Abstract
Introduction
Risk minimization measures are one of the most important tools in ensuring safe use of medicines. The evaluation of their effectiveness in clinical practice is a big challenge. Diclofenac is a medicine indicated for relief of all grades of pain and inflammation, in a wide range of conditions. Certain cardiovascular diseases are contraindications, whilst certain diseases require precautions for diclofenac prescribing.
Objective
The aim of this study is to measure impact of the new digital risk minimization tool, introduced at the level of diclofenac prescribing, on patterns of its prescription in outpatient settings in Montenegro.
Methods
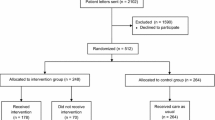
Regulatory impact before/after drug utilization study, using electronic health records of patients with cardiovascular contraindications/precautions for diclofenac prescribing, was conducted. The period of observation was 1 year before and 1 year after introduction of the digital risk minimization tool.
Results
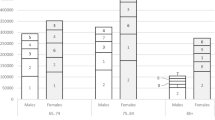
Introduction of the digital risk minimization tool was associated with significant decrease in number of patients on diclofenac and the number of diclofenac prescriptions/packages, in a cohort of patients who were prescribed diclofenac before the digital intervention. Decrease in diclofenac prescription was more obvious in patients with contraindications, as expected, compared with patients with precautions. Decrease in diclofenac prescriptions in patients with other heart diseases, cerebrovascular diseases, and ischemic heart diseases (contraindications) was 38.79%, 37.62%, and 29.85% respectively. There was also a decrease in diclofenac prescriptions in patients with hypertension (22.86%), hyperlipidemia (23.61%), and diabetes mellitus (26.32%; precautions). After the digital intervention, initiation of diclofenac in diclofenac-naive patients was significantly decreased compared with patients who were prescribed diclofenac before the digital intervention.
Conclusions
Although a significant decrease in diclofenac prescription in both cohorts occurred, diclofenac is still prescribed, even in diagnoses contraindicated for its use. Further improvements of existing tools and the creation of new ones are necessary for minimization of cardiovascular and other risks related to diclofenac.




Similar content being viewed by others
References
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP): module V—risk management systems (Rev 2). 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-v-risk-management-systems-rev-2_en.pdf. Accessed 15 June 2023.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP): module XVI—risk minimisation measures: selection of tools and effectiveness indicators, draft for public consultation (Rev 3). 2021. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices. Accessed 15 June 2023.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP): module XVI—risk minimisation measures: selection of tools and effectiveness indicators (Rev 2). 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-xvi-risk-minimisation-measures-selection-tools_en-3.pdf. Accessed 15 Jun 2023.
Ministry of Health of Montenegro. Law on medicines. Official Gazette of Montenegro. 080/2020, 04.08.2020. https://cinmed.me/wp-content/uploads/2022/11/Zakon-o-ljekovima-3.pdf.
European Medicines Agency. Assessment report for diclofenac containing medicinal products (systemic formulations), Procedure under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data. https://www.ema.europa.eu/en/documents/referral/diclofenac-article-31-referral-prac-assessment-report_en.pdf. Accessed 16 June 2023.
European Medicines Agency. New safety advice for diclofenac—new measures aim to minimise cardiovascular risks. 2013. https://www.ema.europa.eu/en/documents/referral/diclofenac-article-31-referral-new-safety-advice-diclofenac_en.pdf. Accessed 18 June 2023.
European Medicines Agency. New safety advice for diclofenac—CMDh endorses PRAC recommendation. 2013. https://www.ema.europa.eu/en/news/new-safety-advice-diclofenac-cmdh-endorses-prac-recommendation. Accessed 15 June 2023.
Stanković M, Turković N, Dobrić S, et al. Prescription patterns of diclofenac in patients with cardiovascular diseases or at high risk for cardiovascular diseases at primary health care level in Montenegro: retrospective, national, drug utilization study. Vojnosanit Pregl. 2023. https://doi.org/10.2298/VSP221229021S.
Institute for Medicines and Medical Devices. Diklofen (diklofenak), tablete sa produženim oslobađanjem, 100 mg, Galenika A.D. Srbija: summary of product characteristics. 2020. https://cinmed.me/humani-lijekovi/humani-lijek/?id=2568. Accessed 10 June 2023.
Institute for Medicines and Medical Devices. Dear healthcare professional communication on safety of medicines containing diclofenac. 2015. https://cinmed.me/wp-content/uploads/2022/12/DiklofenakDHPC.pdf. Accessed 10 June 2023.
Morales DR, Morant SV, MacDonald TM, et al. Impact of EU regulatory label changes for diclofenac in people with cardiovascular disease in four countries: interrupted time series regression analysis. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.14478.
Kasciuškevičiute S, Gumbrevičius G, Vendzelyte A, et al. Impact of the World Health Organization pain treatment guidelines and the European medicines agency safety recommendations on nonsteroidal anti-inflammatory drug use in Lithuania: an observational study. Medicina. 2018. https://doi.org/10.3390/medicina54020030.
Scholle O, Kollhorst B, Haug U. Are prescribers not aware of cardiovascular contraindications for diclofenac? A claims data analysis. J Intern Med. 2020. https://doi.org/10.1111/joim.12990.
Langaas HC, Hurley E, Dyrkorn R, et al. Effectiveness of an academic detailing intervention in primary care on the prescribing of non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2019;75(4):577–86.
Goedecke T, Morales DR, Pacurariu A, et al. Measuring the impact of medicines regulatory interventions—systematic 435 review and methodological considerations: methods for measuring impact of medicines regulatory interventions. Br J Clin Pharmacol. 2018;84(3):419–33.
Sekulic R. Development of Data Warehouse model for prescription of medicines in PHC IS. In: IPA Project on monitoring the prescription of diclofenac with the aim of optimization of its safe use. 2020. https://mgsoft.me/IPAWeb/index.html/Activities/DEV2.1. Accessed 20 June 2023.
World Health Organization. International Classification of Diseases (ICD) 10th revision. 2020. http://www.who.int/standards/classifications. Accessed 19 June 2023.
Uprava za statistiku. Demografija: Procjene stanovništva/Podaci. 2023. https://monstat.org/cg/page.php?id=48&pageid=48. Accessed 20 June 2023.
Krum H, Swergold G, Gammaitoni A, et al. Blood pressure and cardiovascular outcomes in patients taking nonsteroidal antiinflammatory drugs. Cardiovasc Ther. 2012. https://doi.org/10.1111/j.1755-5922.2011.00283.x.
Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–79.
European Medicines Agency. PRAC strategy on measuring the impact of pharmacovigilance activities (Revision 2). 2022. https://www.ema.europa.eu/en/documents/other/prac-strategy-measuring-impact-pharmacovigilance-activities_en.pdf. Accessed 11 June 2023.
Institute for Medicines and Medical Devices. Statistics for the annual sales of medicines in Montenegro. 2022. https://cinmed.me/humani-lijekovi/potrosnja-ljekova/. Accessed 16 June 2023.
European Commission. Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. OJEU. 31.12.2010;348:74–99. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0074:0099:EN:PDF.
European Commission. Regulation 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation 726/2004 and Regulation 1394/2007. OJEU. 31.12.2010;348:1–16. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0001:0016:EN:PDF.
European Commission. Commission Implementing Regulation 520/2012 of the European Parliament and of the Council of 19 June 2012, on the performance of pharmacovigilance activities provided for in Regulation 726/2004 of the European Parliament and of the Council and Directive 2001/83/EC of the European Parliament and of the Council. OJEU. 20.06.2012;159:5–25. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:159:0005:0025:EN:PDF.
European Medicines Agency. Big data. 2023. https://www.ema.europa.eu/en/about-us/how-we-work/big-data. Accessed 17 June 2023.
European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Impact of EU label changes, for systemic diclofenac products: post-referral prescribing trends. 2020. https://www.encepp.eu/encepp/viewResource.htm?id=33662. Accessed 15 June 2023.
European Medicines Agency. Key principles for the use of electronic product information for EU medicines. 2020. https://www.ema.europa.eu/en/news/key-principles-use-electronic-product-information-eu-medicines. Accessed 17 June 2023.
Acknowledgements
We would like to thank Partner in project—IT company MGSOFT—one of the largest IT companies in Montenegro included in establishment and development of the integral healthcare information system in Montenegro.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Financial assistance for conducting the study and analysis described in the manuscript was obtained through a project named “Collaborative grant scheme for innovative project ideas—monitoring the prescription of diclofenac with the aim of optimisation of its safe use” within the budgetary line of IPA II—Multi-annual Programme for Montenegro on Employment, Education and Social Policies, reference EuropeAid/162456+7/ID/ACT/ME.
Conflicts of interest
The authors have no competing interests.
Ethics approval
The permission to use patient medical data from PHCIS was obtained from the MNE Health Insurance Fund (number of consent: 04-2294, dated 04 June 2020), in accordance with national regulations on the conditions for using data from PHCIS. The permission for the introduction of the digital tool into PHCIS was obtained from the Ministry of Health of MNE (number of consent: 1-427/21-2450/3, dated 24 September 2021), the competent institution in MNE for the development of PHCIS. The personal data of patients included in study were unknown (anonymous data).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The availability of data from Primary Healthcare Institutions are subject to the restrictions in accordance with applicable regulations in Montenegro.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design, including the methods used. Maja Stanković and Nemanja Turković were in charge of data collection and processing. Statistical analysis was performed by Nemanja Rančić. The first draft of the manuscript was written by Maja Stanković, and all authors critically reviewed and interpreted the data in previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stankovic, M., Turkovic, N., Dobric, S. et al. Evaluation of diclofenac utilization patterns before and after digital risk minimization intervention in outpatient settings in Montenegro. Drugs Ther Perspect 40, 90–100 (2024). https://doi.org/10.1007/s40267-024-01049-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-024-01049-w