Abstract
Background
The acetylcholinesterase inhibitors (AChEIs) donepezil, galantamine, and rivastigmine are commonly used in the management of various forms of dementia.
Objectives
While these drugs are known to induce classic cholinergic adverse events such as diarrhea, their potential to cause psychiatric adverse events has yet to be thoroughly examined.
Methods
We sought to determine the risk of psychiatric adverse events associated with the use of AChEIs through a systematic review and meta-analysis of double-blind randomized controlled trials involving patients with Alzheimer’s dementia and Parkinson’s dementia.
Results
A total of 48 trials encompassing 22,845 patients were included in our analysis. Anorexia was the most commonly reported psychiatric adverse event, followed by agitation, insomnia, and depression. Individuals exposed to AChEIs had a greater risk of experiencing appetite disorders, insomnia, or depression compared with those who received placebo (anorexia: odds ratio [OR] 2.93, 95% confidence interval [CI] 2.29–3.75; p < 0.00001; decreased appetite: OR 1.93, 95% CI 1.33–2.82; p = 0.0006; insomnia: OR 1.55, 95% CI 1.25–1.93; p < 0.0001; and depression: OR 1.59, 95% CI 1.23–2.06, p = 0.0004). Appetite disorders were also more frequent with high-dose versus low-dose therapy. A subgroup analysis revealed that the risk of insomnia was higher for donepezil than for galantamine.
Conclusions
Our findings suggest that AChEI therapy may negatively impact psychological health, and careful monitoring of new psychiatric symptoms is warranted. Lowering the dose may resolve some psychiatric adverse events, as may switching to galantamine in the case of insomnia.
Clinical Trial Registration
The study was pre-registered on PROSPERO (CRD42021258376).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This comprehensive review and meta-analysis examines the prevalence of psychiatric adverse events in patients with Alzheimer’s disease and Parkinson’s dementia taking acetylcholinesterase inhibitors (AChEIs). |
Psychiatric adverse events are relatively common during AChEI treatment, with appetite disorders, depression, and insomnia occurring more frequently during AChEI treatment than during placebo treatment. |
Physicians should be aware of these psychotropic effects and monitor their patients carefully during AChEI treatment. |
1 Introduction
Currently, more than 55 million people worldwide are living with dementia [1]. Dementias predominantly affect the elderly [2], as only 5.5% of patients with dementia develop symptoms before the age of 65 years [3]. Because of the increasing proportion of older people in most industrialized countries [4], dementia is becoming a serious public health challenge. It is estimated that the number of people with dementia will almost triple to more than 152 million by 2050 [5]. Alzheimer’s disease (AD) is the most common cause of dementia and contributes to 60–70% of cases [1]. The acetylcholinesterase inhibitors (AChEIs) donepezil, rivastigmine, and galantamine are approved by the US Food and Drug Administration as well as the European Medicines Agency for the treatment of mild-to-moderate AD [6, 7]. In addition, rivastigmine is also approved for the treatment of Parkinson’s dementia (PDD) [8]. All three AChEIs increase the synaptic availability of acetylcholine (ACh) by inhibiting its degradation through the enzyme acetylcholinesterase [9]. Furthermore, rivastigmine also inhibits the enzyme butyrylcholinesterase, and galantamine acts as an allosteric modulator on nicotinic ACh receptors [10]. Despite these differences in pharmacodynamics, the AChEIs are usually considered to be equally effective [11]. The therapeutic benefit in dementia, however, is small, with no disease-modifying or even curative effect [12,13,14]. Given the small therapeutic effects, the safety of AChEI treatment is of particular importance. While AChEIs are most commonly associated with classic cholinergic effects, such as nausea, diarrhea, vomiting, dizziness, and bradycardia [15], there is a lack of research on their potential psychiatric adverse events (PAEs). However, as psychiatric symptoms significantly affect the quality of life of patients with dementia [16, 17], PAEs of AChEIs should also be a focus of interest. Brain ACh has been linked with the regulation of several psychological functions, including mood [18, 19], anxiety [19], sleep [20], and appetite [21, 22]. Therefore, increasing ACh levels may adversely affect these functions. Indeed, psychiatric symptoms, such as decreased appetite, insomnia, and agitation, are frequently registered as suspected adverse events (AEs) in both the Food and Drug Administration and European Medicines Agency surveillance systems (Table S1 of the Electronic Supplementary Material [ESM]). In addition, the prescribers’ information for the three substances also lists PAEs, such as anorexia, insomnia, confusion, and depression, as common symptoms that occurred during controlled clinical trials [23,24,25]. However, this information originates solely from the manufacturer-conducted approval studies. Previous reviews have examined the safety of AChEIs but have not investigated the incidence of specific PAEs [26], are limited to classic cholinergic symptoms, such as bradycardia, diarrhea, nausea, and vomiting [27], or investigated PAEs in unapproved conditions, such as vascular dementia [28] or mild cognitive impairment [29]. With our systematic review and meta-analysis, we therefore aim to fill this gap and provide a comprehensive synthesis of data from double-blind parallel-arm studies about the risk of PAEs during AChEI treatment in AD and PDD. Furthermore, we investigate whether there are differences between the three substances regarding the risk of PAEs.
2 Methods
2.1 Overview
This meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [30]. Our study was pre-registered on PROSPERO (registration number: CRD42021258376).
2.2 Search Strategy
Relevant double-blind randomized controlled trials (RCTs) with a parallel-arm design were identified through a keyword-based search in the literature databases PubMed and Web of Science from database inception to 28 February, 2023. The following search term was used: (donepezil OR galantamine OR rivastigmine) AND (random*).
Only English or German publications were considered. In addition, unpublished studies were identified by searching the trial registries ClinicalTrials.gov, ClinicalStudyDataRequest.com, the Yale University Open Data Access Project, as well as the Food and Drug Administration and European Medicines Agency drug approval packages. Finally, we scanned the references from the cited articles to identify additional RCTs and contacted authors of eligible trials who incompletely reported AEs in order to obtain additional data.
2.3 Study Eligibility
We used the principles of the Population–Intervention–Comparator–Outcomes–Study (PICOS) framework [31] to define eligibility criteria for the inclusion of RCTs. All included trials met the following criteria: (1) double-blind parallel-arm RCTs; (2) applying AChEI monotherapy in at least one arm systemically for at least 4 weeks; (3) comparing the AChEI to either placebo or an active comparator (different AChEI, different dose of the same AChEI, or other drug); (4) in patients diagnosed with either AD or PDD; (5) not preselected for AChEI tolerance or intolerance prior to the study; and (6) reporting at least one PAE during either AChEI or comparator therapy. A detailed list of inclusion and exclusion criteria can be found in the Appendix (Table S2 of the ESM). For the meta-analysis, both placebo-controlled trials and trials using active comparators (other AChEIs or other AChEI doses) were considered eligible. All inclusion criteria were determined a priori.
2.4 Data Extraction
Relevant data, such as trial design and duration, treatment (AChEI type, dose, and frequency; comparator), sample characteristics (sample size, demographics, type of dementia), and AEs (types and numbers of relevant AEs, AE assessment methodology), were systematically extracted from the full-text articles by two reviewers (NB and CSMF). In addition to PAEs, we also extracted data on nausea and diarrhea, two well-known frequent side effects of AChEIs [32], to serve as a positive control to further validate our findings.
2.5 Data Synthesis
We observed that different studies reported similar symptoms using different terms. These symptoms were grouped into clusters based on a consensus of psychiatrists, clinical psychologists, and pharmacologists and guided by the Medical Dictionary for Regulatory Activities Terminology [33]; see Table S3 of the ESM).
2.6 Quality Assessment
Four reviewers (NB and CSMF, AS, or TGR) rated all included studies using the revised Cochrane Collaboration’s risk of bias assessment tool [34]. The quality of AE measuring and reporting was evaluated using a self-designed rating instrument (Table S4 of the ESM) [35]. Finally, we checked whether there was clear evidence of industry sponsorship. Any discrepancies between the evaluations were resolved by discussion.
2.7 Statistical Analysis
Meta-analyses were performed using Review Manager 5.4 [36]. Comparisons of interest were AChEIs versus placebo, AChEI high dose versus AChEI low dose, and AChEIs versus different AChEIs. In the case of placebo-controlled studies having separate arms for different doses of the same AChEI, these arms were pooled for the main analysis of AChEIs versus placebo. The comparison of high-dose versus low-dose therapy was done to further strengthen a possible causal relationship between PAEs and drug therapy, as most AEs are dose related. As we anticipated possible between-study heterogeneity, a random-effects model was employed to pool effect sizes. We intended to directly compare different AChEIs but were unable to do so because of an insufficient number of eligible trials. The analyses were conducted if at least five placebo-controlled or active-controlled trials were available. It has been shown that five or more studies are required to achieve a statistical power that is likely to exceed that of individual contributing studies in random-effect models [37]. Pair-wise meta-analyses were calculated, measuring odd ratios (ORs) with 95% confidence intervals (CIs) and respective p-values. A p-value of 0.05 or less was considered statistically significant. While ORs were our primary measure of effect owing to advantages in the case of low event rates and stability across studies with different baseline risks [38], absolute risks, risk ratios, and risk differences were also calculated to enhance interpretability of the results. Heterogeneity was assessed using the I2 statistic, and the following thresholds were employed in accordance with the Cochrane Handbook: 0–40% for low heterogeneity, 30–60% for moderate heterogeneity, 50–90% for substantial heterogeneity, and 95–100% for considerable heterogeneity [39]. Furthermore, funnel plots were created to visually estimate the potential role of publication bias for meta-analyses with at least ten contributing studies [39].
2.8 Sensitivity Analyses
Five a priori planned sensitivity analyses were conducted: (1) exclusion of studies with a duration of less than 8 weeks, as the manifestation of some symptoms may only occur after prolonged exposure; (2) exclusion of studies with a high risk of bias in the Cochrane Collaboration’s risk of bias assessment tool; (3) exclusion of the study with the greatest weight; (4) exclusion of studies that did not specifically measure frequencies of AEs in an active and structured manner (e.g., direct questions, symptom checklist); and finally (5) exclusion of studies sponsored by the industry (presence or absence of clear indicators). Sensitivity analyses were conducted if at least five studies remained after excluding studies by the respective method. Table S5 of the ESM provides an overview of the rationale for the sensitivity analyses performed.
2.9 Subgroup Analyses
In placebo-controlled trials, subgroup analyses were performed, investigating whether the outcomes were moderated by the type of AChEI (donepezil vs galantamine vs rivastigmine). We also intended to consider the indication for AChEI use (AD vs PDD), dementia severity, and participant age for subgroup analyses but the numbers of eligible studies was insufficient. Subgroup analyses were conducted if at least three studies per group were available.
3 Results
3.1 Overview
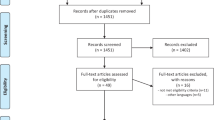
In total, 2954 records, excluding duplicates, were identified in our search. A total of 302 records were reviewed in full text. The remaining records were excluded at the abstract level because they did not meet the inclusion criteria (e.g., unsuitable trial design, absence of AChEI monotherapy, or study population other than AD/PDD). After reviewing the full texts, another 254 studies were excluded for reasons such as a lack of AE or PAE reporting, unsuitable study design (e.g., cross-over trials, single-blind trials), or population-based reasons (e.g., patients preselected for AChEI tolerance or intolerance). In the end, 48 studies including 22,845 patients were eligible to be included in this review and meta-analysis (Fig. 1). Tables S6 and S7 of the ESM provide a list of all trials included in our review displaying clinical characteristics and the results of the bias rating. A reference list for all included studies can be found in the ESM as well.
3.2 Studies
Of these 48 studies, 40 were placebo controlled, with 14 of them also reporting PAEs at different dose levels. In a further six studies, different dose levels were compared with each other without a placebo comparison. Two studies tested an AChEI against another AChEI. One trial compared donepezil to galantamine in a total of 233 patients, and the other trial tested donepezil against rivastigmine with 994 patients exposed. The vast majority of these studies included patients diagnosed with AD (46 trials, 21,754 patients), followed by PDD (two trials, 1091 patients). In total, 15,875 patients received AChEIs (donepezil 27 trials/6523 patients, galantamine 11 trials/4552 patients, rivastigmine 12 trials/4800 patients), and 6970 patients received placebos. The overall study quality was good, with only eight studies being rated as having a high risk of bias (Table S6 of the ESM). Fifteen studies provided information on how the occurrence of AEs during the trial was assessed. Only three out of these 15 trials used active assessment methods (Table S6 of the ESM). In a large fraction of the studies, not all side effects were reported, as many studies applied a frequency threshold for their report. For 46 studies, there is sufficient evidence to assume that they were sponsored by industry. The data of six of the included studies have not yet been published.
3.3 Qualitative Analysis
Across all studies, anorexia was the most commonly reported PAE, with data stemming from 27 trials and 720 affected patients out of 10,123 exposed patients. Other commonly reported symptoms were agitation (21 trials, 594 out of 9262 patients), insomnia (23 trials, 444 out of 9770 patients), depression (13 trials, 310 out of 6605 patients), decreased appetite (12 trials, 238 out of 5223 patients), confusion (14 trials, 191 out of 5863 patients), anxiety (13 trials, 185 out of 5596 patients), somnolence (ten trials, 162 out of 6196 patients), and aggression (seven trials, 127 out of 3488 patients). Three other PAEs were rarely reported: hallucination (seven trials, 71 out of 3379 patients), nervousness (three trials, 32 out of 2361 patients), and nightmares (one trial, three out of 151 patients). The positive control symptoms occurred frequently (nausea: 43 trials, 2312 out of 15,323 patients; diarrhea: 44 trials, 1258 out of 15,200 patients). Frequencies for all PAEs investigated are shown in Table 1 grouped by individual AChEI, and grouped by included study in Table S7 of the ESM.
3.4 Quantitative Analysis
3.4.1 AChEIs Versus Placebo
Meta-analyses with data from 46 trials were performed in order to quantify the risk of PAEs for AChEIs versus placebo (Fig. 2). The estimated pooled effects for AChEIs were significant for the following AEs, indicating a higher risk during AChEI treatment compared with placebo treatment: anorexia (OR 2.93, 95% CI 2.29–3.75; p < 0.00001; I2 13%; Fig. S1 of the ESM), decreased appetite (OR 1.93, 95% CI 1.33–2.82; p = 0.0006; I2 0%; Fig. S2 of the ESM), insomnia (OR 1.55, 95% CI 1.25–1.93; p < 0.0001; I2 5%; Fig. S3 of the ESM), and depression (OR 1.59, 95% CI 1.23–2.06; p = 0.0004; I2 0%; Fig. S4 of the ESM). We distinguished anorexia and decreased appetite as distinct PAEs, as they are often reported separately in trials, and categorized by the Medical Dictionary for Regulatory Activities as different severities of appetite disorders, with anorexia signifying a complete loss of appetite.
No higher risk during AChEI treatment was detected for agitation (OR 1.01, 95% CI 0.78–1.30; p = 0.95; I2 40%; Fig. S5 of the ESM), anxiety (OR 1.16, 95% CI 0.86–1.58; p = 0.33; I2 5%; Fig. S6 of the ESM), confusion (OR 0.84, 95% CI 0.65–1.09; p = 0.19; I2 0%; Fig. S7 of the ESM), hallucination (OR 0.74, 95% CI 0.45–1.22; p = 0.24; I2 31%; Fig. S8 of the ESM), and somnolence (OR 1.52, 95% CI 0.95–2.43; p = 0.08; I2 11%; Fig. S9 of the ESM). The positive control symptoms nausea and diarrhea were significantly associated with the use of AChEIs (OR 3.13, 95% CI 2.66–3.69; p <0.00001; I2 36%; Fig. S10 of the ESM and OR 1.58, 95% CI 1.31–1.90; p <0.00001; I2 44%; Fig. S11 of the ESM), respectively. A table showing the absolute risks, risk ratios, and risk differences for all PAEs investigated through meta-analyses can be found in the Appendix (Table S8 of the ESM). Heterogeneity was low to moderate for the investigated PAEs and moderate to substantial for the positive control symptoms [39]. Visual inspection of funnel plots for comparisons with ten or more eligible studies was not suggestive of a publication bias (Fig. S12–17 of the ESM).
3.5 Sensitivity Analyses
The association between AChEI use and anorexia, decreased appetite, depression, and insomnia remained stable in all viable analyses. Sensitivity analyses for active AE assessment as well as industry sponsorship could not be performed for any of the symptoms including the positive control symptoms nausea and diarrhea. For all other symptoms, sensitivity analyses were unremarkable (Table S10 of the ESM).
3.6 Subgroup Analyses
The full results of viable subgroup analyses are presented in Table S11 of the ESM. For insomnia, we found a difference between donepezil and galantamine, with galantamine exhibiting a more favorable risk profile. The analyses did not indicate differences regarding the risk of any other PAEs between the different AChEIs. However, both positive control symptoms showed significant subgroup effects: for nausea, the risk was higher during rivastigmine or galantamine than during donepezil treatment. Regarding diarrhea, the risk was higher during donepezil or rivastigmine than during galantamine treatment, respectively.
3.6.1 Analysis of Dose Effects
A dose–response relationship (with higher doses of AChEIs leading to an increased risk compared with lower doses) was detected for anorexia (OR 1.91, 95% CI 1.33–2.76; p = 0.0005; I2 55%; Fig. S18 of the ESM) and decreased appetite (OR 2.60, 95% CI 1.85–3.64; p <0.00001; I2 0%; Fig. S19 of the ESM). No effect was found for agitation (OR 0.87, 95% CI 0.65–1.15; p = 0.33; I2 17%; Fig. S20 of the ESM), anxiety (OR 0.81, 95% CI 0.53–1.24; p = 0.34; I2 0%; Fig. S21 of the ESM), confusion (OR 0.88, 95% CI 0.61–1.25; p = 0.47; I2 0%; Fig. S22 of the ESM), depression (OR 1.17, 95% CI 0.83–1.64; p = 0.38; I2 0%; Fig. S23 of the ESM), insomnia (OR 1.30, 95% CI 0.95–1.79; p = 0.11; I2 35%; Fig. S24 of the ESM), and somnolence (OR 1.47, 95% CI 0.97–2.22; p = 0.07; I2 0%; Fig. S25 of the ESM). A dose–response effect was also found for the positive control symptoms nausea (OR 2.56, 95% CI 1.96–3.34; p < 0.00001; I2 72%; Fig. S26 of the ESM) and diarrhea (OR 1.64, 95% CI 1.41–1.92; p <0.00001; I2 0%; Fig. S27 of the ESM). Absolute risks, risk ratios, and risk differences for all for all dose–effect analyses can be found in the Appendix (Table S9 of the ESM). Heterogeneity was low to moderate for all investigated PAEs with the exception of anorexia, which had substantial heterogeneity. For the positive control symptoms nausea and diarrhea, heterogeneity was substantial and low, respectively [40]. Visual inspection of funnel plots was not suggestive of a publication bias (Figs. S28–29 of the ESM).
3.6.2 Comparison of different AChEIs
As only two studies compared an AChEI against an active comparator, available data did not allow for a meta-analytic comparison of AChEIs against other active treatments for frequencies of different PAEs.
4 Discussion
Our meta-analysis aimed to fill a gap in the literature by investigating the risks of PAEs during treatment with AChEIs, an area that has previously received little attention. We synthesized the most up-to-date data on PAEs during AChEI treatment from double-blind parallel-arm RCTs. Our sample based on 48 studies included populations with AD as well as PDD. Psychiatric AEs such as anorexia, agitation, insomnia, and depression were found to commonly occur in the standardized setting of RCTs. These results of our investigation are largely consistent with the suspected AEs documented in the American and European ADR surveillance systems (Table S1 of the ESM). In addition, our meta-analyses indicate that several PAEs, including appetite disorders, insomnia, and depression, indeed occur more often during AChEI treatment compared with placebo treatment.
The cholinergic system has been shown or proposed to play an integral role in the regulation of several psychological functions. A treatment with AChEIs increases ACh levels in both the central nervous system and the periphery. First, cholinergic signaling has been associated with appetite and weight gain through the central activation of nicotinic and muscarinic ACh receptors in a variety of ways [21, 41]. However, the findings are ambiguous. It can be assumed that the feeling of satiety is induced by an increase in ACh. This is supported by the findings of Herman et al., who observed that impairment of cholinergic signaling increases food intake and enhanced cholinergic signaling decreases food consumption [21]. Avena et al. also concluded in their review that increased levels of ACh in the nucleus accumbens promote satiety [42]. In contrast, Jeong et al. stated that activation of cholinergic neurons in the dorsomedial hypothalamus increases food intake [43]. This effect can be blocked by muscarinic antagonism but not by the nicotinic AChR antagonist mecamylamine. This suggests that cholinergic activation of hypothalamic muscarinic receptors appears to increase food intake, while the activation of hypothalamic nicotinic receptors appears to decrease feeding [21, 41, 44]. The increased risk for the occurrence of anorexia during administration of AChEIs may therefore be attributed to increased satiety signaling via activation of nicotinic ACh receptors.
The physiological mechanisms of sleep and wakefulness are modulated by the cholinergic system as well [20, 45]. This explains the association between the administration of AChEIs and the frequent occurrence of sleep disturbances such as insomnia in patients with AD. The natural sleep cycle has five different stages (wake, non-rapid eye movement (REM) sleep N1 [relaxed wakefulness], N2 [light sleep], N3 [deep sleep], and REM sleep). Individuals cycle through these stages about four to six times every night, with each cycle approximately 90 minutes in length [46]. The periodic change between the phases is modulated by transmitters such as ACh. In particular, cholinergic signaling, especially through the M2 subtype of muscarinic receptors, plays a crucial role in the regulation of REM sleep [47, 48]. The release of cortical ACh through cholinergic neurons of the basal forebrain is greatest during waking and REM sleep in order to promote the waking process [45, 49]. Therefore, an increased level of ACh during therapy with AChEIs prolongs the REM sleep period and leads to longer periods of wakefulness, which consequently causes changes in the sleep architecture [50]. Another study that examined the effects of donepezil on sleep in patients with AD showed that not only did REM sleep increase significantly after 3 and 6 months of donepezil treatment compared with baseline and placebo, but also the total sleep time was reduced in patients with AD after donepezil treatment [51]. However, the latter finding was not statistically significant. A similar result for donepezil was shown previously by Mizuno et al. [52]. Rivastigmine has also been reported to significantly decrease REM latency in a study on healthy subjects. Furthermore, in older participants it also increased the number of REM sleep periods and REM density [53]. A statistically significant increase in REM sleep density after the administration of rivastigmine was earlier found by Holsboer-Trachsler et al. as well [54]. For galantamine, both an increased tonic REM sleep and shorter REM latency could be found in one study on young healthy participants [55]. Previously, Riemann et al. stated that galantamine shortens REM latency and increases REM density in healthy subjects [56]. As REM sleep plays an important role in memory consolidation [57, 58], various studies were able to prove that the REM sleep-stimulating effects of AChEIs are related to an enhancement of cognitive functioning in patients with AD [52, 59, 60].
Some of the PAEs of AChEI treatment mentioned above had also been detected in a previous meta-analysis, which investigated the general AE profile of AChEIs: Birks reported data on anorexia and insomnia, both of which occurred more frequently during AChEI therapy [61]. Our meta-analysis of PAEs in placebo-controlled studies extend these findings by providing important evidence for an association between AChEI and depression. To the best of the authors’ knowledge, no other study has collected sufficient data to make a statement about this specific PAE. For instance, in their recent meta-analysis, Li et al. cited a lack of data as the reason not to investigate individual AEs such as depression [26].
A relevant role of ACh in the development of mood disorders was first proposed by Janowsky et al. 50 years ago [62]. The theory was further elaborated on in 2015 to include the role of dopamine and published as the catecholaminergic-cholinergic balance hypothesis of mania and depression. According to this hypothesis, depressed mood is caused by a predominance of ACh to catecholamines [63]. A recent review by Fitzgerald et al. summarized the evidence of AChEIs on depression collected in both animal and human studies, including studies with elderly subjects with and without AD [64]. According to them, AChEIs show both depression-promoting and antidepressant qualities under different experimental conditions. The authors assume a Janus-faced dose–response relationship, with relatively low levels of AChEIs being therapeutic with regard to depressive symptoms and high levels promoting depression or depression-like behavior. Several RCTs have investigated the effect of AChEIs on symptoms of depression in AD, as measured via a neuropsychiatric inventory. While some studies showed positive effects on depressive symptoms [65,66,67,68,69], others did not detect a change in neuropsychiatric inventory scores [70,71,72]. Some authors have suggested that AChEIs may have a modest impact on neuropsychiatric symptoms such as depression in AD and may therefore be a pharmacological treatment approach in the management of neuropsychiatric symptoms in AD [73, 74]. The results of our meta-analysis suggest that AChEIs are not suitable for adjunctive treatment of depression as part of AD or PDD, as they may even induce such depressive symptoms in some patients.
Concerning differences between the three AChEIs regarding the risk of PAEs, our subgroup analyses indicate that the overall severity and types of PAEs due to the use of AChEIs in AD and PDD are largely comparable between the AChEIs. However, our results suggest that the risk of insomnia may be lower with galantamine than with donepezil. The positive control symptoms nausea and diarrhea showed significant subgroup effects as well. Some previous authors stated that donepezil generally has a better side-effect profile and especially with regard to gastrointestinal side effects, such as diarrhea and nausea [15, 61, 75, 76]. We can confirm this only for nausea. With regard to diarrhea, donepezil does not differ from rivastigmine. The risk was lowest for galantamine, which is in agreement with Zhang et al., who stated that galantamine had fewer gastrointestinal AEs than donepezil [77].
Regarding dose–effect relationships for PAEs, our results indicate that higher doses of AChEIs indeed increase the risk for PAEs, including anorexia and decreased appetite. A dose–effect relationship has previously been demonstrated for AEs of AChEIs in general. For example, Espiritu et al. and Farlow et al. reported a higher prevalence of AEs among those receiving greater doses of donepezil [78, 79]. Takeda et al. were able to demonstrate this for the other two substances as well [80].
In the overall assessment, the strengths of the present review are as follows: we specifically focused on a group of important side effects that had previously received little attention. Compared with similar reviews, our study population is comparatively large, allowing for stronger and more reliable results. We used nausea and diarrhea, known AEs of AChEIs, as positive controls, which indeed showed the expected results (a higher risk of nausea and diarrhea, with subgroup differences between higher and lower doses). Furthermore, we performed a series of sensitivity and subgroup analyses to test the robustness of our results and find potential causes of heterogeneity. Finally, data from published and unpublished studies were included in the review in order to reduce the risk of a potential publication bias. Despite the aforementioned strengths, several limitations of our study need to be considered. First, many studies had to be excluded because the side effect report was insufficient. Often, no side effects were reported or no precise information on the frequency of certain AEs was given. Requests for information from the authors usually remained unanswered. We performed analyses for each symptom or symptom cluster reported in at least five placebo-controlled trials or five active-controlled trials. As this number was not always reached, analyses could not be performed for all reported PAEs. Furthermore, the way AEs are assessed has a significant impact on AE detection: passive monitoring methods may yield fewer events than active monitoring and the lack of standardization in the passive collection of AE data may further increase the likelihood of bias. We also intended to consider indication for AChEI use (AD vs PDD), dementia severity, and participant age for subgroup analyses, but the number of eligible studies was insufficient. With only two studies, the number of studies on PDD in particular was insufficient to obtain meaningful results.
Finally, it is important to consider that the terms used by the authors of the original studies to describe PAEs may not necessarily align with psychiatric disorders denoted by similar terms. For instance, the PAE ‘depression’ was often assessed through passive data collection methods such as non-leading questions or spontaneous complaints. As a result, it could not be ensured that reported ‘depression’ met the necessary criteria for diagnosis, and some of the affected patients may have experienced subsyndromal depressive symptoms instead.
5 Conclusions
This meta-analysis summarizes the available data on the risks of PAEs during AChEI treatment. In previous studies, psychiatric safety of AChEIs was insufficiently addressed despite numerous studies investigating efficacy or general tolerability. With our work, we provide conclusive evidence that certain PAEs, including appetite disorders, insomnia, and depression, occur more frequently during AChEI treatment in the standardized setting of RCTs. Consequently, careful monitoring of newly occurring PAEs using active surveillance methods as well as regular weight checks are recommended. In our analysis, no clear differences between the substances could be demonstrated. Therefore, no individual AChEI can be specifically recommended over the others regarding psychiatric safety, except for insomnia that occurred less often during galantamine than during donepezil treatment. Finally, there appears to be a dose dependence related to certain PAEs, hence lowering the dose may resolve emerging PAEs.
References
Gauthier S, Webster C, Servaes S, Morais JA, Rosa-Neto P. World Alzheimer report 2022. Life after diagnosis: navigating treatment, care and support. London: Alzheimer’s Disease International; 2022.
Farfel JM, Yu L, Boyle PA, Leurgans S, Shah RC, Schneider JA, et al. Alzheimer’s disease frequency peaks in the tenth decade and is lower afterwards. Acta Neuropathol Commun. 2019;7(1):104.
Zhu XC, Tan L, Wang HF, Jiang T, Cao L, Wang C, et al. Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Ann Transl Med. 2015;3(3):38.
United Nations Department of Economic and Social Affairs. Population division: world population ageing 2020 highlights: living arrangements of older persons. ST/ESA/SER.A/451, 2020.
GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):105–25.
Yiannopoulou KG, Sokratis GP. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397.
Dekker MJHJ, Bouvy JC, O’Rourke D, Thompson R, Makady A, Jonsson P, et al. Alignment of European regulatory and health technology assessments: a review of licensed Products for Alzheimer’s disease. Front Med (Lausanne). 2019;6:73.
Chitnis S, Rao J. Rivastigmine in Parkinson’s disease dementia. Expert Opin Drug Metab Toxicol. 2009;5(8):41–55.
Kumar A, Singh A. Ekavali: a review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67(2):195–203.
Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging. 2004;21(7):453–78.
Čolović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315–35.
Folch J, Ettcheto M, Petrov D, Abad S, Pedrós I, Marin M, et al. Review of the advances in treatment for Alzheimer disease: strategies for combating β-amyloid protein. Neurologia (Engl Ed). 2018;33(1):47–58.
Florindo S, Radanovic M, Canineu PR, de Paula VJR, Forlenza OV. Anti-dementia medications: current prescriptions in clinical practice and new agents in progress. Ther Adv Drug Saf. 2015;6(4):151–65.
O’Brien, J.T. and Burns, A; BAP Dementia Consensus Group: Clinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(8):997–1019.
Mohammad D, Chan P, Bradley J, Lanctôt K, Herrmann N. Acetylcholinesterase inhibitors for treating dementia symptoms: a safety evaluation. Expert Opin Drug Saf. 2017;16(9):1009–19.
Magierski R, Sobow T, Schwertner E, Religa D. Pharmacotherapy of behavioral and psychological symptoms of dementia: state of the art and future progress. Front Pharmacol. 2020;11:1168.
Gómez-Esteban JC, Tijero B, Somme J, Ciordia R, Berganzo K, Rouco I, et al. Impact of psychiatric symptoms and sleep disorders on the quality of life of patients with Parkinson’s disease. J Neurol. 2011;258(3):494–9.
Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694–709.
Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015;96:235–43.
Platt B, Riedel G. The cholinergic system, EEG and sleep. Behav Brain Res. 2011;221(2):499–504.
Herman AM, Ortiz-Guzman J, Kochukov M, Herman I, Quast KB, Patel JM, et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature. 2016;538(7624):253–6.
Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53(4):618–32.
Eisai Inc. Prescribing information Aricept® (donepezil hydrochloride tablets). https://www.accessdata.fda.gov/drugsatfdadocs/label/2012/020690s035021720s008022568s005lbl.pdf. Accessed 20 Mar 2023.
Janssen Pharmaceuticals, Inc. Prescribing information Razadyne® (galantamine hydrobromide). https://www.accessdata.fda.gov/drugsatfdadocs/label/2015/021615s021lbl.pdf. Accessed 20 Mar 2023.
Novartis Pharmaceuticals Corporation. Prescribing information Exelon® (rivastigmine tartrate). https://www.novartis.com/us-en/sites/novartisus/files/exelonpatch.pdf. Accessed 20 Mar 2023.
Li DD, Zhang YH, Zhang W, Zhao P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front Neurosci. 2019;13:472.
Tricco AC, Ashoor HM, Soobiah C, Rios P, Veroniki AA, Hamid JS, et al. Comparative effectiveness and safety of cognitive enhancers for treating Alzheimer’s disease: systematic review and network metaanalysis. J Am Geriatr Soc. 2018;66(1):170–8.
Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a metaanalysis of randomised controlled trials. Lancet Neurol. 2007;6(9):782–92.
Matsunaga S, Fujishiro H, Takechi H. Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta-analysis. J Alzheimer’s Dis. 2019;71(2):513–23.
Moher D, Shamsee L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P). Syst Rev. 2015;4(1):1.
Costantino G, Montano N, Casazza G. When should we change our clinical practice based on the results of a clinical study? Searching for evidence: PICOS and PubMed. Intern Emerg Med. 2015;10(4):525–7.
Gauthier S. Cholinergic adverse effects of cholinesterase inhibitors in Alzheimer’s disease: epidemiology and management. Drugs Aging. 2001;18(11):853–62.
MedDRA Maintenance and Support Services Organization. Introductory Guide to MedDRA Version 25.1. McLean, Virginia [updated 2022 September; cited 2023 Febr]. http://www.meddra.org/how-to-use/support-documentation.
Sterne JACSJ, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Riemer TG, Villagomez Fuentes LE, Algharably EAE, Schäfer MS, Mangelsen E, Fürtig MA, et al. Do β-blockers cause depression? Systematic review and meta-analysis of psychiatric adverse events during β-blocker therapy. Hypertension. 2021;77(5):1539–48.
The Cochrane Collaboration. Review Manager (RevMan) [computer program]. Version 5.4. The Cochrane Collaboration; 2020. Chichester (UK).
Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res Synth Methods. 2017;8(3):290–302.
Doi SA, Furuya-Kanamori L, Xu C, Lin L, Chivese T, Thalib L. Controversy and debate: questionable utility of the relative risk in clinical research. Paper 1: a call for change to practice. J Clin Epidemiol. 2022;142:271–9.
Simmonds M. Quantifying the risk of error when interpreting funnel plots. Syst Rev. 2015;4:24.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions. Version 6.3 (updated February 2022). Cochrane; 2022. Chichester (UK).
Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gu¨ndisch D, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332(6035):1330–2.
Avena NM, Rada PV. Cholinergic modulation of food and drug satiety and withdrawal. Physiol Behav. 2012;106(2):332–6.
Jeong JH, Lee DK, Jo YH. Cholinergic neurons in the dorsomedial hypothalamus regulate food intake. Mol Metab. 2017;6(3):306–12.
Falk S, Lund C, Clemmensen C. Muscarinic receptors in energy homeostasis: physiology and pharmacology. Basic Clin Pharmacol Toxicol. 2020;126(6):66–76.
Watson CJ, Baghdoyan HA, Lydic R. Neuropharmacology of sleep and wakefulness. Sleep Med Clin. 2010;5(4):513–28.
Patel AK, Reddy V, Shumway KR, Araujo JF. Physiology, sleep stages. StatPearls. Treasure Island: StatPearls Publishing; 2022
Baghdoyan HA, Lydic R. M2 muscarinic receptor subtype in the feline medial pontine reticular formation modulates the amount of rapid eye movement sleep. Sleep. 1999;22(7):835–47.
Hobson JA, Stickgold R, Pace-Schott EF. The neuropsychology of REM sleep dreaming. NeuroReport. 1998;9(3):1–14.
Vazquez J, Baghdoyan HA. Basal forebrain acetylcholine release during REM sleep is significantly greater than during waking. Am J Physiol Regulatory Integr Comp Physiol. 2001;280(2):598–601.
Cooke JR, Loredo JS, Liu L, Marler M, Corey-Bloom J, Fiorentino L, et al. Acetylcholinesterase inhibitors and sleep architecture in patients with Alzheimer’s disease. Drugs Aging. 2006;23(6):503–11.
Moraes WS, Poyares DR, Guilleminault C, Ramos LR, Bertolucci PH, Tufik S. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep. 2006;29(2):199–205.
Mizuno S, Kameda A, Inagaki T, Horiguchi J. Effects of donepezil on Alzheimer’s disease: the relationship between cognitive function and rapid eye movement sleep. Psychiatry Clin Neurosci. 2004;58(6):660–5.
Schredl M, Weber B, Braus D, Gattaz WF, Berger M, Riemann D, et al. The effect of rivastigmine on sleep in elderly healthy subjects. Exp Gerontol. 2000;35(2):243–9.
Holsboer-Trachsler E, Hatzinger M, Stohler R, Hemmeter U, Gray J, Müller J, et al. Effects of the novel acetylcholinesterase inhibitor SDZ ENA 713 on sleep in man. Neuropsychopharmacology. 1993;8(1):87–92.
Biard K, Douglass AB, De Koninck J. The effects of galantamine and buspirone on sleep structure: implications for understanding sleep abnormalities in major depression. J Psychopharmacol. 2015;10:1106–11.
Riemann D, Gann H, Dressing H, Müller WE, Aldenhoff JB. Influence of the cholinesterase inhibitor galanthamine hydrobromide on normal sleep. Psychiatry Res. 1994;51(3):253–67.
Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69(1–2):137–45.
Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;256(5172):679–82.
Hornung OP, Regen F, Danker-Hopfe H, Schredl M, Heuser I. The relationship between REM sleep and memory consolidation in old age and effects of cholinergic medication. Biol Psychiatry. 2007;61(6):750–7.
Schredl M, Weber B, Leins ML, Heuser I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Exp Gerontol. 2001;36(2):353–61.
Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;1:CD005593.
Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632–5.
van Enkhuizen J, Janowsky DS, Olivier B, Minassian A, Perry W, Young JW, et al. The catecholaminergic-cholinergic balance hypothesis of bipolar disorder revisited. Eur J Pharmacol. 2015;753:14–126.
Fitzgerald PJ, Hale PJ, Ghimire A, Watson BO. Repurposing cholinesterase inhibitors as antidepressants? Dose and stress-sensitivity may be critical to opening possibilities. Front Behav Neurosci. 2021;14: 620119.
Cummings J, Lai TJ, Hemrungrojn S, Mohandas E, Yun Kim S, Nair G, et al. Role of donepezil in the management of neuropsychiatric symptoms in Alzheimer’s disease and dementia with lewy bodies. CNS Neurosci Ther. 2016;22(3):159–66.
Holmes C, Wilkinson D, Dean C, Vethanayagam S, Olivieri S, Langley A, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology. 2004;63(2):214–9.
Gauthier S, Feldman H, Hecker J, Vellas B, Ames D, Subbiah P, Whalen E, et al. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer’s disease. Int Psychogeriatr. 2002;14(4):389–404.
Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57(4):613–20.
Mega MS, Masterman DM, O’Connor SM, Barclay TR, Cummings JL. The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol. 1999;56(11):1388–93.
Jawaid A, Pawlowicz E, Schulz P. Do acetylcholinesterase inhibitors increase anxiety and depression in elderly adults with dementia? J Am Geriatr Soc. 2015;63(8):1702–4.
Winblad B, Grossberg G, Frölich L, Farlow M, Zechner S, Nagel J, et al. IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology. 2007;69(4):14–22.
Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2004;363(9427):2105–15.
Nagata T, Shinagawa S, Nakajima S, Noda Y, Mimura M. Pharmacological management of behavioral disturbances in patients with Alzheimer’s disease. Expert Opin Pharmacother. 2020;21(9):1093–102.
Rodda J, Morgan S, Walker Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int Psychogeriatr. 2009;21(5):813–24.
Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(2):615–31.
Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3(2):211–25.
Zhang Z, Yu L, Gaudig M, Schäuble B, U., R.: Galantamine versus donepezil in Chinese patients with Alzheimer’s disease: results from a randomized, double-blind study. Neuropsychiatr Dis Treat. 2012;8:571–7.
Espiritu AI, Cenina ARF. The effectiveness and tolerability of the high dose donepezil at 23 mg tablet per day for Alzheimer’s disease: a meta-analysis of randomized controlled trials. Acta Med Philipp. 2020;54(3):296–304.
Farlow MR, Salloway S, Tariot PN, Yardley J, Moline M, Wang Q, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32(7):1234–51.
Takeda A, Loveman E, Clegg A, Kirby J, Picot J, Payne E, et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;21(1):17–28.
Acknowledgements
This study, carried out under the Yale University Open Data Access Project #2021-4836, used data obtained from the Yale University Open Data Access Project, which has an agreement with Janssen Research & Development, L.L.C. The interpretation and reporting of research using this data are solely the responsibility of the authors and do not necessarily represent the official views of the Yale University Open Data Access Project or Janssen Research & Development, L.L.C. We acknowledge the provision of relevant data by principal investigators and study sponsors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for the preparation of this article.
Conflicts of interest/competing interests
Reinhold Kreutz reports modest honoraria for consultancy and lectures and support for research from Bayer AG, CinCor Menarini, Merck, Sanofi, and Servier outside the submitted work. Eva J. Brandl has received speaker fees from Medice. Nadine Bittner, Cleo S.M. Funk, Alexander Schmidt, Felix Bermpohl, Engi E.A. Algharably, and Thomas G. Riemer have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Availability of data and material
Upon a reasonable request, data supporting the conclusions can be provided.
Code availability
Not applicable.
Authors’ contributions
NB and TGR conceptualized the study; acquired, curated, and analyzed the data; and collaborated on the original draft. CSMF and AS performed supporting literature research and assisted in assessing the quality of the trials. EEAA visualized the data. RK, FB, and EJB contributed to the interpretation of the results and provided research resources. All authors provided critical feedback, approved the final submitted manuscript, and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bittner, N., Funk, C.S.M., Schmidt, A. et al. Psychiatric Adverse Events of Acetylcholinesterase Inhibitors in Alzheimer’s Disease and Parkinson’s Dementia: Systematic Review and Meta-Analysis. Drugs Aging 40, 953–964 (2023). https://doi.org/10.1007/s40266-023-01065-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-023-01065-x