Abstract
Background
The off-label use of medicines is a common practice that occurs over a wide range of therapeutic areas in both adults and children, but occurs much more frequently in pediatric population. So far, the extent of off-label use among children in Bulgaria has not been studied.
Objective
The aim of this nested, retrospective, non-interventional, single-center study is to provide data on the frequency, type, and the most common situations in which off-label medicines are prescribed in daily pediatric practice in Bulgaria.
Methods
The data on prescriptions of 360 pediatric outpatients, treated during a 1-year period, were recorded and provided for analysis. The summaries of product characteristics (SmPC) were used as reference documents for the assessment of prescriptions. Descriptive statistics, with absolute frequencies, means, and standard deviation, were used to analyze the processed data.
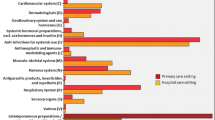
Results
The results from this study show that most pediatric patients (78%) were exposed to off-label use. Half of the medicines prescribed off-label were used for a therapeutic indication other than the one listed in the SmPC. We found that certain medicines were used 100% off-label, and certain diseases were also 100% treated with off-label medications.
Conclusion
Although the study was limited to one center, it deserves attention as it reveals many different aspects of the off-label use of medications in pediatric patients in Bulgaria. Further studies involving a larger number of medical centers are needed to establish more accurate data on off-label prescribing in pediatric patients at a national level.
Similar content being viewed by others
References
Weda M, et al. Study on off-label use of medicinal products in the European Union. European Commission; 2017. Available from: https://ec.europa.eu/commission/index_en.
Gazarian M, Kelly M, McPhee JR, et al. Off-label use of medicines: consensus recommendations for evaluating appropriateness. Med J Aust. 2006;185(10):544–8.
European Medicines Agency. Guideline on good pharmacovigilance practices: annex I—definitions (Rev 4); 2017. Available from: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices.
Lenk C, Duttge G. Ethical and legal framework and regulation for off-label use: European perspective. Ther Clin Risk Manag. 2014;10:537–46.
Shiel Jr W. Medical definition of Hippocratic oath. MedicineNet; 2018. Available from: https://www.medicinenet.com/medterms-medical-dictionary/article.htm.
Blanco-Reina E, Muñoz-García A, Cárdenas-Aranzana MJ, et al. Assessment of off-label prescribing: profile, evidence and evolution. Farm Hosp. 2017;41(4):458–69.
Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021–6.
Drenska M, Getov I. Research on approaches for regulation of the “off-label” use of medicinal products in the European Union. Acta Med Bulga. 2017;44(1):17–21.
European Medicines Agency and its Paediatric Committee. 10-year report to the European Commission: general report on the experience acquired as a result of the application of the Paediatric Regulation. European Commission; 2017. Available from: https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/paediatrics_10_years_ema_technical_report.pdf.
Balan S, Hassali MAA, Mak VSL. Two decades of off-label prescribing in children: a literature review. World J Pediatr. 2018;14(6):528–40.
Wimmer S, Rascher W, McCarthy S, et al. The EU paediatric regulation: still a large discrepancy between therapeutic needs and approved paediatric investigation plans. Paediatr Drugs. 2014;16(5):397–406.
European Medicines Agency. Pharm655. Human Pharmaceutical Committee - Meetings; 2014 March 26; European Commission; Available from: https://ec.europa.eu/commission/index_en.
European Commission. A guideline on summary of product characteristics. European Medicines Agency; 2009. Available from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/product-information/how-prepare-review-summary-product-characteristics#scientific-guidelines-with-smpc-recommendations-section.
Tabarrok A. Assessing the FDA via the anomaly of off-label drug prescribing. Indep Rev. 2000;5(1):25–53.
Miller K. Off-Label drug use: what you need to know. WebMD; 2009. Available from: https://www.webmd.com/a-to-z-guides/features/off-label-drug-use-what-you-need-to-know#1.
Solère P. Diabetes drug benfluorex linked to thousands of hospitalizations, hundreds of deaths for valvular disease. Medscape; 2010. Available from: https://www.medscape.com/pharmacists.
Gonçalves MG, Heineck I. Frequency of prescriptions of off-label drugs and drugs not approved for pediatric use in primary health care in a southern municipality of Brazil [in Portuguese]. Rev Paul Pediatr. 2016;34(1):11–7.
Chalumeau M, Tréluyer JM, Salanave B, et al. Off label and unlicensed drug use among French office based paediatricians. Arch Dis Child. 2000;83(6):502–5.
Turner S, Longworth A, Nunn AJ, et al. Unlicensed and off label drug use in paediatric wards: prospective study. BMJ. 1998;316(7128):343–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Drenska, V. Grigorova, S. Elitova, E. Naseva, I. Getov have no conflicts of interest that are directly relevant to the content of this article.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Ethical approval
This retrospective study is based on recorded data and approval from owners of the data was received.
Data confidentiality
Data confidentiality of all prescription records were respected at all time. Information about the identity of the patients was not collected.
Rights and permissions
About this article
Cite this article
Drenska, M., Grigorova, V., Elitova, S. et al. The off-label use of medicines in pediatric outpatients in Bulgaria based on an analysis of their prescription data. Drugs Ther Perspect 35, 391–395 (2019). https://doi.org/10.1007/s40267-019-00638-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-019-00638-4