Abstract
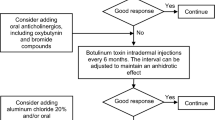
Hyperhidrosis (HH) includes chronic focal primary HH (PHH) stemming from sweat gland overactivity, and generalised secondary HH (SHH), which warrants investigation for an underlying cause. PHH, which may be axillary, plantar, palmar and/or craniofacial, decreases patients’ quality of life and often goes undiagnosed. Topical therapies, starting with aluminium chloride, are often effective. Additional recommendations for severe or recalcitrant PHH, and in craniofacial HH, are iontophoresis, topical or systemic anticholinergic agents and onabotulinum toxin injections.

Similar content being viewed by others
References
Henning MAS, Bouazzi D, Jemec GBE. Treatment of hyperhidrosis: an update. Am J Clin Dermatol. 2022;23(5):635–46.
McConaghy JR, Fosselman D. Hyperhidrosis: management options. Am Fam Physician. 2018;97(11):729–34.
Kisielnicka A, Szczerkowska-Dobosz A, Purzycka-Bohdan D, et al. Hyperhidrosis: disease aetiology, classification and management in the light of modern treatment modalities. Postepy Dermatol Alergol. 2022;39(2):251–7.
Solish N, Bertucci V, Dansereau A, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33(8):908–23.
International Hyperhidrosis Society. Primary focal palmar. 2012. https://www.sweathelp.org. Accessed 20 Apr 2023.
Wilke K, Martin A, Terstegen L, et al. Neurobiology of skin appendages: eccrine, apocrine, and apoeccrine sweat glands. In: Granstein RD, Luger TA, editors., et al., Neuroimmunology of the skin. Berlin: Springer; 2009.
Hornberger J, Grimes K, Naumann M, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. 2004;51(2):274–86.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
Thianboonsong T, Kanokrungsee S, Paichitrojjana A, et al. Efficacy and tolerability of 20% aluminum sesquichlorohydrate vs 20% aluminum chloride for the treatment of axillary hyperhidrosis: a randomized controlled trial. Dermatol Ther. 2020;33(6):e14354.
Streker M, Reuther T, Hagen L, et al. Hyperhidrosis plantaris—a randomized, half-side trial for efficacy and safety of an antiperspirant containing different concentrations of aluminium chloride. J Dtsch Dermatol Ges. 2012;10(2):115–9.
Masur C, Soeberdt M, Kilic A, et al. Safety and efficacy of topical formulations containing 0·5, 1 and 2% glycopyrronium bromide in patients with primary axillary hyperhidrosis: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2020;182(1):229–31.
Abels C, Soeberdt M, Kilic A, et al. A glycopyrronium bromide 1% cream for topical treatment of primary axillary hyperhidrosis: efficacy and safety results from a phase IIIa randomized controlled trial. Br J Dermatol. 2021;185(2):315–22.
Yokozeki H, Fujimoto T, Wanatabe S, et al. Topical glycopyrronium tosylate in Japanese patients with primary axillary hyperhidrosis: a randomized, double-blind, vehicle-controlled study. J Dermatol. 2022;49(1):86–94.
Glaser DA, Hebert AA, Nast A, et al. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: results from the ATMOS-1 and ATMOS-2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2019;80(1):128-38.e2.
Pariser DM, Hebert AA, Drew J, et al. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: patient-reported outcomes from the ATMOS-1 and ATMOS-2 phase III randomized controlled trials. Am J Clin Dermatol. 2019;20(1):135–45.
Kirsch B, Smith S, Cohen J, et al. Efficacy and safety of topical sofpironium bromide gel for the treatment of axillary hyperhidrosis: a phase II, randomized, controlled, double-blinded trial. J Am Acad Dermatol. 2020;82(6):1321–7.
Yokozeki H, Fujimoto T, Abe Y, et al. A phase 3, multicenter, randomized, double-blind, vehicle-controlled, parallel-group study of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis. J Dermatol. 2021;48(3):279–88.
Artzi O, Loizides C, Zur E, et al. Topical oxybutynin 10% gel for the treatment of primary focal hyperhidrosis: a randomized double-blind placebo-controlled split area study. Acta Derm Venereol. 2017;97(9):1120–4.
Lueangarun S, Sermsilp C, Tempark T. Topical botulinum toxin type A liposomal cream for primary axillary hyperhidrosis: a double-blind, randomized, split-site, vehicle-controlled study. Dermatol Surg. 2018;44(6):e14354.
Wade R, Llewellyn A, Jones-Diette J, et al. Interventional management of hyperhidrosis in secondary care: a systematic review. Br J Dermatol. 2018;179(3):599–608.
Obed D, Salim M, Bingoel AS, et al. Botulinum toxin versus placebo: a meta-analysis of treatment and quality-of-life outcomes for hyperhidrosis. Aesthetic Plast Surg. 2021;45(4):1783–91.
International Hyperhidrosis Society. Systemic medication. 2018. https://www.sweathelp.org. Accessed 20 Apr 2023.
Dr Reddy’s Laboratories Ltd. Glycopyrrolate tablets: US prescribing information. 2016. https://dailymed.nlm.nih.gov. Accessed 20 Apr 2023.
Rising Pharmaceuticals Inc. Oxybutynin chloride tablets: US prescribing information. 2018. https://dailymed.nlm.nih.gov. Accessed 20 Apr 2023.
National Institute for Health and Care Excellence. Hyperhidrosis: oral glycopyrronium bromide. 2013. www.nice.org.uk/guidance/esuom16. Accessed 20 Apr 2023.
Wolosker N, Campos JRM, Kauffman P, et al. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696–700.
Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis: a randomized, placebo-controlled trial. Br J Dermatol. 2015;173(5):1163–8.
ESTEVE. Vagantin® RIEMSER 50 mg coated tablets: German prescribing information. 2020. https://www.esteve.com/de/produkte/vagantin. Accessed 20 Apr 2023.
Müller C, Berensmeier A, Hamm H, et al. Efficacy and safety of methantheline bromide (Vagantin(®) ) in axillary and palmar hyperhidrosis: results from a multicenter, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2013;27(10):1278–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
C. Fenton, a contracted employee of Adis International Ltd/Springer Nature, and C. Kang, a salaried employee of Adis International Ltd/Springer Nature, declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fenton, C., Kang, C. Treat hyperhidrosis with topical therapies first, then dermatological or systemic therapies. Drugs Ther Perspect 39, 200–206 (2023). https://doi.org/10.1007/s40267-023-00996-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-023-00996-0