dose 2) of Cmax and AUCt was 97.75% (95% confidence interval [CI] 93.36–102.35%) and 98.86% (95% CI 91.05–107.34%), respectively (non-baseline corrected), and 96.56% (95% CI 88.86–104.92%) and 96.07% (95% CI 80.44–114.73%), respectively (baseline corrected). The degree of assurance for GMRs of Cmax and AUCt was > 99.99% and 99.54%, respectively (non-baseline corrected), and 98.44% and 34.19%, respectively (baseline corrected).
Mean serum concentration-time curves in patients with CKD-5HD. A Mean Fetot concentration-time plot (absolute); B mean Fetot concentration-time semi-log plot (absolute); C mean Fetot concentration-time plot (baseline correction); D mean Fetot concentration-time semi-log plot (baseline correction). CKD-5HD hemodialysis-dependent stage 5 chronic kidney disease, Fetot total serum iron
3.3 Safety
Among healthy subjects, two (14.29%) subjects reported three AEs of mild severity, which were cured. In patients with CKD-5HD, two (16.7%) patients reported two AEs, of which one (8.3%) patient reported an SAE of acute myocardial infarction, which may not be related to the study drug, and the outcome was stable (Table 4). In the healthy subjects and patients with CKD-5HD, no SAEs or AEs leading to withdrawal from the trial occurred and there was no incidence of death during the study period. No clinically relevant abnormalities were observed in physical examination, ECG, and laboratory test results in both studies.
4 Discussion
Treatment of anemia and maintaining Hgb levels is highly important in patients receiving HD. The available treatments of oral and intravenous iron have their own limitations. While oral iron may lead to iron overload and other AEs related to the gastrointestinal tract, intravenous iron, mostly available as iron carbohydrate complex, is associated with an elevated risk of anaphylactic reaction, which also requires metabolism in the reticuloendothelial system to release free iron. Furthermore, its absorption is limited by elevated hepcidin levels [16, 17]. These limitations can be addressed by using FPC, a soluble source of iron that is usually administered through the dialysate during HD. Its unique mechanism of action helps bypass the hepcidin barrier and presents iron directly to erythropoiesis. Furthermore, its highly soluble nature may make it easy to formulate for intravenous administration [18]. FPC administered via dialysate helps to maintain the hemoglobin levels without increasing iron stores, and exhibits a tolerable safety profile in patients with CKD-5HD [14, 15]. Since assessment of the PK of FPC in a particular population precedes its use clinically, the current study evaluated the PK and safety of FPC in healthy Chinese subjects and Chinese patients with CKD-5HD.
Among healthy adults, administration of 6.5 mg of intravenous FPC was well tolerated. Since healthy populations were included, the subjects were on a strict diet to avoid iron intake. The peak plasma concentration was reached by the end of the infusion, i.e. about 4 h, and the terminal half-life (T½Z) was 1.93 h, with a terminal rate constant (λZ) of 0.41 h−1. The results obtained in the study were in accordance with the study by Pratt et al., which reported a peak plasma concentration at 4.8 h, T½Z of 1.3 h, and terminal rate constant of 0.7 h−1 after intravenous administration of 7.5 mg FPC in healthy subjects [10]. In the present study, other PK parameters such as Cmax, AUCt, AUCend, AUC∞, and CLZ were found to be in par with reported studies [10, 19]. Previous studies also reported that FPC was safe and well tolerated in healthy populations from other geographic areas [10, 19]. The common AEs observed were gastrointestinal disorders and pain at the injection site, in accordance with a previous trial [19].
In patients with CKD-5HD, FPC was administered through a pre-dialysate blood circuit or via dialysate. The results showed that the peak serum iron concentration was attained at the end of intravenous administration or dialysis and returned to baseline levels 12 h after administration. The results of the present study were consistent with a previous study, where mean serum concentrations reached the maximum at 3–4 h for intravenous administration before dialysis and 2–4 h for dialysate administration, and the concentrations returned to baseline levels at approximately 10 h for both routes of administration [20].
All PK parameters, with the exception of Tmax and AUCt, were similar in both routes of administration of FPC, suggesting that both routes could be explored for clinical application. Tmax and AUCt differed significantly between the two administration methods, indicating that the cycle factor could be a variable factor that needs to be accounted for in clinical application. The GMRs of Cmax and AUCt were within the set limits of 85–125%, indicating no significant difference between the two administration methods. Furthermore, no significant changes were observed in TSAT, TIBC, unsaturated iron binding capacity, and ferritin between the two administration methods. The changes in these parameters from baseline to end of the study were similar to the results presented in previous studies [9, 14, 15]. AEs were reported in two (16.7%) of the patients, and only one patient from the pre-dialyzer group had an SAE which was not related to the study drug. No deaths were reported, indicating the safety and tolerability of FPC in CKD-5HD patients when administered through the dialysate or pre-dialyzer [14, 15].
To the best of our knowledge, this is the first study to evaluate the PK parameters of FPC in the Chinese population. The study further evaluated the PK parameters in both healthy and diseased subjects using a comprehensive set of PK evaluation methods, and also compared two different administration methods in patients with CKD-5HD. However, the study was not without limitations. First, concomitant medications and comorbid conditions may affect the metabolism of the drug and follow-up observation is required. However, in the current study, due to the stringent inclusion and exclusion criteria, individuals received FPC either intravenously or via dialysate, therefore evaluation of the effect of concomitant medications and other diseased states were excluded. Verification of this in future studies is therefore warranted. Second, only the short-term safety assessment of FPC was performed, hence validation of safety in future studies with long term-follow up is required.
5 Conclusion
The results of the present study suggest that the PK profile of FPC after administration through the dialysate or pre-dialyzer in Chinese patients with CKD-5HD are similar and is well tolerated. The findings from this study may help in guiding the best administration method of FPC in Chinese adult CKD-5HD patients.
References
Gafter-Gvili A, Schechter A, Rozen-Zvi B. Iron deficiency anemia in chronic kidney disease. AHA. 2019;142:44–50.
Portolés J, Martín L, Broseta JJ, Cases A. Anemia in chronic kidney disease: from pathophysiology and current treatments, to future agents. Front Med. 2021;8:328.
Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, et al. Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2013;28:2570–9.
Mikhail A, Brown C, Williams JA, Mathrani V, Shrivastava R, Evans J, et al. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017;18:345.
Vaziri ND. Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis. 2013;61:992–1000.
Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J Am Soc Nephrol. 2004;15(Suppl 2):S93-98.
Garcia-Fernandez N, Echeverria A, Sanchez-Ibarrola A, Páramo JA, Coma-Canella I. Randomized clinical trial on acute effects of i.v. iron sucrose during haemodialysis. Nephrology (Carlton). 2010;15:178–83.
Kuo K-L, Hung S-C, Wei Y-H, Tarng D-C. Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J Am Soc Nephrol. 2008;19:1817–26.
Shah HH, Hazzan AD, Fishbane S. Ferric pyrophosphate citrate: a novel iron replacement agent in patients undergoing hemodialysis. Semin Nephrol. 2016;36:124–9.
Pratt RD, Swinkels DW, Ikizler TA, Gupta A. Pharmacokinetics of ferric pyrophosphate citrate, a novel iron salt, administered intravenously to healthy volunteers. J Clin Pharmacol. 2017;57:312–20.
Zhang L, Zhao M-H, Zuo L, Wang Y, Yu F, Zhang H, et al. China Kidney Disease Network (CK-NET) 2015 annual data report. Kidney Int Suppl. 2011;2019(9):e1-81.
Zhao X, Niu Q, Gan L, Hou FF, Liang X, Ni Z, et al. Baseline data report of the China Dialysis Outcomes and Practice Patterns Study (DOPPS). Sci Rep. 2021;11:873.
Qian J, Zhang W. Shanghai dialysis registration and analysis of results. Chin Blood Purif. 2012;11:233–6.
Fishbane SN, Singh AK, Cournoyer SH, Jindal KK, Fanti P, Guss CD, et al. Ferric pyrophosphate citrate (TrifericTM) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant. 2015;30:2019–26.
Gupta A, Lin V, Guss C, Pratt R, Ikizler TA, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients. Kidney Int. 2015;88:1187–94.
Fishbane S, Shah HH. Ferric pyrophosphate citrate as an iron replacement agent for patients receiving hemodialysis: ferric pyrophosphate citrate in dialysis. Hemodial Int. 2017;21:S104–9.
Cançado RD, Muñoz M. Intravenous iron therapy: how far have we come? Rev Bras Hematol Hemoter. 2011;33:461–9.
Gupta A. FP673structural, physical and functional characterisation of ferric pyrophosphate citrex (FPC, TRIFERIC), a novel iron compound for pharmaceutical applications. Nephrol Dial Transplant. 2015;30:299.
A Single Ascending Dose Study of Soluble Ferric Pyrophosphate Administered Intravenously in Healthy Volunteers. Report No.: NCT01920854. https://clinicaltrials.gov/ct2/show/results/NCT01920854. Accessed 18 Nov 2021
Pratt RD, Grimberg S, Zaritsky JJ, Warady BA. Pharmacokinetics of ferric pyrophosphate citrate administered via dialysate and intravenously to pediatric patients on chronic hemodialysis. Pediatr Nephrol. 2018;33:2151–9.
Acknowledgements
The authors acknowledge Dr. Satya Lavanya Jakki and Dr. Kaushik Subramanian (Indegene, Bangalore, India) for medical writing and editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was funded by Wanbang Biopharmaceuticals.
Conflicts of interest
Liangying Gan, Panpan Xie, Kexin Li, Yi Fang and Li Zuo declare no conflicts of interest related to this study. Yan Tan, Gang Wei and Ai-Min Hui are full-time employees of Shanghai Fosun Pharmaceutical Development, Co., Ltd. Zhifei Lu is a full-time employee of Beijing Fosun Pharmaceutical Development, Co., Ltd. Xiaojuan Yuan and Yongchun Zhou are full-time employees of Wanbang Biopharmaceuticals. Raymond Pratt is a full-time employee of Rockwell Medical Inc. USA and received salary and stock options.
Ethics approval
The single-arm study was approved by the Ethics Committee of Beijing Hospital, and the two-sequence, randomized study was approved by the Medical Ethics Committee of Peking University People’s Hospital. The studies were carried out in accordance with the ethical principles of the Helsinki Declaration and according to the guidelines of the Chinese NMPA for clinical trial application.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Consent for publication
Not applicable.
Consent for participate
All participants provided written informed consent before participating in the study.
Informed Consent
Written informed consent was provided by the patients.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LG, PX, KL, YF and LZ. All authors commented on the draft of the manuscript and read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
40268_2022_384_MOESM1_ESM.tif
Supplementary file1 Study designs for (a) healthy subjects and (b) patients with CKD-5HD. CKD-5HD hemodialysis-dependent stage 5 chronic kidney disease (TIF 85 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gan, L., Xie, P., Tan, Y. et al. Pharmacokinetics and Safety of Ferric Pyrophosphate Citrate in Chinese Subjects with and without Hemodialysis-Dependent Stage 5 Chronic Kidney Disease. Drugs R D 22, 119–129 (2022). https://doi.org/10.1007/s40268-022-00384-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-022-00384-5