Abstract
Purpose
Gemigliptin is approved for the treatment of type II diabetes mellitus. Sulfonylureas are commonly used in combination with other antidiabetic drugs to improve glycemic control. The objective of this study was to evaluate the pharmacokinetics, safety, and tolerability of gemigliptin and glimepiride combination therapy compared with those of monotherapies.
Methods
A randomized, open-label, crossover study was performed on healthy Korean male volunteers. Each subject received the following treatments (A and B) with a 7-day washout period: treatment A consisted of gemigliptin 50 mg once daily administered orally for 6 days, followed by concomitant oral dosing of glimepiride 4 mg and gemigliptin 50 mg on day 7; treatment B consisted of a single dose of glimepiride 4 mg. Blood samples were collected up to 24-h postdose on day 6 (gemigliptin) and day 7 (gemigliptin and glimepiride) following treatment A, and on day 1 (glimepiride) following treatment B. Concentrations of gemigliptin, glimepiride, and metabolites were determined using validated liquid chromatography–tandem mass spectrometry (LC–MS/MS). Safety assessments were performed throughout the study.
Results
Twenty-three subjects completed the study. The geometric mean ratios (GMRs) of C max,ss and AUC τ,ss for gemigliptin were 1.0097 [90 % confidence interval (CI) 0.924–1.103] and 0.9997 (90 % CI 0.976–1.024), respectively. For glimepiride, the GMRs of C max and AUClast were 1.031 (90 % CI 0.908–1.172) and 0.995 (90 % CI 0.902–1.097), respectively. Both combination and monotherapy were well tolerated, and no serious adverse events were reported.
Conclusion
Gemigliptin and glimepiride did not alter the pharmacokinetic properties of each other when they were co-administered in healthy volunteers, and were generally tolerated.
Similar content being viewed by others
1 Introduction
Type 2 diabetes mellitus (T2DM)—a chronic and progressive disorder that is characterized by the insufficient production of insulin and/or reduced responsiveness to its effects—is difficult to effectively treat long-term [1]. Because intensive glucose control using oral antidiabetic agents or insulin significantly reduces microvascular complications compared with dietary modification, one of the major therapeutic targets of T2DM is the maintenance of a normal glucose level [2]. However, a previous study reported that only 50 % of patients are able to maintain the target level during 3 years of monotherapy; by 9 years, this figure declines to 25 % [3]. Therefore, the majority of T2DM patients require multiple therapies in order to achieve their therapeutic goals and prevent complications. Several antiglycemic agents are now available that directly target one or more of the pathophysiological processes of T2DM. Furthermore, the optimal therapeutic strategy depends on individual clinical conditions [1].
Sulfonylurea is the oldest oral class of drugs that stimulates insulin release by inhibiting ATP-regulated potassium channels in the β-cells of the pancreas, thereby leading to cell membrane depolarization [4]. Unfortunately, many patients are unable to maintain glycemic control with sulfonylurea monotherapy (or even combination therapy) because of treatment failure or hypoglycemia. From previous studies, primary treatment failure (i.e. no therapeutic response) has been reported in up to 41 % of patients, and secondary failure occurs at an estimated annual rate of 5–7 % [5].
Accordingly, combination therapy could demonstrate the additional benefit of reducing the risk of adverse events (AEs) because lower doses of sulfonylurea may be required in comparison with monotherapy, and synergistic glycemic control can be expected [6–8].
Meanwhile, new antiglycemic agents that target the incretin system were recently introduced [9]. Incretins are endogenous hormones, such as glucagon-like peptide-1 (GLP-1), that potently stimulate glucose-dependent insulin secretion and suppress glucose-dependent glucagon secretion, thereby lowering prandial plasma glucose. Because GLP-1 is rapidly degraded by dipeptidyl-peptidase 4 (DPP-4), DPP-4 inhibitors can increase active circulating incretins, thereby reducing blood glucose [9, 10]. Also, preliminary studies show that DPP-4 inhibitors could preserve pancreatic β-cell mass and function by reducing apoptosis. Considering the fact that β-cell exhaustion is associated with excessive demand, DPP-4 inhibitors could mitigate the drawbacks of sulfonylurea administration [11, 12]. Some randomized clinical trials previously reported improved postprandial glucose levels as well as β-cell function following the addition of DPP-4 inhibitors and sulfonylurea [13, 14].
Gemigliptin is a novel, selective, and competitive inhibitor of DPP-4 that has been approved for the treatment of T2DM [15]. The pharmacokinetic characteristics of gemigliptin were previously reported. In a single ascending-dose study on healthy volunteers, gemigliptin was absorbed with t max at 0.5–5.1 h, was eliminated after a mean t ½ of 16.7–21.3 h, and demonstrated dose-linear C max and area under the curve (AUC) values that were in the range of 50–400 mg [16]. Following multiple once-daily administration to healthy volunteers, the mean accumulation index at steady state ranged between 1.22 and 1.31, and the mean fraction of the unchanged drug that was excreted in urine (fe) ranged between 0.40 and 0.48 (Gemigliptin IB version 6.0, September 2012). According to preclinical studies, the inhibitory or induction potential of gemigliptin and its metabolites was very low, and the major metabolic route is via cytochrome P450 (CYP) 3A4 (Gemigliptin IB version 6.0, September 2012). A recent study reported that the addition of gemigliptin 50 mg (or twice daily 25 mg) to daily metformin 1,000 mg significantly improved glycemic control in patients who have inadequately controlled T2DM when taking metformin alone [17]. No studies have reported combination gemigliptin and sulfonylurea for treating T2DM patients, but this combination could be required in certain clinical circumstances. Recently, some studies added the DPP-4 inhibitor to metformin and/or sulfonylurea treatment and reported significant and well-tolerated glycemic control [14, 18].
Glimepiride is a second-generation sulfonylurea that is widely used to treat T2DM—usually administered once daily to patients with glycemia that is poorly controlled by metformin monotherapy [19]. Glimepiride demonstrates known dose-linear pharmacokinetics. After oral administration, glimepiride is completely absorbed and the maximum concentration is reached after 0.7–2.8 h (t max) in healthy volunteers and 2.4–3.75 h in T2DM patients. Terminal half-life was increased from 3.2 to 8.8 h over the range of doses from 1 to 8 mg in healthy volunteers. There are no major differences between C max, t max, or AUC after 1 day, and after 7 days of administration of multiple doses of glimepiride to T2DM patients; glimepiride does not accumulate [20, 21]. Glimepiride is primarily metabolized in the liver, and the major metabolites are the cyclohexyl hydroxyl methyl derivative (M1) and the carboxyl derivative (M2); the M1 metabolite is mainly formed by CYP2C9, and M1 is further oxidized to the inactive form, M2. Therefore, the interactions between glimepiride and the CYP2C9 inhibitor and/or inducer are expected. For example, fluconazole is known to increase plasma concentrations of glimepiride, but other clinically significant drug interactions mediated by the metabolizing enzymes have not yet been proven [22].
Because gemigliptin and glimepiride demonstrate different major elimination pathways, the use of these drugs in combination could be considered safe and potentially demonstrate complementary effects on T2DM patients. Accordingly, the present study was conducted to investigate the pharmacokinetic interactions and tolerability of gemigliptin and glimepiride in healthy volunteers.
2 Methods
2.1 Subjects
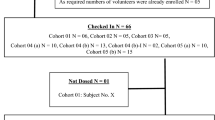
This study enrolled healthy Korean male volunteers between 20 and 45 years of age with body mass indexes (calculated from height and weight) between 18 and 27 kg/m2. All volunteers were assessed by physicians using their medical histories, physical examination results, laboratory test results (e.g. hematology, blood chemistry, urinalysis, human immunodeficiency virus antibody test, hepatitis B surface antigen, hepatitis C virus, syphilis high-quality reagin test), electrocardiography (ECG) results, and vital signs. Participants were excluded for the following reasons: any chronic, clinically significant medical histories, including drug hypersensitivity; blood donation <60 days prior to study drug administration; taken any drugs that could influence drug metabolism (e.g. barbiturates) <30 days and/or prescription drugs <14 days prior to dosing; positive for opiates, barbiturates, amphetamines, cocaine, and/or benzodiazepines at screening; abnormal liver function test results (e.g. aspartate aminotransferase, alanine aminotransferase, total bilirubin >1.5 times the upper normal limit); low or high blood pressure [BP; systolic BP (SBP) ≤90 or ≥140 mmHg; diastolic BP (DBP) ≤60 or ≥95 mmHg]; abnormal creatinine clearance (<80 mL/min as calculated using the Cockcroft–Gault equation); and/or abnormal results on ECG, especially corrected QT (QTc) >450 ms. All laboratory tests were performed at the Department of Laboratory Medicine of Asan Medical Center, which is accredited by the Korean Association of Quality Assurance for Clinical Laboratories and certified by the College of American Pathologists. All volunteers provided written informed consent prior to any screening, and this trial was conducted in accordance with the Declaration of Helsinki and International Conference of Harmonization (ICH) guidelines for good clinical practice [23, 24]. The Institutional Review Board of Asan Medical Center approved the study protocol prior to the start of the trial (NCT01768455).
2.2 Study Design
This randomized, open-label, two-period, two-sequence crossover study was conducted at the Asan Medical Center (Seoul, Republic of Korea). Twenty-four volunteers were assigned to one of two sequence groups according to a randomization table that was generated using R version 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria). Subjects received gemigliptin 50 mg once daily for 6 days, followed by glimepiride that was co-administered on day 7 (treatment A); in the other period, a single 4-mg dose of glimepiride was administered (treatment B). For treatment B, participants were admitted to hospital on day −1 and discharged on day 2 after all blood samples were collected at 24 h postdose. After receiving glimepiride 4 mg on day 1, participants were seated on a bed at 45° for 4 h. Food was restricted for 1 h. Water was not allowed during the 1 h predose and 2 h after study drug administration.
For treatment A, subjects visited the hospital on days −1, 1, 2, 3, and 4, were admitted on day 5, and discharged on day 8. Participants received gemigliptin 50 mg once daily on an empty stomach on days 1–4, and then remained in hospital until 2 h after administration under the supervision of the medical staff, who assessed the occurrence of any AEs. Subjects were required to stay in a sitting position and could not eat any food until 1 h postdose. On the morning of day 5, subjects were admitted and administered gemigliptin. On day 6 (received gemigliptin) and day 7 (received gemigliptin + glimepiride), subjects were seated on the bed at 45° for 4 h and food was restricted for 1 h after drug administration. Water was not allowed for 1 h predose and 2 h after the administration of study drugs.
Throughout the entire study period, smoking, the ingestion of beverages containing caffeine or alcohol, and heavy exercise were not allowed. During the admission period, food was strictly controlled and standardized.
2.3 Blood Sample Collection
When receiving treatment B, blood samples (8 mL) were collected prior to and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 14, and 24 h after glimepiride dosing. When receiving treatment A, blood samples (8 mL) were collected predose, on day 5 at 0 h, on days 6 and 7 at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 14 h, and on day 8 at 0 h after 7-day repeated dosing. Samples were collected in heparinized tubes, and 1.5 mL blood was discarded before obtaining samples from an inserted angiocatheter. Plasma was extracted by centrifugation at 1,800 g for 8 min at 4 °C, and 0.5 mL was immediately transferred to two Eppendorf tubes and mixed by vortexing with 5 % formic acid (FA; 98 %) in 0.5 mL water. The remaining plasma was divided and 1 mL was transferred to two Eppendorf tubes. The four Eppendorf tubes containing plasma were frozen at −70 °C until they were shipped to the Chemical Structure Analysis Team of LG Life Sciences (Daejeon, Republic of Korea), where gemigliptin and glimepiride concentrations were assayed.
2.4 Bioanalytical Methods
2.4.1 Gemigliptin and LC15-0636 Analysis
Plasma concentrations of gemigliptin and its active metabolite (LC15-0636) were determined using a validated liquid chromatography–tandem mass spectrometry (LC–MS/MS) method (Chemical Structure Analysis Team, LG Life Sciences Ltd, Daejeon, Korea). An internal standard (IS) solution was prepared by dissolving LC15-0510 in 2 % FA/acetonitrile. An aliquot of 50 μL plasma and 100 μL IS solution were mixed, vortexed, and centrifuged in a precooled (4 °C) centrifuge for 5 min at 14,000 rpm. An aliquot of 100 μL supernatant was mixed with 100 μL water, vortexed, and centrifuged in a precooled (4 °C) centrifuge for 5 min at 14,000 rpm. 150 μL of each sample was injected into the LC–MS/MS system for analysis. The sample extracts were analyzed using high-performance liquid chromatography (HPLC) [Shiseido NASCA; Shiseido, Tokyo, Japan] and a Gemini C18 column (3 μm, 50.0 × 3.0 mm; Phenomenex, Torrance, CA, USA) under binary gradient mode [the mobile phase consisted of solvent A (water with 0.1 % FA) and solvent B (methanol with 0.1 % FA)]. The MS system was AB Sciex TQ 5500 (AB Sciex, Framingham, MA, USA) that was operated in positive electrospray ionization mode with multiple reaction monitoring (MRM). For gemigliptin and LC15-0636, the precursor-to-production reactions monitored were m/z 490.1 → 338.1 and 506.28 → 175.1, respectively. Calibration standards covered the theoretical concentration range of 0.5–200 ng/mL gemigliptin (R 2 > 0.996) and 0.5–100 ng/mL LC15-0636 (R 2 > 0.996). Using this assay, the accuracy of the calibration standard curve for gemigliptin was between 91.3 and 113.6 %, and the coefficient of variation (CV) of the back-calculated concentration was <6.2 %. The accuracy of the quality control (QC) samples for gemigliptin was between 103.2 and 105.6 %, with CVs between 6.0 and 6.5 %. The accuracy of the calibration standard curve for LC15–0636 was between 87.4 and 114.0 %, and the CV of the back-calculated concentration was <5.7 %. The accuracy of the QC samples for LC15-0636 was between 101.0 and 104.1 %, with CVs between 7.3 and 7.7 %.
The lower limit of quantifications (LLOQ) for gemigliptin and LC15-0636 were 0.5 ng/mL. All assays were conducted in a blinded manner in terms of treatment, sequence, and period.
2.4.2 Glimepiride Analysis
Plasma concentrations of glimepiride and its metabolite M1 were determined using LC–MS/MS. An IS solution (50 ng/mL) was prepared by dissolving glimepiride-d5 and trans-hydroxy glimepiride-d5 in methanol. A sample aliquot (50 μL) and aliquot of IS solution (150 μL) were mixed. The mixture was vortexed and then centrifuged in a precooled (4 °C) centrifuge for 5 min at 14,000 rpm. An aliquot of the supernatant (100 μL) was taken, mixed with 50 μL water, vortexed, and centrifuged at 14,000 rpm for 5 min at 4 °C. Five microliters of each sample was injected into the LC–MS/MS system for analysis. The sample extracts were analyzed using HPLC (Shimadzu Prominence, Shimadzu Scientific Instruments, Columbia, MD, USA; autosampler: Shiseido Z3133, Shiseido, Tokyo, Japan) over a Thermo Fisher Scientific Hypersil Gold column (5 μm, 100.0 × 2.1 mm; Thermo Fisher Scientific Inc, Waltham, MA, USA) in binary mode [the mobile phase consisted of solvent A (water with 0.1 % FA) and solvent B (methanol with 0.1 % FA)]. The MS system was an AB Sciex QTRAP 4000 (AB Sciex, Framingham, MA, USA) that was operated in positive electrospray ionization mode with MRM. For glimepiride and M1, the precursor-to-production reactions monitored were m/z 491.4 → 352.2 and 507.3 → 352.2, respectively. Calibration standards covered 1–200 ng/mL of the theoretical concentration range of glimepiride (R 2 > 0.996); 0.5–100 ng/mL of M1 (R 2 > 0.998). For glimepiride, the accuracy was between 97.5 and 102.0 %, and CV of the back-calculated concentration was <8.7 %. For the metabolite M1, the accuracy was between 98.7 and 101.2 %, and the CV of the back-calculated concentration was <7.6 %. The accuracy of the QC samples was between 97.2 and 100.4 %, with CVs of 5.5–8.2 % for glimepiride, while the accuracy of the QC samples was between 98.1 and 101.7 %, and the CVs were between 3.9 and 6.2 % for M1. LLOQ was 1 ng/mL for glimepiride and 0.5 ng/mL for M1. All assays were conducted in a blinded manner in terms of treatment, sequence, and period.
2.5 Pharmacokinetic Assessments
Pharmacokinetic parameters were determined using non-compartmental analysis (Phoenix WinNonlin, version 6.1; Pharsight, Mountain View, CA, USA). Only data from subjects who completed the entire sampling schedule were used; the actual sampling time points were applied to determine the pharmacokinetic parameters. During analysis, set the concentration below the LLOQ to the zero. Gemigliptin, LC15-0636, glimepiride, and M1 concentrations versus time profiles were plotted for each subject on linear and log-linear graphs. The C max and t max of gemigliptin, LC15-0636, glimepiride, and M1 were directly determined from the observed values, and the terminal elimination rate constants (λ z ) were estimated by linear regression of the log-linear decline of individual plasma concentration–time data. AUClast was obtained using the trapezoidal method (linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations), AUCinf was calculated as AUClast + C last/λ z , and t ½β was calculated as ln(2)/λ z [25]. To compare the pharmacokinetic profiles of gemigliptin and glimepiride when administered as monotherapy and combination therapy, log-transformed individual C max (C max,ss for gemigliptin) and AUC values (AUC τ,ss for gemigliptin; AUClast for glimepiride) were compared using mixed-effects model analysis of variance (SAS version 9.3, SAS Institute Inc., Cary, NC, USA; and R version 2.15.0, R Foundation for Statistical Computing, Vienna, Austria). Sequence, period, and treatment were considered fixed effects, and subjects were nested within the sequences as random effects. Treatment effects are presented as the ratios and 90 % CIs of the geometric means for the pharmacokinetic parameters of each drug during combination therapy and monotherapy. If the 90 % CI of the geometric mean ratio (GMR) for each treatment comparison was contained within the bioequivalence limits of 80.0–125.0 % for the primary pharmacokinetic parameters, no drug–drug interactions were pharmacologically indicated [26].
2.6 Tolerability Assessments
All subjects who received more than one dose of the study drug were included in the tolerability analyses. All AEs were noted regardless of the suspected relationship with the study drugs. All AEs were determined by unmasked investigators who assessed the investigators’ questions, observations, subjects’ spontaneous reports, and the severity, course, outcome, seriousness, and relationship with the study drugs. Vital signs, physical examinations, 12-lead ECG recordings, and clinical laboratory tests (e.g. hematology, biochemistry, urinalysis) were also included in the tolerability assessments. Vital signs were measured in the sitting position, and subjects rested ≥5 min before measurement. ECG was performed before blood sampling, and all laboratory test results were obtained after fasting for >8 h. If abnormal vital signs, ECGs, and/or clinical laboratory test results were observed, the investigators subsequently assessed the clinical significance and relationship to the study drug and considered further evaluation and/or treatments if needed.
3 Results
3.1 Demographics
A total of 27 healthy male volunteers were enrolled, and 23 volunteers were administered the study drugs and completed the study. Four subjects withdrew consent before administration. The mean [standard deviation (SD)] age of study participants was 29.3 (5.6) years, the mean (SD) height was 174.2 (4.7) cm, and the mean (SD) weight was 70.8 (7.8) kg. The baseline demographic characteristics of the sequence groups were similar across all groups (p > 0.05; Table 1). Because 23 subjects completed the study without protocol violation, all were included in the tolerability and pharmacokinetics assessments.
3.2 Pharmacokinetic Analysis
To evaluate the pharmacokinetic drug–drug interactions between gemigliptin and glimepiride, the pharmacokinetic properties of gemigliptin, glimepiride, LC15-0636 (gemigliptin metabolite), and M1 (glimepiride metabolite) were separately assessed. The mean plasma concentration profiles of gemigliptin, glimepiride, LC15-0636, and M1 following monotherapy or combination therapy are shown in Figs. 1 and 2, respectively. The mean pharmacokinetic properties are summarized in Table 2.
The mean (SD) C max,ss of gemigliptin was 80.17 (15.67) ng/mL, demonstrating a median (range) t max,ss value of 1.5 (0.5–6.0) h following repeated administration of gemigliptin only. The mean (SD) AUC τ,ss value was 797.93 (122.08) ng·h/mL, and t ½β was 8.77 (0.79) h. When gemigliptin was administered with glimepiride, the mean (SD) C max,ss value of gemigliptin was 81.37 (18.66) ng/mL, demonstrating a median (range) t max of 3.0 (0.5–5.0) h. The mean (SD) AUC τ,ss value was 799.26 (133.90) ng·h/mL, and t ½β was 10.45 (0.09) h. The mean (SD) C max of glimepiride was 227.05 (72.64) ng/mL, demonstrating a median (range) t max of 3.0 (2.0–5.0) h after the single administration of glimepiride. The mean (SD) AUClast value was 1,104.95 (365.00) ng·h/mL. When glimepiride was administered with gemigliptin, the mean (SD) C max value was 231.32 (71.58) ng/mL and demonstrated a median (range) t max value of 4.0 (2.0–5.0) h. The mean (SD) AUClast value was 1,086.49 (323.76) ng·h/mL.
The mean (SD) C max,ss values of LC15-0636 were 17.71 (4.45) and 17.83 (3.99) ng/mL after administering monotherapy and combined therapy, respectively. Median t max,ss values were 5.00 (range 1.0–12.0) and 4.00 (range 1.0–5.0) h, and the mean (SD) AUC τ,ss values were 233.32 (34.24) and 247.55 (36.35) ng·h/mL, respectively. The mean (SD) metabolic ratio (MR; calculated by dividing LC15-0636 AUC τ,ss by gemigliptin AUC τ,ss) was 0.31 (0.05) following the repeated dosing of gemigliptin only, while 0.30 (0.05) was calculated after combination gemigliptin and glimepiride dosing. The mean (SD) C max value of M1 was 28.26 (8.40) ng/mL, demonstrating a median (range) t max value of 4.0 (3.0–6.0) h following the single-dose administration of glimepiride. Mean (SD) AUClast was 189.88 (52.77) ng·h/mL. In comparison, the mean (SD) C max of M1 following combination glimepiride and gemigliptin therapy was 29.58 (8.23) ng/mL, demonstrating a median t max value of 4.0 (3.0–6.0) h. The mean (SD) AUClast value was 191.85 (46.85) ng·h/mL. The mean (SD) MR of M1 was 0.18 (0.03), regardless of gemigliptin administration.
The GMRs (combined/monotherapy) and 90 % CIs of the primary pharmacokinetic parameters for gemigliptin and glimepiride are shown in Table 3. For gemigliptin, the point estimates (PEs) (90 % CI) of the C max,ss and AUC τ,ss were 1.0097 (0.924–1.103) and 0.9997 (0.976–1.024), respectively. In the case of glimepiride, the PEs (90 % CI) of C max and AUClast were 1.031 (0.908–1.172) and 0.995 (0.902–1.097), respectively. Thus, all primary parameters were within the range of 0.8–1.25, suggesting no pharmacokinetic drug–drug interactions between gemigliptin and glimepiride.
3.3 Tolerability
No deaths, serious AEs, or AEs that resulted in premature discontinuation were reported. In total, eight AEs were experienced by 6 of 23 study participants (26.1 %). Among these, two AEs (excoriation and headache) occurred in two participants before administration of the study drug. The other six AEs occurred in four participants during repeated gemigliptin dosing. Of these, three AEs in three participants were considered possibly related to the study drug, including rhinorrhea, constipation, and headache. Other AEs were assessed as unlikely to be or unrelated to the study drugs. No severe AEs were reported, and participants spontaneously recovered without additional treatment (Table 4).
During the study period, no trends were seen in terms of the regularly measured vital signs. One subject instantly showed clinically significant decreased BP with dizziness right after venous catheter insertion for blood sampling, but his vital signs recovered in less than 5 min without treatment. Compared with baseline, no significant changes in vital signs were seen following the administration of either combination therapy or monotherapy. No clinically important changes in the laboratory test results were observed in any of the 23 participants, and no clinically significant ECG results were reported. Throughout the study, all subjects demonstrated normal findings on physical examination, except three participants who developed abnormal skin lesions (e.g. scar, discoloration, abrasion). All abnormal findings on physical examination were due to injuries before study drug administration, and these lesions demonstrated no changes, or partially recovered, by the end of the study period. Study drug administration did not seem to deteriorate or delay the recovery of the skin lesions. No subjects used any other concomitant medications for AEs or developed other clinically significant signs.
4 Discussion
Both the prevalence and incidence of T2DM have steadily increased worldwide [27]. Moreover, diabetes is a well-known major cause of heart disease, stroke, kidney failure, non-traumatic lower-limb amputation, and new cases of blindness among adults [28]. Previous studies have established that the risk of developing many of these vascular complications is related to hyperglycemia, which is the main target of diabetes therapy [29]. There are various oral antiglycemic agents that lower blood glucose by affecting various pathways in the complex pathogenesis of diabetes, and drug treatment should be determined after taking into account individual conditions and treatment goals. Most of these drugs can reduce hemoglobin A1c by 0.5–2.0 % as monotherapies, but many patients eventually require combination therapy [30, 31].
DPP-4 inhibitors demonstrate different target mechanisms than sulfonylureas, and combination treatment not only improves glycemic control but minimizes the weaknesses of each drug. Previous studies report the effects of combination treatment without significant increases in the risk of AEs such as hypoglycemia [32, 33]. A recent study reports the efficacy on glucose fluctuation when added to DPP-4 inhibitors and administered to patients receiving ongoing sulfonylurea-based therapy [34].
Glimepiride is one of the most commonly used sulfonylureas due to its convenient once-daily dosing regimen and tissue selectivity. Although some potential interactions with glimepiride have been predicted, such as some drugs that are metabolized by CYP2C9 (e.g. phenytoin, diclofenac, naproxen) and protein-binding drugs (e.g. sulfonamides, probenecid, β-blocking agents), no clinically significant drug interactions have been reported [22].
Theoretically, gemigliptin could also be administered with glimepiride, but there are no reported interactions between these drugs. Therefore, this study was conducted to assess the pharmacokinetic interactions and tolerability of gemigliptin and glimepiride when administered in combination to healthy volunteers. It is unlikely that pharmacokinetic interactions occur between these two drugs because it is known that gemigliptin demonstrates no significant effects on cytochromes, operates via different metabolic pathways, and demonstrates no strong protein-binding characteristics, but clinically confirming this lack of interactions is important given the fact that combination therapy might help some patients.
In this study, glimepiride demonstrated no pharmacokinetic effects on steady-state gemigliptin, nor did gemigliptin affect the pharmacokinetics of single-dose glimepiride. Also, the time to maximum concentration and the half-life of the combination therapies were comparable to each monotherapy. In the case of gemigliptin, the half-life was somewhat shorter than previously reported by multiple-dose studies (16.6–20.1 h); we determined a mean value of 8.77 h for monotherapy and 10.45 h for combination therapy. However, as mentioned in the previous studies, differences in sampling time affected this value; in this study, blood sampling was performed ≤24 h after the last dose, but previous studies obtained blood samples ≤72 h after the last dose. In fact, day 1 of a previous study using 24 h sampling to calculate half-life showed similar (7.4–9.3 h) results to our study [16]. Therefore, terminal half-life calculated in this study could be somewhat biased. Because the pharmacokinetic profile of each drug is well known, and we should consider the safety concerns of blood sampling from healthy volunteers, we planned to obtain the minimum number of samples required to evaluate pharmacokinetic interactions. Therefore, blood sampling was limited to the dosing interval (24 h). Although this design is sufficient for assessing main pharmacokinetic parameters (AUC and C max) to compare (Table 5), data from some participants were insufficient for calculating the terminal slope of the concentration–time plot. The mean pharmacokinetic values related to the terminal slope (AUCinf and t ½β) were therefore excluded because some participants demonstrated %AUCextrapolation >20 % (% of extrapolation part of AUCinf); in particular, only two subjects could be included for calculating half-life in the gemigliptin + glimepiride treatment group, and most subjects were excluded by this extrapolation (Table 2). Moreover, from this study, there might be a difference in the half-life of gemigliptin between treatment groups because almost all subjects were excluded from the analysis of the half-life in the combination group compared with the monotherapy group. However, pharmacokinetic comparisons between treatment groups were based on AUC τ,ss (gemigliptin) or AUClast (glimepiride) and C max by protocol, and which values were calculated only observed data, not extrapolated. Therefore, further evaluation would be needed to obtain accurate pharmacokinetic parameters of gemigliptin related to the AUCinf and apparent terminal half-life.
The MRs of LC15-0636 to gemigliptin are also similar to previously reported MR values (0.27 ± 0.10; Gemigliptin IB version 6.0, September 2012). As expected, glimepiride did not seem to affect the production of gemigliptin metabolites. Similarly, the MRs of M1 were the same (0.18 ± 0.03), regardless of the coadministration of gemigliptin. A previous study indicated that M1 is mainly formed by CYP2C9, and there are a number of reported genetic variants of CYP2C9. Among these, the CYP2C9*2 and 3 alleles are known to markedly reduce the metabolism of glimepiride [35, 36]. The CYP2C9 polymorphism also demonstrates inter-ethnic differences. Among Caucasians, CYP2C9*2 demonstrates an allele frequency of 10–19 %, but is rare among East Asians [37]. The CYP2C9*3 heterozygous allele is only found in East Asians at a frequency of 1–6 % [38, 39]. This might be part of the reason for the differences in the pharmacokinetic values of glimepiride between previous studies and our own. Malerczyk et al. reported the pharmacokinetic parameters for glimepiride following the single-dose administration of 4 mg to healthy volunteers: mean C max of 307.8 μg/L and mean AUC of 1,297 μg/L · h for glimepiride, which were slightly higher than the results of our present study. Another study reported a geometric C max mean of 1,084 ng/mL and AUClast of 8,753 ng · h/mL, and the subjects were all Caucasian [20, 40]. Because the participants in this study were all Korean, most were expected to express the CYP2C9*1 allele, but we did not evaluate genotypes. Hence, differences between genotypes should be further evaluated. However, this is a crossover study, and the finding that glimepiride did not change due to gemigliptin administration is still valid even without genotype testing.
Up to 8 mg/day of glimepiride can be administered, but the usual maintenance dose is 1–4 mg once daily. Clinically, when treatment failure occurs following the administration of 4 mg, other treatments such as insulin or combination therapy should be considered [22, 41]. Accordingly, the single-dose administration of glimepiride 4 mg was evaluated in this study. This is somewhat reasonable in terms of safety considering the fact that the participants were healthy volunteers who could also experience hypoglycemic symptoms. Since both gemigliptin and glimepiride do not seem to induce or inhibit CYP enzymes, repeated dosing regimens that evaluate interactions might not be significantly essential. However, gemigliptin demonstrates a relatively long half-life (approximately 17 h), and accumulation was reported in a previous multiple-dose study [42]. Meanwhile, glimepiride demonstrates a short half-life (<5 h) without accumulation after multiple dosing [22]. Therefore, this study was designed to evaluate the pharmacokinetic interactions of steady-state gemigliptin and single-dose glimepiride. A similar study on sitagliptin and glyburide was also previously reported, and this study concluded that sitagliptin does not affect the pharmacokinetics of glyburide [43]. However, that study did not assess the effects of sulfonylurea on the pharmacokinetics of DPP-4 inhibitors. Also, according to another study on linagliptin (5 mg/day × 6 days) and glyburide (single-dose 1.75 mg), the pharmacokinetics of linagliptin are not affected, whereas exposure to glyburide is slightly reduced by coadministration with linagliptin [44]. Compared with these results, our study indicates that neither gemigliptin nor glimepiride alters pharmacokinetic characteristics when administered in combination.
Although this study assessed healthy volunteers, all participants tolerated treatment throughout the study period. No serious AEs were reported, and no hypoglycemic symptoms developed during the study. One participant experienced short-term dizziness, but his blood sugar level was considered normal (86 mg/dL). Symptoms occurred prior to administration and right after venous catheter reinsertion, and naturally disappeared after <5 min. Serial laboratory tests, including glucose level, were also stable; no clinically significant trends were observed throughout the study. Considering that hypoglycemic events could present in healthy people receiving antidiabetic agents, the results of this study show that adding gemigliptin to glimepiride might not increase hypoglycemic risk.
This study has some limitations. First, some pharmacokinetic parameters of gemigliptin related to the terminal slope (i.e. terminal half-life and AUCinf) could not be calculated precisely because only 24-h blood samplings after administration were conducted. Also, because the dosing duration of this study was short and only healthy volunteers were included, further evaluation of long-term tolerability in T2DM patients is needed.
5 Conclusions
A combination treatment with gemigliptin and glimepiride demonstrates no clinically relevant pharmacokinetic interactions in healthy volunteers. In addition, both agents are generally well tolerated. Accordingly, these two drugs could be safely administered together, and it is expected that they would demonstrate similar pharmacokinetic characteristics compared with the monotherapy of each drug.
References
Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60:1–23. doi:10.1016/j.metabol.2010.09.010.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. pii: S0140673698070196.
Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12 pii: joc72221.
Kramer W, Muller G, Geisen K. Characterization of the molecular mode of action of the sulfonylurea, glimepiride, at beta-cells. Horm Metab Res. 1996;28:464–8. doi:10.1055/s-2007-979838.
Bell DS, Ovalle F. How long can insulin therapy be avoided in the patient with type 2 diabetes mellitus by use of a combination of metformin and a sulfonylurea? Endocr Pract. 2000;6:293–5 pii: ep99064.or.
DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303 pii: 199908170-00008.
Erle G, Lovise S, Stocchiero C, Lora L, Coppini A, Marchetti P, Merante D. A comparison of preconstituted, fixed combinations of low-dose glyburide plus metformin versus high-dose glyburide alone in the treatment of type 2 diabetic patients. Acta Diabetol. 1999;36:61–5 pii: 90360061.592.
Tosi F, Muggeo M, Brun E, Spiazzi G, Perobelli L, Zanolin E, Gori M, Coppini A, Moghetti P. Combination treatment with metformin and glibenclamide versus single-drug therapies in type 2 diabetes mellitus: a randomized, double-blind, comparative study. Metabolism. 2003;52:862–7 pii: S002604950300101X.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi:10.1016/S0140-6736(06)69705-5.
Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diab Vasc Dis Res. 2006;3:159–65. doi:10.3132/dvdr.2006.024.
Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi:10.1210/er.2007-0015.
Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–58. doi:10.1210/en.2003-0323.
Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjornsdottir S, Camisasca RP, Couturier A, Baron MA. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–56. doi:10.1111/j.1463-1326.2008.00859.x.
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–45. doi:10.1111/j.1463-1326.2007.00744.x.
Yang SJ, Min KW, Gupta SK, Park JY, Shivane VK, Pitale SU, Agarwal PK, Sosale A, Gandhi P, Dharmalingam M, Mohan V, Mahesh U, Kim DM, Kim YS, Kim JA, Kim PK, Baik SH. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:410–6. doi:10.1111/dom.12042.
Lim KS, Kim JR, Choi YJ, Shin KH, Kim KP, Hong JH, Cho JY, Shin HS, Yu KS, Shin SG, Kwon OH, Hwang DM, Kim JA, Jang IJ. Pharmacokinetics, pharmacodynamics, and tolerability of the dipeptidyl peptidase IV inhibitor LC15-0444 in healthy Korean men: a dose-block-randomized, double-blind, placebo-controlled, ascending single-dose, phase I study. Clin Ther. 2008;30:1817–30. doi:10.1016/j.clinthera.2008.10.013.
Rhee EJ, Lee WY, Min KW, Shivane VK, Sosale AR, Jang HC, Chung CH, Nam-Goong IS, Kim JA, Kim SW. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes Metab. 2013;15:523–30. doi:10.1111/dom.12060.
Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28:1352–61. doi:10.1111/j.1464-5491.2011.03387.x.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79. doi:10.2337/dc12-0413dc12-0413.
Malerczyk V, Badian M, Korn A, Lehr KH, Waldhausl W. Dose linearity assessment of glimepiride (Amaryl) tablets in healthy volunteers. Drug Metabol Drug Interact. 1994;11:341–57.
Rosenkranz B. Pharmacokinetic basis for the safety of glimepiride in risk groups of NIDDM patients. Horm Metab Res. 1996;28:434–9. doi:10.1055/s-2007-979833.
Sanofi-aventis (2013). AMARYL (glimepiride) tablets. FDA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020496s027lbl.pdf. Accessed 3 Dec 2013.
Declaration of Helsinki. Ethical principles for medical research involving human subjects. World Medical Association; 2008. http://www.wma.net/en/30publications/10policies/b3/17c.pdf. Accessed 3 Dec 2013.
ICH. Guideline for Good Clinical Practice E6 (R1). ICH Harmonised Tripartite Guideline; 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf. Accessed 2 Dec 2013.
Rowland M, Tozer T. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. Philadelphia: Lippincott Williams & Wilkins; 2011.
FDA. Guidance for Industry. Drug interaction studies: study design, data analysis, implications for dosing, and labeling recommendations. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf. Accessed 3 Dec 2013.
Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. 2012;8:228–36. doi:10.1038/nrendo.2011.183nrendo.2011.183.
CDC. National diabetes fact sheet. US Department of Health and Human Services; 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi:10.2337/dc08-9025.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–31. doi:10.2337/dc09-9029.
Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395–406. doi:10.1111/j.1742-1241.2009.02143.x.
El-Ouaghlidi A, Rehring E, Holst JJ, Schweizer A, Foley J, Holmes D, Nauck MA. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab. 2007;92:4165–71. doi:10.1210/jc.2006-1932.
Yoshioka K. Efficacy of initial basal-supported oral therapy with sitagliptin in untreated type 2 diabetes. Diabetes Ther. 2013;. doi:10.1007/s13300-013-0043-x.
Niemi M, Cascorbi I, Timm R, Kroemer HK, Neuvonen PJ, Kivisto KT. Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin Pharmacol Ther. 2002;72:326–32. doi:10.1067/mcp.2002.127495.
Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12:251–63.
Zainuddin Z, Teh LK, Suhaimi AW, Salleh MZ, Ismail R. A simple method for the detection of CYP2C9 polymorphisms: nested allele-specific multiplex polymerase chain reaction. Clin Chim Acta. 2003;336:97–102 pii: S000989810300319X.
Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Park YS, Lee SY. Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol. 2005;60:418–22. doi:10.1111/j.1365-2125.2005.02448.x.
Myrand SP, Sekiguchi K, Man MZ, Lin X, Tzeng RY, Teng CH, Hee B, Garrett M, Kikkawa H, Lin CY, Eddy SM, Dostalik J, Mount J, Azuma J, Fujio Y, Jang IJ, Shin SG, Bleavins MR, Williams JA, Paulauskis JD, Wilner KD. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther. 2008;84:347–61. doi:10.1038/sj.clpt.61004826100482.
Kasichayanula S, Liu X, Shyu WC, Zhang W, Pfister M, Griffen SC, Li T, LaCreta FP, Boulton DW. Lack of pharmacokinetic interaction between dapagliflozin, a novel sodium-glucose transporter 2 inhibitor, and metformin, pioglitazone, glimepiride or sitagliptin in healthy subjects. Diabetes Obes Metab. 2011;13:47–54. doi:10.1111/j.1463-1326.2010.01314.x.
HanDok Amaryl Tab 4 mg (Glimepiride) label. Korean Pharmaceutical Information Center. http://www.health.kr/images/insert_pdf/IN_A11AGGGGA5812_00.pdf. Accessed 3 Dec 2013.
Lim KS, Cho JY, Kim BH, Kim JR, Kim HS, Kim DK, Kim SH, Yim HJ, Lee SH, Shin SG, Jang IJ, Yu KS. Pharmacokinetics and pharmacodynamics of LC15-0444, a novel dipeptidyl peptidase IV inhibitor, after multiple dosing in healthy volunteers. Br J Clin Pharmacol. 2009;68:883–90. doi:10.1111/j.1365-2125.2009.03376.xBCP3376.
Mistry GC, Bergman AJ, Zheng W, Hreniuk D, Zinny MA, Gottesdiener KM, Wagner JA, Herman GA, Ruddy M. Sitagliptin, an dipeptidyl peptidase-4 inhibitor, does not alter the pharmacokinetics of the sulphonylurea, glyburide, in healthy subjects. Br J Clin Pharmacol. 2008;66:36–42. doi:10.1111/j.1365-2125.2008.03148.xBCP3148.
Graefe-Mody U, Rose P, Ring A, Zander K, Iovino M, Woerle HJ. Assessment of the pharmacokinetic interaction between the novel DPP-4 inhibitor linagliptin and a sulfonylurea, glyburide, in healthy subjects. Drug Metab Pharmacokinet. 2011;26:123–9 pii: JST.JSTAGE/dmpk/DMPK-10-RG-091.
Acknowledgments
This study was funded by LG Life Sciences Ltd (Seoul, Republic of Korea), the manufacturer of gemigliptin. This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. HI07C0001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Choi, H.Y., Kim, Y.H., Kim, M.J. et al. Evaluation of Pharmacokinetic Drug Interactions Between Gemigliptin (Dipeptidylpeptidase-4 Inhibitor) and Glimepiride (Sulfonylurea) in Healthy Volunteers. Drugs R D 14, 165–176 (2014). https://doi.org/10.1007/s40268-014-0054-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-014-0054-8