Abstract
Background and Objective
Childhood tuberculosis remains a global public health threat despite being preventable and curable. Childhood tuberculosis prevention using strategies including isoniazid preventive therapy is evidence based and cost effective. This study aimed to determine the isoniazid preventive therapy completion rate and predictors of hepatotoxicity of isoniazid among children who sought human immunodeficiency virus (HIV)/acquired immune deficiency syndrome care in public hospitals in Sokoto, Nigeria.
Methods
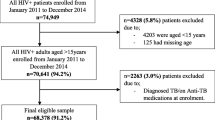
This was a 4-year (2016–19) retrospective chart review of 429 paediatric patients with HIV/acquired immune deficiency syndrome aged ≤18 years, accessing isoniazid preventive therapy from Usmanu Danfodiyo University Teaching Hospital and Specialist Hospital, Sokoto, Nigeria. The study commenced in January 2020 and lasted for 6 months. Regression models were used to determine predictors of the isoniazid preventive therapy completion rate and the development of hepatotoxicity.
Results
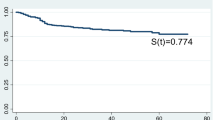
Of the 429 HIV-infected children initiated on isoniazid preventive therapy, 244 (56.9%) successfully completed isoniazid preventive therapy. Young age and low socioeconomic status were the strongest predictors of the isoniazid preventive therapy completion rate. Only 37 (18.0%; 37/205) were found to have their alanine transaminase raised to a grade 1 level (47.5–95.0 U/L) with the median (interquartile range) being 71 (53–87) U/L. Advanced HIV (HIV clinical stages 3 and 4) predicted isoniazid-induced hepatotoxicity.
Conclusions
The isoniazid preventive therapy completion rate was sub-optimal and the incidence rate of developing grade 1 liver injury was low among the study cohort. We recommend community-appropriate and tailored strategies to promote the isoniazid preventive therapy completion rate and that their liver enzymes be monitored especially for children with advanced HIV disease.
Plain Language Summary
The burden of childhood tuberculosis continues to remain unacceptably high in developing countries despite the preventability and curability of the disease. Tuberculosis and human immunodeficiency virus (HIV) often coexist and impact each other and thus form a highly lethal partnership. Consequently, measures such as isoniazid preventive therapy (IPT) that can prevent such coexistence is highly warranted. However, low uptake and completion rate, and hepatotoxicity threaten the beneficial effects of IPT. The study investigated the completion rate of a 6-month course of IPT among HIV/acquired immune deficiency syndrome-infected children on antiretroviral drugs and the predictors of hepatotoxicity of the IPT. The 4-year retrospective chart review revealed a less-than-adequate IPT completion rate of 56.9% (244/429) that was predicted by young age (≤10 years) and low socioeconomic status of the children. A grade 1 level elevation of alanine transaminase (47.5–95.0 U/L: mild hepatotoxicity) was also recorded among 18.0% (37/205) of the children and this was predicted by HIV clinical stage. The alanine transaminase levels of the remaining children (82.0%; 168/205) were within the normal range. Interventions designed to improve the IPT completion rate and periodic measurement of alanine transaminase for those with advanced HIV (HIV clinical stages 3 and 4) were recommended.



Similar content being viewed by others
References
World Health Organization. WHO consolidated guidelines on tuberculosis. Module 1: prevention: tuberculosis preventive treatment. Geneva: World Health Organization; 2020: licence: CC BY-NC-SA 3.0 IGO.
Liu L, Villavicencio F, Yeung D, et al. National, regional, and global causes of mortality in 5–19-year-olds from 2000 to 2019: a systematic analysis. Lancet Glob Health. 2022;10:e337–47.
Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. 2022;6:106–15.
World Health Organization. Global tuberculosis report 2021. Geneva: World Health Organization; 2021: licence: CC BY-NC-SA 3.0 IGO.
Federal Ministry of Health. National tuberculosis and leprosy control programmes. NTBLCP annual report 2020; 2021. Abuja: Federal Ministry of Health.
Adamu AL, Aliyu MH, Galadanci NA, et al. Deaths during tuberculosis treatment among paediatric patients in a large tertiary hospital in Nigeria. PLoS ONE. 2017;12(8):e0183270.
Federal Ministry of Health. National Tuberculosis and Leprosy Control programmes. NTBLCP Annual Report 2017. Abuja: Federal Ministry of Health. www.health.gov.ng/doc/NTBLCP%202017%20Annual%20report-2.pdf. Accessed 5 Feb 2022.
Chretien J. Tuberculosis and HIV: the cursed duet. Bull Int Union Tuberc Lung Dis. 1990;65:25–8.
Chamla D, Asadu C, Davies A, et al. Patching the gaps towards the 90–90-90 targets: outcomes of Nigerian children receiving antiretroviral treatment who are co-infected with tuberculosis. J Int AIDS Soc. 2015;18(Suppl. 6):20251.
Ogbudebe CL, Adepoju V, Ekerete-Udofia C, et al. Health Service Insights. 2018;11:1178632918757490. (1177/1178632918757490).
Dawit Z, Abebe S, Dessu S, et al. Incidence and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at public hospitals in Southern Ethiopia. PLoS ONE. 2021;16(6):e0253449.
Joint United Nations Programme on HIV/AIDS. Fact sheet: world TB day 2022. https://www.unaids.org/en/resources/documents/2022/tb-fact-sheet. Accessed 26 May 2022.
Oxlade O, Rochon H, Campbell JR, et al. Tuberculosis preventive treatment in people living with HIV: is the glass half empty or half full? PLoS Med. 2021;18(9):e1003702.
Kay AW, Rabie H, Maleche-Obimbo E, et al. HIV-associated tuberculosis in children and adolescents: evolving epidemiology, screening, prevention and management strategies. Pathogens. 2022;11:33.
World Health Organization. End TB strategy. Geneva: WHO; 2015. Available from: http://www.who.int/tb/post2015_TBstrategy.pdf?ua=1. Accessed 26 May 2022.
Tigabu Z, Taye BW. Isoniazid preventive therapy uptake and completion among HIV infected children in two referral hospitals, Northwest Ethiopia. Ethiopian Med J. 2018;56(3):1–4. Retrieved from https://emjema.org/index.php/EMJ/article/view/632
Adepoju AV, Ogbudebe CL, Adejumo OA, et al. Implementation of isoniazid preventive therapy among people living with HIV in Northwestern Nigeria: completion rate and predictive factors. J Global Infect Dis. 2020;12:105–11.
Melgar M, Nichols C, Cavanaugh JS, et al. Tuberculosis preventive treatment scale-up among antiretroviral therapy patients: 16 countries supported by the U.S. President’s Emergency Plan for AIDS Relief, 2017–2019. Morbidity Mortality Wkly Rep. 2020;69(12):329–34.
Bastos M, Melnychuk L, Campbell JR, et al. The latent tuberculosis cascade-of-care among people living with HIV: a systematic review and meta-analysis. PLoS Med. 2021;18(9):e1003703.
Olajide O, Okonkwo P, Ajayi O, et al. Determinants of isoniazid preventive therapy completion among people living with HIV in Oyo and Ogun states, Southwest Nigeria. J Community Med Primary Health Care. 2022;34(1):23–37.
Cerrone M, Bracchi M, Wasserman S, et al. Safety implications of combined antiretroviral and anti-tuberculosis drugs. Expert Opin Drug Saf. 2020;19(1):23–41.
Gray D, Nuttall J, Lombard C, et al. Low rates of hepatotoxicity in HIV-infected children on anti-retroviral therapy with and without isoniazid prophylaxis. J Trop Pediatr. 2010;56:159e65.
Le Roux SM, Cotton MF, Myer L, et al. Safety of long-term isoniazid preventive therapy in children with HIV: a comparison of two dosing schedules. Int J Tuberc Lung Dis. 2013;17(1):26–31.
Mudzviti T, Shamu T, Chimbetete C, et al. Tolerability of isoniazid preventive therapy in an HIV-infected cohort of paediatric and adolescent patients on antiretroviral therapy from a resource-limited setting: a retrospective cohort study. Drugs Real World Outcomes. 2019;6(1):37–42.
Umeokonkwo CD, Segun B, Nguku P, et al. Effectiveness of isoniazid preventive treatment among patients on antiretroviral treatment in Southeast Nigeria: a retrospective cohort study. J Interv Epidemiol Public Health. 2018;1(1):11.
Jalo IR, Ibrahim UM. Adherence to isoniazid preventive therapy for tuberculosis among HIV patients in Kano. Nigeria New Nig J Clin Res. 2019;8:91–6.
Yunusa F, Bello M, Kayode GA, et al. Uptake of tuberculosis prevention therapy in people living with HIV/AIDS in northern Nigeria: a programme to increase use of isoniazid preventive therapy. Lancet Global Health. 2020. https://doi.org/10.1016/S2214-109X(20)30178-9.
Busari AA, Oshikoya KA, Adejumo IA, et al. Low prevalence of isoniazid preventive therapy uptake among HIV-infected patients attending tertiary health facility in Lagos, Southwest Nigeria. Pan Afr Med J. 2021;39:123.
Ijeoma NH, Onuka O, Uloaku E-U, et al. Use of isoniazid preventive therapy on HIV/AIDS patient in a tertiary health facility South Eastern Nigeria. Sci J Public Health. 2015;3(2):265–8.
Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297.
Oshikoya KA, Ogunyinka IA, Adamaigbo C, et al. Surgical antimicrobial prophylaxis and its dose appropriateness among paediatric patients in a Nigerian teaching hospital. J Chemother. 2019;31(6):329–42.
Oyedeji GA. Socio-economic and cultural background of hospitalized children in Ilesa. Nig J Paediatr. 1985;12:111–7.
Tweed CD, Wills GH, Crook AM, et al. Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Med. 2018;16(1):46.
Rai S, Mishra P, Ghoshai UC. Survival analysis: a primer for the clinician scientist. Indian J Gastroenterol. 2021;40(5):541–9.
Pallant J. SPSS survival manual: a step by step guide to data analysis using IBM SPSS. 6th ed. Berkshire: McGraw-Hill Education; 2016.
Masini E, Sitienei J, Weyeinga H. Outcomes of isoniazid prophylaxis among HIV-infected children attending routine HIV care in Kenya. Public Health Action. 2013;3(3):204–8.
Yotebieng M, Edmonds A, Lelo P, et al. High completion of isoniazid preventive therapy among HIV-infected children and adults in Kinshasa, Democratic Republic of Congo. AIDS. 2015;29:2055–60.
Ngugi SK, Muiruri P, Theresa Odero T, et al. Factors affecting uptake and completion of isoniazid preventive therapy among HIV-infected children at a national referral hospital, Kenya: a mixed quantitative and qualitative study. BMC Infect Dis. 2020;20:294.
National Bureau of Statistics. National literacy survey 2010. Available from: www.nigerianstat.gov.ng. Accessed 26 May 2022.
Ajiboye TA, Oyetunde MO, Tijani AW, et al. Effects of health literacy on medication adherence among patients with glaucoma in two ophthalmic clinics in Oyo state. J Community Mental Health Nursing. 2016;2(1):1–8.
Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: a meta-analysis. Patient Educ Couns. 2016;99(7):1079–86.
Adekoya-Cole TO, Akinmokun OI, Enweluzo GO, et al. Poor health literacy in Nigeria: causes, consequences and measures to improve it. Nig Q J Hosp Med. 2015;25:112–7.
Mwangi PM, Wamalwa D, Marangu D, et al. Implementation of of isoniazid preventive therapy among HIV-infected children at health facilities in Nairobi county, Kenya: a cross-sectional study. East Afr Health Res J. 2019;32:141–50.
Muller P, Velez LL. Mixed methods systematic review and metasummary about barriers and facilitators for the implementation of cotrimoxazole and isoniazid-preventive therapies for people living with HIV. PLoS ONE. 2022;17(3):e0251612.
Oleribe OS, Udofia D, Oladipo O, et al. Healthcare workers’ industrial action in Nigeria: a cross-sectional survey of Nigerian physicians. Hum Resour Health. 2018;16:54.
Punch Newspaper. Health sector gulps N2.5tn in six years, say stakeholders. Available from: https://punchng-com.cdn.amproject.org/v/s/punchng.com/health-sector-gulps-n2-5tn-in-six-years-say-stakeholders/?amp=&_gsa=1&_js_v=a9&usqp=m. Accessed 16 Aug 2022.
Kopanoff DE, Snider DE, Caras GJ. Isoniazid-related hepatitis: a U.S. Public health service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001.
Metushi I, Uetrecht J, Phillips E. Mechanism of isoniazid-induced hepatotoxicity: then and now. Br J Clin Pharmacol. 2016;81(6):1030–6.
Russom M, Debesai M, Zeregabr M, et al. Serious hepatotoxicity following use of isoniazid preventive therapy in HIV patients in Eritrea. Pharmacol Res Perspect. 2018;6(4): e00423.
Nsobya L, Nsobya H, Nakawesi J, et al. Isoniazid preventive therapy associated hepatotoxicity among children living with HIV: case series at Mildmay Uganda HIV/AIDS clinic, Uganda. Int J Clin Med. 2015;6(6):384–91.
Walker K, Ginsberg G, Hattis D, et al. Genetic polymorphism in N-acetyltransferase (NAT): population distribution of NAT1 and NAT2 activity. J Toxicol Environ Health. 2009;12(5–6):440–72.
Kiser JJ, Zhu R, D’Argenio DZ, et al. Isoniazid pharmacokinetics, pharmacodynamics and dosing in South African infants. Ther Drug Monit. 2012;34(4):446–51.
Mahomed N, Reubenson G. Immune reconstitution inflammatory syndrome. SA J Radiol. 2017;21(2): a1257.
Cotton MF, Rabie H, Nemes E, et al. A prospective study of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected children from high prevalence countries. PLoS ONE. 2019;14(7): e0211155.
Ibadin MO, Akepede GO. A revised scoring scheme for the classification of socio-economic status in Nigeria. Niger J Paediatr. 2021;48(1):26–33.
Ogunsola OO, Ajayi O, Ojo O, et al. Improving coverage and completion rate of isoniazid preventive therapy among eligible HIV patients using quality improvement approaches: a case study of State Hospital, Ijebu Ode, Ogun State, Nigeria. Pan African Med J. 2019;34:193.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This paper was not funded.
Conflicts of interest/competing interests
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethics approval
Approval was sought and received from the Health Research and Ethics Committees of Usmanu Danfodiyo University Teaching Hospital (UDUTH/HREC/2019/No.845) and Specialist Hospital (SHS/SUB/133/VOL.1), Sokoto, Nigeria. The procedures used in this study adhere to the tenets of the Declaration of Helsinki and the ethical standards of the relevant national guidelines on human experimentation.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Availability of data and material
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
OIA and OKA were involved in the conception and design of this work. All authors were involved with data collection. OIA undertook the literature search and the first draft of the work. All authors approved the final version of the manuscript submitted for publication consideration.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ogunyinka, I.A., Wada, Y.H., Bolajoko, T. et al. Completion Rates and Hepatotoxicity of Isoniazid Preventive Therapy Among Children Living with HIV/AIDS: Findings and Implications in Northwestern Nigeria. Drugs Ther Perspect 38, 455–466 (2022). https://doi.org/10.1007/s40267-022-00946-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00946-2