Abstract
Background
Doxylamine succinate, an ethanolamine-based antihistamine, is used in the short-term management of insomnia because of its sedative effects. No data on the dose proportionality of the pharmacokinetics of doxylamine are available, although this drug has been marketed in European countries for more than 50 years.
Objective
The objective of this study was to evaluate and compare the dose proportionality between two marketed strengths (12.5 mg and 25 mg) of doxylamine hydrogen succinate after a single oral dose administration under fasting conditions in healthy human subjects.
Study Design
This was a single-center, randomized, single dose, laboratory-blinded, two-period, two-sequence, crossover study.
Setting
The study was conducted in a phase I clinical unit.
Subjects and Methods
A single oral dose of doxylamine hydrogen succinate of 12.5 mg (equivalent to 8.7 mg of doxylamine base) or 25 mg (equivalent to 17.4 mg of doxylamine base) was administered to healthy volunteers under fasting conditions in each study period. The drug administrations were separated by a wash-out period of 7 calendar days. Blood samples were collected for up to 60 h post-dose, and plasma doxylamine levels were determined by an ultra high-performance liquid chromatography method with tandem mass spectrometry detection. Pharmacokinetic parameters were calculated using non-compartmental analysis. Dose proportionality was assessed based on the parameter area under the concentration–time curve (AUC t normalized). Safety was evaluated through assessment of adverse events, standard laboratory evaluations, vital signs and 12-lead electrocardiogram (ECG).
Results
In total, 12 healthy volunteers (3 male; 9 female) were included in the study. Mean maximum observed plasma concentration (C max) and area under the concentration–time curve from time zero to time t (AUC t ) of doxylamine hydrogen succinate 12.5 mg and 25 mg tablets increased linearly and dose-dependently [12.5 mg: mean C max 61.94 ng/mL, coefficient of variation (CV) 23.2 %; mean AUC t 817.33 ng·h/mL, CV 27.4 %; and 25 mg: mean C max 124.91 ng/mL, CV 18.7 %; mean AUC t 1630.85 ng·h/mL, CV 22.8 %]. Mean AUC t normalized was 815.43 ng·h/mL, CV 22.8 % for 25 mg. The dose-normalized geometric mean ratio (%, 12.5 mg/25 mg) of AUC t was 98.92 (90 % CI: 92.46, 105.83). The most common adverse event was somnolence.
Conclusions
Exposure to doxylamine was proportional over the therapeutic dose range of 12.5–25 mg in healthy volunteers. Based on the results, a predictable and linear increase in systemic exposure can be expected. Doxylamine hydrogen succinate was safe and well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Doxylamine succinate, an ethanolamine-based antihistamine, shares the actions and uses of other antihistamines. Because of its sedative effect, doxylamine medicinal products (alone or in combination with other drugs) have been authorized for more than 50 years with an appropriate extent of use for short-term management of insomnia [1–5]. Currently, it is a medical product with a legal base of well-established use in Europe.
Based on clinical practice, the recommended adult dose for doxylamine hydrogen succinate as a nighttime sleep aid is 25 mg, once daily, taken orally up to half an hour before bedtime. If drowsiness is excessive, the dosage should be reduced to 12.5 mg. Doses higher than 25 mg are not recommended. Dormidina® has been marketed in Spain since 1990 with a unique active ingredient: doxylamine hydrogen succinate, 12.5 mg or 25 mg. Because its marketing authorization was approved before the implementation of the present regulatory standards, a new pharmacokinetic study of doxylamine hydrogen succinate in its current pharmaceutical presentation (film-coated tablets) has been recently published [6]. This study provides updated data on the pharmacokinetic parameters of doxylamine following a 25 mg dose in both fasting and fed conditions. The results indicate that the kinetic parameters of doxylamine were not affected by a high-fat, high-calorie food intake, and the drug was safe and well tolerated by the subjects. Furthermore, no differences between genders were observed [6].
No data on the dose proportionality of doxylamine were available. Therefore, the main objective of this study was to evaluate and compare the bioavailability with regard to dose proportionality between the two marketed strengths (12.5 mg and 25 mg) of doxylamine hydrogen succinate film-coated tablets after a single oral dose administration under fasting conditions in healthy subjects.
2 Subjects and Methods
2.1 Study Design
This was a single-center, randomized, single-dose, laboratory-blinded, two-period, two-sequence, crossover study. A single oral dose of doxylamine hydrogen succinate, 12.5 mg (Dormidina®, equivalent to 8.7 mg of doxylamine base) or 25 mg (Dormidina®, equivalent to 17.4 mg of doxylamine base), was administered under fasting conditions in each study period. Since the Physician’s Desk Reference rates doxylamine as being in pregnancy category B, it was acceptable to include women in the present study. To ensure that no carryover effect was observed, a wash-out period of 7 calendar days was observed between drug administrations, corresponding to more than 10 times the expected half-life of the moiety to be measured. It should be noted that the randomization code was not made available to the personnel in charge of the determination of plasma drug concentrations (Bioanalytical and Development ADME Department, Laboratorios del Dr. Esteve, S.A., Barcelona-Catalonia) until results were audited by the quality assurance department.
The protocol and the informed consent forms were approved by an independent review board (ETHIPRO) on 27 September 2012. All subjects voluntarily agreed to participate in this study and signed the informed consent form after having fully comprehended its contents and prior to initiation of study procedures. This study was performed in compliance with Good Clinical Practice [7].
2.2 Study Population
Subject screening procedures included informed consent, inclusion/exclusion check, demography, medical history, medication history, physical examination, height, weight, body mass index and a concomitant medication check. Subjects were in good health as determined by a medical history, physical examination (including vital signs), electrocardiogram (12-lead ECG) and the usual clinical laboratory tests (hematology, biochemistry, urinalysis) including negative HIV, hepatitis B and hepatitis C tests, negative screening for ethanol and drugs of abuse in urine and negative pregnancy test (for female subjects).
All participating subjects were judged to be eligible for the study when assessed against the inclusion and exclusion criteria. Tolerability and safety were evaluated through assessment of adverse events (AEs), standard laboratory evaluations and vital signs.
The predetermined reason for removing subjects from the study was for any safety issues as determined by the investigator. Also, subjects could be withdrawn because of protocol violations, administrative problems, difficulties in blood collection, occurrence of emesis during the time interval described in the protocol or other reasons described in the protocol. Furthermore, subjects were allowed to discontinue their participation in the study at any time. In the event of such a discontinuation, the subject was requested to complete a safety assessment at the time of discontinuation, and the reason for discontinuation was to be documented.
2.3 Blood Sample Collection; Method of Measurement
Blood samples were collected into tubes containing K2-EDTA prior to and 0.33, 0.67, 1, 1.33, 1.67, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 16, 24, 36, 48 and 60 h after drug administration. This sampling was planned in order to provide a reliable estimate of the extent of absorption, as well as the terminal elimination half-life, and to ensure that the area under the plasma concentration–time curve (AUC) from time zero to time t (AUC t ) was at least 80 % of the AUC from time zero extrapolated to infinity (AUC ∞ ). Samples were processed and stored under conditions (frozen) that have been shown not to cause significant degradation of the analyte.
The experimental samples were assayed for doxylamine, using a validated bioanalytical ultra-high-performance liquid chromatography method with tandem mass spectrometry detection (UPLC/MS/MS method, Xevo TQ MS, Waters Corp., Milford MA), which involved the solid-phase extraction of doxylamine and the deuterium-labeled internal standard (Doxylamine-d5) from plasma samples (150 μL). The calibration curve ranged from 1.0 to 300.0 ng/mL and the limit of quantification was 1.0 ng/mL. A gradient elution with 0.1 % formic acid in acetonitrile and 0.1 % formic acid in water was used for the mobile phase. A volume of 10 μL was injected into an Acquity UPLC BEH C18 column (1.7 μm particle size, 2.1 mm id × 50 mm length) and the transitions (m/z) for both doxylamine (271.22/167.02) and internal standard (276.24/171.28) were monitored using MRM ion mode ESI+.
The parameters evaluated during the validation were linearity and range, selectivity including hemolysed and hyperlipidemic plasma, specificity in the presence of common OTC, intra- and inter-run precision and accuracy, limit of quantification, dilution integrity, carryover, recovery, matrix effect, stock solution stability, autosampler stability, short-term stability in human plasma at room temperature, freeze-thaw and long-term stability in human plasma. All the evaluated parameters met the acceptance criteria of the current guidelines.
For past analytical batches run during the validation, the precision expressed as %CV of calibration standards ranged from 0.8 to 3.7 %, and the % mean accuracy of the back-calculated value of the calibration standards ranged from 94.2 to 103.4 %. The mean correlation coefficient for these analytical batches was 0.9992.
The intra-run precision expressed as %CV for all concentration levels of quality control samples ranged from 0.9 to 12.7 %, and the inter-run precision ranged from 1.1 to 7.9 %. The intra-run accuracy expressed as % nominal for all concentration levels of quality control samples ranged from 96.4 to 113.7 %, and the inter-run accuracy ranged from 102.8 to 108.8 %.
Each batch of test samples was analyzed together with a set of calibration standards and quality control samples. Quality control samples were prepared in blank plasma at low, medium and high concentration of the calibration curve. Acceptance criteria based on current guidelines were used for each analytical batch. Batches not meeting these acceptance criteria were rejected and the samples repeated.
2.4 Treatments Schedule
Subjects received the investigational products—doxylamine hydrogen succinate 12.5 mg (Dormidina® 12.5-mg film-coated tablets, Laboratorios del Dr. Esteve, S.A, Barcelona, Spain) or doxylamine hydrogen succinate 25 mg (Dormidina® 25-mg film-coated tablets, Laboratorios del Dr. Esteve, S.A, Barcelona, Spain)—at each period of the study under fasting conditions according to the randomization list. The randomization scheme was computer generated.
Food was controlled and standardized during the housing period and for all subjects. Subjects fasted overnight for at least 10 h prior to drug administration. A single dose of the Investigational Product was thereafter administered orally with approximately 240 mL of water at ambient temperature. Fasting continued for at least 4 h following drug administration, after which a standardized lunch was served. A supper and a light snack were also served at appropriate times thereafter, but not before 9 h after dosing. Water was allowed ad libitum until 1 h pre-dose and beginning 1 h from drug administration.
2.5 Statistical Analysis
2.5.1 Sample Size
Based on the result of a previous study, the intra-subject variability of AUC t for this product is around 6.2 % [6]. Assuming the expected geometric mean ratio of dose-normalized AUC t is within 95–115 %, to meet the 80–125 % bioequivalence range with a statistical power of at least 80 %, it is estimated that the minimum number of subjects required is 6. On the other hand, the minimum number of subjects for a standard bioequivalence study according to EMA’s guideline is 12. Therefore, it should be sufficient for this study to include 12 healthy volunteers.
2.5.2 Statistical Comparison
Descriptive statistics were used to summarize adverse events, safety results and demographic variables (age, height, weight and BMI). Pharmacokinetic parameters such as C max, the time to reach C max (t max), AUC t , AUC ∞ , AUC t :AUC ∞ , the elimination rate constant (k e) and elimination half-life (t ½) were calculated for each strength tested.
According to EMA’s Guideline on the Investigation of Bioequivalence [8], dose proportionality in terms of extent of exposure was assessed based on the parameter AUC t normalized (i.e. dose-adjusted AUC t ). Moreover, dose proportionality in terms of rate of exposure was also assessed using the parameter C max normalized. The natural logarithmic transformation of AUC t was used for all statistical inference using an Analysis of Variance (ANOVA) model. The fixed factors included in this model were the subject effect (nested within sequence), the treatment received, the period at which it was given as well as the sequence in which each treatment was received. Proportionality was assumed if the 90 % confidence interval of the dose-normalized geometric mean ratio of AUC t was within the 80.00 to 125.00 % range.
The main absorption and disposition parameters [C max (-t max-), AUC t , AUC ∞ , k e and t ½] were estimated using a non-compartmental approach with a log-linear terminal phase assumption. The trapezoidal rule was used to estimate the area under the concentration–time curve, and the terminal phase was estimated by maximizing the coefficient of determination estimated from the log-linear regression model. They were not to be estimated for individual concentration–time profiles, where the terminal log-linear phase could not be reliably characterized. Furthermore, the mean, median, minimal value, maximal value, standard deviation and coefficient of variation were calculated for plasma concentrations at each individual timepoint and for all pharmacokinetic parameters. Between-treatment comparisons were performed using the ANOVA model mentioned above for all parameters except t max, which was analyzed using a non-parametric approach.
Statistical and pharmacokinetic analyses were generated using Kinetic (version 9.01), an application developed at Algorithme Pharma and SAS® (version 9, GLM procedure).
3 Results
3.1 Subject Recruitment
A total of 12 healthy volunteers were included (3 male, 9 female), with a median age of 43 years (range 28, 58), weight of 66.1 kg (range 51.6, 96.3), height of 167 cm (range 157, 184) and body mass index of 24.0 kg/m2 (range 20.2, 28.4). All (100 %) subjects were white, and all of them completed the crossover design and received a single oral dose of the assigned treatment on day 1 and day 8.
3.2 Treatment Compliance
All subjects took the study medication according to the protocol. The investigational product was administered under the supervision of the qualified investigator or his designees. The film-coated tablet was to be swallowed whole and was not to be chewed or broken. Following administration of the drug, each subject’s hands and mouth were checked in order to confirm the consumption of the medication. The physician in charge remained at the clinical site for at least the first 4 h following each drug administration and remained available at all times during the entire period of the study.
3.3 Pharmacokinetic Assessments
Table 1 depicts the doxylamine pharmacokinetic results: C max, t max, AUC t , AUC t normalized, AUC ∞ , AUC t :AUC ∞ , k e and t ½ for both strengths of doxylamine hydrogen succinate, and Table 2 shows the comparison results with standards for bioequivalence. Proportionality was assumed given that the 90 % confidence interval of the dose-normalized geometric mean ratio of AUC t was within the 80.00 to 125.00 % range [98.92 % (90 % CI: 92.46, 105.83)]. No statistically significant between-treatment differences were observed for the following parameters: AUC t normalized, ln(AUC t normalized), AUC t : AUC∞, t max, k e and t ½. Likewise, C max normalized was also calculated, and the ratio between normalized doses was 101.45 (90 % CI: 96.17–107.01).
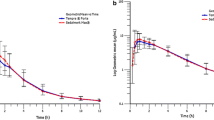
Figure 1 shows the linear profile of the mean ± standard deviation (SD) plasma concentrations of doxylamine.
3.4 Tolerability and Safety
No deaths or serious AEs were reported during this study. Eight (67 %) of the 12 subjects included in the study experienced a total of 13 AEs. Nervous System Disorders (69 %) was the most commonly reported of the System Organ Classes (SOCs).
After the administration of doxylamine hydrogen succinate 12.5 mg, three subjects (25 %) reported five AEs [2 different SOCs and 3 different MedDRA Preferred Terms (PTs)]; after the administration of doxylamine hydrogen succinate 25 mg, seven subjects (58 %) reported eight AEs (2 different SOCs and 3 different MedDRA PTs). The adverse events reported during the study were all of mild severity. No moderate or severe adverse events were observed during the study.
The most commonly reported AE of this study was somnolence. Of the 13 AEs reported during the study, 6 subjects reported 8 occurrences of somnolence (62 %, 8/13): 2 subjects reported 2 occurrences following the administration of doxylamine hydrogen succinate 12.5 mg (17 %, 2/12) and 6 subjects reported 6 occurrences following the administration of doxylamine hydrogen succinate 25 mg (50 %, 6/12), p = 0.083. The two subjects who presented somnolence with the 12.5-mg dose also reported the event with the 25-mg dose.
No significant alterations were found in the laboratory evaluations and the electrocardiogram repeated at the end of the study.
4 Discussion
This is the first time that the dose proportionality of the pharmacokinetics of doxylamine hydrogen succinate in film-coated tablets has been studied. According to EMA’s Guideline on the Investigation of Bioequivalence [8], dose proportionality is to be assessed based on the AUC t parameter. The results of this study showed that the 90 % confidence interval of the dose-normalized geometric mean ratio of AUC t was within the range of 80 to 125 %. Consequently, this result indicates that the two strength formulations of doxylamine hydrogen succinate [12.5 mg (Dormidina® 12.5 mg film-coated tablets) and 25 mg (Dormidina® 25 mg film-coated tablets) exhibited linear pharmacokinetics and that 12.5 mg and 25 mg of doxylamine hydrogen succinate were dose proportional in healthy subjects. Likewise, the pharmacokinetics of doxylamine show relatively low intra-subject variability.
Updated data on the pharmacokinetic profile of doxylamine in humans after an oral dose of doxylamine hydrogen succinate 25 mg in film-coated tablets have recently been published [6]. As expected, the pharmacokinetic parameters after an oral dose of doxylamine hydrogen succinate 25 mg obtained in the present study were comparable to the ones in the abovementioned study [6]. Likewise, the overall results of this study are in line with studies performed with oral doses of 25-mg doxylamine succinate tablets [5, 9, 10] and with oral doses of 20-mg doxylamine succinate solution [11, 12].
Doxylamine hydrogen succinate is available as an over-the-counter agent and is indicated for the symptomatic treatment of occasional insomnia in adults of 18 years of age and over. Overall, the two formulations tested (12.5- and 25-mg film-coated tablets) in this study were generally safe and well tolerated. It should be noted that most of the subjects reported somnolence mainly when administered the 25 mg strength. In fact, 50 % (6 out of 12) of the subjects presented somnolence when administered the 25-mg dose, but only 17 % (2 out of 12) with 12.5 mg. It is to be note that the two subjects who presented somnolence with the 12.5-mg strength also reported somnolence with the 25-mg dose. Actually, in the case of doxylamine, somnolence has to be considered as a pharmacodynamic effect associated with clinical efficacy in the short-term management of insomnia. Although this study was not designed to study the dose-proportional effect of doxylamine on somnolence, this result may suggest it. In clinical practice, the usual adult dose as nighttime sleep aid is 25 mg once daily, taken 30 min before bedtime. In fact, in clinical practice, the preponderance of side effects associated with this dose is related to a carryover to the next day of the hypnotic effects [13, 14]. This may be experienced primarily as continued drowsiness, tiredness or grogginess, “hangover” effect, sluggishness or lethargy. Therefore, given that the two strength formulations (12.5 mg and 25 mg) exhibited linear pharmacokinetics and since these adverse events may be dose-dependent, in case of presenting these symptoms, it is advisable to reduce the dose to 12.5 mg or to take the dose earlier (more than 30 min) to ensure at least an 8-h elapsed time before awaking.
Certain aspects of the study design should be considered before drawing conclusions for future users of doxylamine hydrogen succinate, as the open-label, single-dose design and the fact that the study population consisted of healthy subjects could lead to under- or overestimation of the generalizability of the results beyond the population and conditions that were studied. Likewise, the criteria used to assess dose proportionality (only 2 strengths were tested to study the dose-proportionality) could also lead to under- or overestimation of the generalizability of the results. Nevertheless, these two doses (12.5 mg and 25 mg of doxylamine hydrogen succinate) represent the two approved formulations commonly used in Spain.
5 Conclusion
Exposure to doxylamine was proportional over the therapeutic dose range of 12.5–25 mg in healthy volunteers with a dose proportional increase in the overall amount of doxylamine and its maximum concentration achieved. The time to peak concentration in plasma was the same for the 12.5 and 25 mg doses of doxylamine hydrogen succinate. Based on the results, a predictable and linear increase in systemic exposure can be expected. Doxylamine hydrogen succinate was safe and well tolerated.
References
Zimmerman DR. Sleep aids. In: Zimmerman’s complete guide to non-prescription drugs. 2nd ed. Detroit (MI): Gale Research Inc.; 1992. p. 870–5.
Brunton LL, Parker JK. Drugs acting on the central nervous system. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s The pharmacological basis of therapeutics. 11th ed. New York: McGraw Hill; 2006. p. 422–7.
Montoro J, Sastre J, Bartra J, et al. Effect of H1 antihistamines upon the central nervous system. J Investig Allergol Clin Immunol. 2006;16(Suppl 1):24–8.
Garrison J. Histamine, bradykinin, 5-hydroxytryptamine and their antagonists. In: Gilman AC, Rall TW, Nies AS, Taylor P, editors. The pharmacological basis of therapeutics. New York: Pergamon; 1990.
Sjöqvist F, Lasagna L. The hypnotic efficacy of doxylamine. Clin Pharmacol Ther. 1967;8:48–54.
Videla S, Lahjou M, Guibord P, Xu Z, Tolrà C, Encina G, Sicard E, Sans A. Food effects on the pharmacokinetics of doxylamine hydrogen succinate 25 mg film-coated tablets: a single-dose, randomized, two-period crossover study in healthy volunteers. Drugs R D. 2012;12:217–25.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: guideline for good clinical practice E6(R1) [online]. Available from URL: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf. [Accessed 2012 Nov 27].
European Medicines Agency. Committee for medicinal products for human use (CHMP): Guideline on the Investigation of Bioequivalence (CPMP/EWP/QWP/1401/98 Rev. 1). Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf.
Friedman H, Greenblatt DJ. The pharmacokinetics of doxylamine: use of automated gas chromatography with nitrogen-phosphorus detection. J Clin Pharmacol. 1985;25:448–51.
Friedman H, Greenblatt DJ, Scavone JM, et al. Clearance of the antihistamine doxylamine. Reduced in elderly men but not in elderly women. Clin Pharmacokinet. 1989; 16:312–6.
Luna BG, Scavone JM, Greenblatt DJ. Doxylamine and diphenhydramine pharmacokinetics in women on low-dose estrogen oral contraceptives. J Clin Pharmacol. 1989;29:257–60.
Nulman I, Koren G. Pharmacokinetic comparison of a delayed-release combination of doxylamine succinate and pyridoxine hydrocholoride (Diclectin) and oral solutions of these drugs in healthy women of childbearing age. Can J Clin Pharmacol. 2009; 16:e400–6.
Dormidina® 25 mg film-coated tablets. Summary of Product Characteristics. http://www.aemps.gob.es/cima/especialidad.do?metodo=verFichaWordPdf&codigo=58658&formato=pdf&formulario=FICHAS&file=ficha.pdf.
Dormidina® 12.5 mg film-coated tablets. Summary of Product Characteristics. http://www.aemps.gob.es/cima/especialidad.do?metodo=verFichaWordPdf&codigo=60154&formato=pdf&formulario=FICHAS&file=ficha.pdf.
Acknowledgments
This work was supported by Laboratorios del Dr. Esteve.
F. Wagner, J. Cebrecos, and A. Sans designed and wrote the study protocol; E. Sicard visited and controlled the healthy volunteers and was the person in charge of the clinical part of the study; A. Sans monitored the study; A. Cabot, M. Encabo, Z. Xu and G. Encina were in charge of analytical results; P. Guibord was in charge of statistical analysis and the data management; S. Videla, M. Lahjou and A. Sans wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest
SV, JC, ZX, AC, ME, GE and AS are employees of Laboratorios del Dr Esteve. ML, FW, PG and ES are employees of the clinical research organization Algorithme Pharma contracted by Laboratorios del Dr Esteve.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited. The exclusive right to any commercial use of the article is with Springer.
About this article
Cite this article
Videla, S., Cebrecos, J., Lahjou, M. et al. Pharmacokinetic Dose Proportionality Between Two Strengths (12.5 mg and 25 mg) of Doxylamine Hydrogen Succinate Film-Coated Tablets in Fasting State: A Single-Dose, Randomized, Two-Period Crossover Study in Healthy Volunteers. Drugs R D 13, 129–135 (2013). https://doi.org/10.1007/s40268-013-0015-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-013-0015-7