Abstract
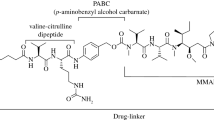
Tisotumab vedotin-tftv (TIVDAK®), a tissue factor-directed antibody-drug conjugate, is a valuable option for the treatment of adult patients with recurrent or metastatic cervical cancer experiencing disease progression on or after chemotherapy in the USA. In a clinical trial in patients with previously treated recurrent or metastatic cervical cancer, treatment with tisotumab vedotin-tftv intravenous infusion led to clinically meaningful and durable antitumor activity. The tolerability profile of tisotumab vedotin-tftv was manageable, with most adverse events being mild or moderate in severity. The most frequently reported treatment-related adverse events included certain unique toxicities (e.g. ocular toxicity, hemorrhage), in addition to toxicities more common among patients receiving chemotherapy (e.g. alopecia, nausea, fatigue, anemia, peripheral neuropathy). Adherence to premedication and required eye care before, during, and after the infusion is essential.
Plain Language Summary
Cervical cancer is the fourth most common female malignancy. Recurrent or metastatic disease is associated with poor survival outcomes. While several cytotoxic treatment options are available for patients with recurrent or metastatic cervical cancer, response rates associated with these agents are low, and as such these patients represent an unmet medical need. Commonly in cervical cancer, tissue factor is highly expressed and has been linked to a poor prognosis; tissue factor is also the main initiator of the extrinsic coagulation pathway. Tisotumab vedotin-tftv (TIVDAK®) is a tissue factor-directed antibody-drug conjugate. Administered as an intravenous infusion, tisotumab vedotin-tftv demonstrated clinically meaningful tumor response rates and durability of response in a clinical trial in patients with previously treated recurrent or metastatic cervical cancer. Tisotumab vedotin-tftv had a manageable tolerability profile, with most adverse events being mild or moderate in severity and manageable with preventive care, supportive care, and dose adjustments. Tisotumab vedotin-tftv is a valuable treatment option for previously treated recurrent or metastatic cervical cancer.
Similar content being viewed by others
References
Giudice E, Camarda F, Salutari V, et al. Tisotumab vedotin in cervical cancer: current status and future perspectives. US Oncol Hematol Rev. 2021;17(2):1–5.
National Comprehensive Cancer Network. Cervical cancer (version 1.2022). 2022. https://www.nccn.org. Accessed 26 Jul 2022.
Hong DS, Concin N, Vergote I, et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin Cancer Res. 2020;26(6):1220–8.
Mauricio D, Zeybek B, Tymon-Rosario J, et al. Immunotherapy in cervical cancer. Curr Oncol Rep. 2021;23(6):61.
Breij EC, de Goeij BE, Verploegen S, et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014;74(4):1214–26.
Markham A. Tisotumab vedotin: first approval. Drugs. 2021;81(18):2141–7.
Seagen Inc. TIVDAK® (tisotumab vedotin-tftv) for injection: US prescribing information. 2022. https://seagendocs.com/Tivdak_Full_Ltr_Master.pdf. Accessed 26 Jul 2022.
Coleman RL, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(5):609–19.
de Bono JS, Concin N, Hong DS, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(3):383–93.
US Food and Drug Administration. Drug approval package: TIVDAK (application number 761208) multi-discipline review. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761208Orig1s000TOC.cfm. Accessed 26 Jul 2022.
Acknowledgments
The article was reviewed by: Y. Chen, Jining Medical University, Shandong, China; S. Mirkov, Cairns and Hinterland Hospital and Health Service, Cairns, QLD, Australia. During the peer review process, Seagen Inc., the marketing authorization holder of tisotumab vedotin-tftv, was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
E. S. Kim, a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. Z. T. Al-Salama, a salaried employee of Adis International Ltd/Springer Nature and an editor of Drugs & Therapy Perspectives, was not involved in any publishing decisions for the manuscript and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, E.S., Al-Salama, Z.T. Tisotumab vedotin-tftv in previously treated recurrent or metastatic cervical cancer: a profile of its use in the USA. Drugs Ther Perspect 38, 382–388 (2022). https://doi.org/10.1007/s40267-022-00939-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00939-1