Abstract
Acne vulgaris is a common, chronic skin disorder with multifactorial causes. Topical retinoids, benzoyl peroxide and/or antibiotics are the first-line treatments for mild-to-moderate acne. However, efficacy is often limited by intolerance to treatment, such as the presence of skin-related adverse events. Recently approved topical products aimed at improving the efficacy and tolerability of treatment have broadened the treatment landscape for patients; new products include novel formulations of existing agents, a fourth-generation retinoid, a new class of antiandrogen drugs and new combination products.
Similar content being viewed by others
References
Leung AK, Barankin B, Lam JM, et al. Dermatology: how to manage acne vulgaris. Drugs Context. 2021;10:1–18.
Drake L, Reyes-Hadsall S, Barbieri JS, et al. New developments in topical acne therapy. Am J Clin Dermatol. 2022;23(2):125–36.
Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–73.
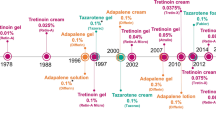
Baldwin H, Webster G, Stein Gold L, et al. 50 years of topical retinoids for acne: evolution of treatment. Am J Clin Dermatol. 2021;22(3):315–27.
Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17(10):1084–91.
Tanghetti EA, Kircik LH, Green LJ, et al. A phase 2, multicenter, double-blind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a novel tazarotene 0.045% lotion and tazarotene 0.1% cream in the treatment of moderate-to-severe acne vulgaris. J Drugs Dermatol. 2019;18(6):542–8.
Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80(6):1691–9.
Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80(1):168–77.
Kalabalik-Hoganson J, Frey KM, Ozdener-Poyraz AE, et al. Clascoterone: a novel topical androgen receptor inhibitor for the treatment of acne. Ann Pharmacother. 2021;55(10):1290–6.
Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156(6):621–30.
Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165(1):177–83.
Mazzetti A, Moro L, Gerloni M, et al. Pharmacokinetic profile, safety, and tolerability of clascoterone (cortexolone 17-alpha propionate, CB-03-01) topical cream, 1% in subjects with acne vulgaris: an open-label phase 2a study. J Drugs Dermatol. 2019;18(6):563.
Del Rosso J, Sugarman J, Levy-Hachman O, et al. Efficacy and safety of microencapsulated benzoyl peroxide 3% and microencapsulated tretinoin 0.1% (E-Bpo/E-Atra) in acne vulgaris: results from two randomized controlled clinical trials [poster presentation]. SKIN. 2021;5(1).
U.S. Food & Drug Administration. FDA approves Differin gel 0.1% for over-the-counter use to treat acne [media release]. 8 Jul 2016. https://www.fda.gov/.
Cunliffe WJ, Poncet M, Loesche C, et al. A comparison of the efficacy and tolerability of adapalene 0.1% gel versus tretinoin 0.025% gel in patients with acne vulgaris: a meta-analysis of five randomized trials. Br J Dermatol. 1998;139(Suppl 52):48–56.
Almirall LLC. AZELEX (azelaic acid) cream, 20%: US prescribing information. 2019. https://dailymed.nlm.nih.gov/. Accessed 17 Aug 2022.
Yang Z, Zhang Y, Lazic Mosler E, et al. Topical benzoyl peroxide for acne. Cochrane Database Syst Rev. 2020;3(3):CD011154.
American Society of Health-System Pharmacists Inc. Clindamycin topical. 2016. https://medlineplus.gov/. Accessed 17 Aug 2022.
Almirall LLC. ACZONE (dapsone) gel, 5%: US prescribing information. 2019. https://dailymed.nlm.nih.gov/. Accessed 17 Aug 2022.
Allergan PLC. ACZONE (dapsone) gel, 7.5%: US prescribing information. 2018. https://dailymed.nlm.nih.gov/. Accessed 17 Aug 2022.
Mayo Foundation for Medical Education and Research. Erythromycin (topical route). 2022. https://www.mayoclinic.org/. Accessed 17 Aug 2022.
Bausch Health US Inc. BENZACLIN (clindamycin phosphate and benzoyl peroxide) gel: US prescribing information. 2017. https://dailymed.nlm.nih.gov/. Accessed 17 Aug 2022.
Almirall LLC. VELTIN (clindamycin phosphate and tretinoin) gel: US prescribing information. 2021. https://dailymed.nlm.nih.gov/. Accessed 17 Aug 2022.
Bausch Health US Inc. BENZAMYCIN (erythromycin and benzoyl peroxide) gel: US prescribing information. 2020. https://dailymed.nlm.nih.gov/. Accessed 17 Aug 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
T. Nie and A. Lee are salaried employees of Adis International Ltd/Springer Nature and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nie, T., Lee, A. Consider new topical treatments alongside existing options when treating acne vulgaris. Drugs Ther Perspect 38, 432–436 (2022). https://doi.org/10.1007/s40267-022-00941-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00941-7