Abstract
Introduction
Pharmacovigilance as a concept is still new to healthcare professionals (HCPs) in Arabian countries. Morbidity and mortality related to adverse drug reactions (ADRs) are health problems that affect both adults and children worldwide, greatly impacting on patients’ health and the costs of healthcare services. Good pharmacovigilance programs can quickly recognize both risks and factors that reduce or prevent harm.
Objective
Our objective was to compare HCPs’ knowledge and practice of and attitudes towards pharmacovigilance in Alexandria, Egypt.
Methods
A cross-sectional survey comprising 20 questions was completed by 547 pharmacists and physicians in three different health sectors between August 2017 and March 2018. Data were analyzed using SPSS version 20. Bivariate analysis was conducted using the Chi-squared test and multivariate logistic regression. The main outcome was measuring HCPs’ knowledge and practice of and attitudes towards pharmacovigilance.
Results
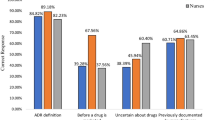
In total, 232 (42.4%) physicians and 315 (57.6%) pharmacists completed the survey. The odds of having a high level of knowledge of and a positive attitude towards pharmacovigilance were nearly six times higher among pharmacists than among physicians (odds ratio [OR] 6.60 [95% confidence interval {CI} 2.31–18.85] and OR 5.66 [95% CI 2.26–14.15], respectively). The odds of high levels of pharmacovigilance practice for pharmacists were more than twice as high as those for physicians (OR 2.62 [95% CI 1.35–5.05]). Major barriers to reporting ADRs were lack of time (71%) and difficulty deciding whether or not an ADR occurred (48%).
Conclusion
In Egypt, physicians had less knowledge and less positive attitudes towards pharmacovigilance than did pharmacists. This limited knowledge among physicians could be affecting the practice of ADR reporting. Health authorities in Egypt should initiate educational interventions and a practical training program primarily targeting physicians to enhance a culture of pharmacovigilance and drug safety in the country.
Similar content being viewed by others
References
Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4(Suppl 1):S73–7.
Gyllensten H, Hakkarainen KM, Hagg S, et al. Economic impact of adverse drug events—a retrospective population-based cohort study of 4970 adults. PLoS ONE. 2014;9(3):e92061.
Impicciatore P, Choonara I, Clarkson A, et al. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52(1):77–83.
Gallagher R, Bird K, Mason J, et al. Adverse drug reactions causing admission to a paediatric hospital: a pilot study. J Clin Pharm Ther. 2011;36(2):194–9.
Paine M. Therapeutic disasters that hastened safety testing of new drugs. Clin Pharmacol Ther. 2017;101(4):430–4.
Olsson S. The role of the WHO programme on International Drug Monitoring in coordinating worldwide drug safety efforts. Drug Saf. 1998;19(1):1–10.
World Health Organization. The importance of pharmacovigilance: safety monitoring of medicinal products. 2002. https://apps.who.int/iris/handle/10665/42493. Accessed 10 Nov 2020.
World Health Orgnization. Safety of medicines—a guide to detecting and reporting adverse drug reactions—why health professionals need to take action. 2002. https://apps.who.int/iris/handle/10665/67378. Accessed 10 Nov 2020.
Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med. 2003;18(1):57–60.
Bawazir SA. Attitude of community pharmacists in Saudi Arabia towards adverse drug reaction reporting. Saudi Pharm J. 2006;14(1):75.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Sweis D, Wong IC. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf. 2000;23(2):165–72.
Green CF, Mottram DR, Rowe PH, et al. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51(1):81–6.
Kelly M, Kaye KI, Davis SR, et al. Factors influencing adverse drug reaction reporting in New South Wales teaching hospitals. J Pharm Pract Res. 2004;34(1):32–5.
Hasford J, Goettler M, Munter KH, et al. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol. 2002;55(9):945–50.
Nadew SS, Beyene KG, Beza SW. Adverse drug reaction reporting practice and associated factors among medical doctors in government hospitals in Addis Ababa, Ethiopia. PLoS ONE. 2020;15(1):e0227712.
Qassim S, Metwaly Z, Shamsain M, et al. Reporting adverse drug reactions: evaluation of knowledge, attitude and practice among community pharmacists in UAE. IOSR J Pharm. 2014;22(30):31–40.
Suyagh M, Farah D, Abu FR. Pharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Saudi Pharm J. 2015;23(2):147–53.
Pirmohamed M. Personalized pharmacogenomics: predicting efficacy and adverse drug reactions. Annu Rev Genom Hum Genet. 2014;15:349–70.
World Health Organization–Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. 2018. https://www.who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf. Accessed 18 May 2020.
Uppsala Monitoring Centre. Countries participating in the WHO programme for International Drug Monitoring, with year of joining. 2018. https://www.who-umc.org/global-pharmacovigilance/who-programme-for-international-drug-monitoring/. Accessed 10 Nov 2020.
Bham B. The first Eastern mediterranean region/Arab countries meeting of pharmacovigilance. Drugs Real World Outcomes. 2015;2(1):111–5.
Alraie NA, Saad AA, Sabry NA, et al. Adverse drug reactions reporting: a questionnaire-based study on Egyptian pharmacists’ attitudes following an awareness workshop. J Eval Clin Pract. 2016;22(3):349–55.
Alshammari TM, Alamri KK, Ghawa YA, et al. Knowledge and attitude of health-care professionals in hospitals towards pharmacovigilance in Saudi Arabia. Int J Clin Pharm. 2015;37(6):1104–10.
Khan TM. Community pharmacists’ knowledge and perceptions about adverse drug reactions and barriers towards their reporting in Eastern region, Alahsa, Saudi Arabia. Ther Adv Drug Saf. 2013;4(2):45–51.
Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5(1):7–13.
World Health Organization. The safety of medicines in public health programmes: pharmacovigilance an essential tool. 2006. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/Pharmacovigilance_B.pdf?ua=1. Accessed 10 Nov 2020.
Pérez García M, Figueras A. The lack of knowledge about the voluntary reporting system of adverse drug reactions as a major cause of underreporting: direct survey among health professionals. Pharmacoepidemiol Drug Saf. 2011;20(12):1295–302.
Herdeiro MT, Figueiras A, Polónia J, et al. Influence of pharmacists’ attitudes on adverse drug reaction reporting. Drug Saf. 2006;29(4):331–40.
Toklu HZ, Uysal MK. The knowledge and attitude of the Turkish community pharmacists toward pharmacovigilance in the Kadikoy district of Istanbul. Pharm World Sci. 2008;30(5):556–62.
Davis DA, Thomson MA, Oxman AD, et al. Evidence for the effectiveness of CME: a review of 50 randomized controlled trials. JAMA. 1992;268(9):1111–7.
Rehan H, Vasudev K, Tripathi C. Adverse drug reaction monitoring: knowledge, attitude and practices of medical students and prescribers. Natl Med J India. 2002;15(1):24–6.
Belton K, Group EPR. Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. Eur J Pharmacol. 1997;52(6):423–7.
Taziaux P, Franck J, Ludovicy R, et al. A study of general practitioners’ prescribing behaviour to the elderly in Wallonia, Belgium. Eur J Public Health. 1996;6(1):49–57.
Granas AG, Buajordet M, Stenberg-Nilsen H, et al. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16(4):429–34.
Gavaza P, Brown CM, Lawson KA, et al. Influence of attitudes on pharmacists’ intention to report serious adverse drug events to the Food and Drug Administration. Br J Clin Pharmacol. 2011;72(1):143–52.
Figueiras A, Herdeiro MT, Polónia J, et al. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA. 2006;296(9):1086–93.
Herdeiro MT, Figueiras A, Polonia J, et al. Physicians’ attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf. 2005;28(9):825–33.
Belton KJ, Lewis SC, Payne S, et al. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39(3):223–6.
Figueiras A, Tato F, Fontaiñas J, et al. Influence of physicians’ attitudes on reporting adverse drug events: a case-control study. Med Care. 1999;37(8):809–14.
Agarwal R, Daher AM, Ismail NM. Knowledge, practices and attitudes towards adverse drug reaction reporting by private practitioners from Klang Valley in Malaysia. Malays J Med Sci. 2013;20(2):52–61.
Herdeiro MT, Polonia J, Gestal-Otero JJ, et al. Factors that influence spontaneous reporting of adverse drug reactions: a model centralized in the medical professional. J Eval Clin Pract. 2004;10(4):483–9.
Acknowledgements
The authors thank Professor Dr. Ramez Bedwany, PhD, Professor Dr. Adel Zaki, PhD (Medical Research Institute, Alexandria University), and Dr. Maged Wasfi, PhD (Faculty of Pharmacy, Alexandria University) for reviewing the manuscript and providing input.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
Fayek Salah ELkhwsky, Iman El Sayed, Omaima Gaber Mohamed Yassine, Sherif Abdelmonem, and Mai Mohamed Salama have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the Medical Research Institute, Alexandria University (Ethics approval number: IORG#: 10RG0008812).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Participants consented to submission of the manuscript to the journal.
Availability of data and material
All data and material are available upon request.
Code availability
Not applicable.
Author contributions
FSE, OGY, ISA, and MMS developed the survey instrument, drafted the manuscript and analyzed the data; MMS suggested the idea, and designed the study; SA and MMS distributed the survey questionnaire, collected the data and conducted pilot testing; FSE, OGY, and ISA contributed towards the reliability and validity of the questionnaire and proofreading the manuscript. All authors approved the version submitted for publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
ELkhwsky, F.S., El Sayed, I., Yassine, O.G.M. et al. Healthcare professionals’ knowledge and practice of and attitudes towards pharmacovigilance in Alexandria, Egypt: a cross-sectional survey. Drugs Ther Perspect 37, 124–136 (2021). https://doi.org/10.1007/s40267-020-00798-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-020-00798-8