Abstract
Background
We previously reported that interventions to optimize medication use reduced adverse drug reactions (ADRs) by 21% and serious ADRs by 36% in older adults. With new evidence, we sought to update the systematic review and meta-analysis.
Method
We searched OVID, Cochrane Library, ClinicalTrials.gov and Google Scholar from 30 April 2017–30 April 2023. Included studies had to be randomized controlled trials of older adults (mean age ≥65 years) taking medications that examined the outcome of ADRs. Two authors independently reviewed all citations, extracted relevant data, and assessed studies for potential bias. The outcomes were any and serious ADRs. We performed subgroup analyses by intervention type and setting. Random-effects models were used to combine the results from multiple studies and create summary estimates.
Results
Six studies are new to the update, resulting in 19 total studies (15,675 participants). Interventions were pharmacist-led (10 studies), other healthcare professional-led (5 studies), technology based (3 studies), and educational (1 study). The interventions were implemented in various clinical settings, including hospitals, outpatient clinics, long-term care facilities/rehabilitation wards, and community pharmacies. In the pooled analysis, the intervention group participants were 19% less likely to experience an ADR (odds ratio [OR] 0.81, 95% confidence interval [CI] 0.68–0.96) and 32% less likely to experience a serious ADR (OR 0.68, 95% CI 0.48–0.96). We also found that pharmacist-led interventions reduced the risk of any ADR by 35%, compared with 8% for other types of interventions.
Conclusion
Interventions significantly and substantially reduced the risk of ADRs and serious ADRs in older adults. Future research should examine whether effectiveness of interventions vary across health care settings to identify those most likely to benefit. Implementation of successful interventions in health care systems may improve medication safety in older patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our systematic review and meta-analysis found that interventions designed to optimize medication use reduced the risk of any and serious ADRs in older adults. |
Although many interventions were examined and were found to be effective, pharmacist-led interventions were particularly effective in reducing ADRs. |
1 Introduction
Adverse drug events (ADEs), defined as “an injury due to a medication”, are a major safety concern for older adults and the focus of research and public policy. The focus of this systematic review and meta-analysis was on a subtype of ADEs, adverse drug reactions (ADRs), which are defined as “a response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function” and excludes therapeutic failure and adverse drug withdrawal events (ADWEs) [1, 2].
ADRs in older adults are common in clinical practice and they can have a wide range of implications, including physical harm to the patient, prolonged illness or hospitalization, increased healthcare costs, and reduced quality of life. ADRs may also cause death [3]. Older adults have greater risk for ADRs because of polypharmacy, altered pharmacokinetics with aging, drug–drug interactions, and multimorbidity [4, 5]. Some ADRs remain undetected by clinicians and are misinterpreted as a new condition that may result in the prescribing of additional medications (i.e., prescribing cascade).
Thus, prevention of ADRs in older adults is necessary as they worsen health outcomes. In our previous systematic review and meta-analysis, we reported that interventions were effective in reducing ADRs in older adults, especially serious ADRs [6]. However, we were unable to perform multiple subgroup analyses because of the limited number of studies. Recently, several new randomized controlled trials of interventions to improve medication use that measured ADRs have been published. Therefore, the objective of the present systematic review and meta-analysis was to update the results to examine the impact of interventions to optimize medication use on ADRs in older adults and examine whether results varied by intervention type. A new objective of this update was to examine whether the intervention effectiveness varied according to the healthcare setting where the intervention was implemented.
2 Methods
2.1 Search Strategy
We used a similar approach as used in the first meta-analysis [6]. Briefly, we performed a comprehensive review of the literature using the OVID, Cochrane Library, ClinicalTrials.gov and Google Scholar databases from 30 April 2017 to 30 April 2023. The latter source was searched to identify any ‘grey literature’. The search terms included a combination of the following keywords: aged, adverse drug events or reaction, randomized controlled trials, and English language. We also examined the citations from seminal studies, reviews, and book chapters, as well as the authors’ files.
2.2 Study Selection
Two authors (SLG and JTH) examined the titles and abstracts of studies to identify those using interventions to optimize medication use or reduce medication errors in any setting compared with usual care. Next, we looked at the full manuscripts for these studies and included only those studies where the average age of participants was ≥ 65 years of age and that used a randomized controlled trial design, and measured ADRs as an independent outcome or as part of an overall assessment of drug-related problems.
2.3 Data Extraction
A standardized data collection form based in part on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was used [7, 8]. Elements of the final data collection form included author name, date of publication, country, sample size, mean age of each study arm, setting (e.g., long-term care facility, outpatient, hospital), study intervention type and details (e.g., pharmacist-led), follow-up time, and rate of any and serious ADRs by group status. Details about ADR assessment included process for detecting ADRs (e.g., chart review, patient interview); method for assessing causality (e.g., whether an explicit causality algorithm was used, such as Naranjo or the World Health Organization–Uppsala Monitoring Centre (WHO–UMC) [9], or implicit methods using just a Likert scale); and number and background of the evaluators. Two reviewers (SLG and JTH) independently extracted study data. Discrepancies were resolved in consensus meetings.
2.4 Assessment of Study Quality
Two authors (SLG, JTH) independently assessed the methodological quality of the six new studies using the RoB 2.0 Cochrane Collaboration tool for assessing the risk of bias [10]. Five separate bias domains were assessed, including the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. One additional domain utilized the results from the five domains to determine overall bias. Discrepancies were resolved in consensus meetings.
2.5 Outcomes
The outcomes include any and serious ADRs [1, 2]. Serious ADRs were defined as those that resulted in death, hospitalization, permanent disability, or the need for an intervention to prevent permanent impairment [11]. The terms ADEs and ADRs are often used interchangeably. We included studies that examined the outcome of ADEs if we were able to determine that the outcome excluded therapeutic failures and ADWEs, often requiring communication with the study author.
2.6 Statistical Analysis
We essentially followed the same methodology as the original meta-analysis and added information from the six new studies to the analyses and examined a subgroup according to study setting. The number of distinct participants with at least one ADR and the total number of participants was extracted for each intervention arm in each new study. From multiple formats of data presented, odds ratios (ORs) were computed as the common metric, representing each study’s intervention versus control group difference. We constructed forest plots to present the results graphically. We used a Chi-square statistic based on Cochran’s Q-statistic and Higgins I2 statistic for assessing heterogeneity [12]. An I2 of 25% is considered low heterogeneity, 50% moderate, and 75% high. Upon observing non-trivial levels of heterogeneity, we used random effects models for pooling the individual log ORs to obtain overall estimates [13]. Leave-one-out and cumulative meta-analyses were performed to identify influential studies and examine evolution of accumulated evidence over time. We used a funnel plot and Egger’s test to assess publication bias [14]. We stratified the analysis for any ADR by whether the intervention studied was led by a pharmacist and according to the healthcare setting, and tested for differences in intervention effectiveness across subgroups. Comprehensive Meta-Analysis® version 3 (Biostat, Inc., Englewood, NJ, USA) and SAS® version 9.4 (SAS Institute, Inc., Cary, NC, USA) software was used for main analyses and graphics. Robvis® was used to create risk-of-bias plots [15]. Data sharing is not applicable to this article as only publicly available information was used.
3 Results
3.1 Characteristics of Included Studies
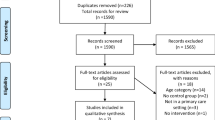
Of the 36,576 studies retrieved, 316 were screened for inclusion, with 27 being assessed for eligibility. After review, 21 studies were excluded. Fourteen were excluded due to age < 65 years (n = 2), non-randomized design (n = 1), duplicate publication (n = 2), and not assessing ADRs or ADEs (n = 9), and seven studies that assessed ADEs or drug-related problems were excluded as ADRs could not be delineated [16,17,18,19,20,21,22]. Thus, we identified six additional studies, which added 8669 patients, to bring the total number to 19 studies, with 21 comparisons involving 15,675 patients included in this review (Fig. 1) [23,24,25,26,27,28]. The six new studies examined interventions implemented in the hospital, with one extending the intervention throughout the post-acute care (PAC) facility stay. For three studies, the outcome of interest was inpatient ADRs [23, 24, 26], whereas the remaining studies examined ADRs 1–12 months following discharge from hospital or PAC facility [25, 27, 28].
The study characteristics are presented in Table 1. The studies were conducted in Europe (11 studies) [23,24,25,26, 29,30,31,32,33,34,35], North America (7 studies) [11, 27, 28, 36,37,38,39], and Australia (1 study) [40] between 1996 and 2023. The interventions were implemented in a variety of clinical settings, including hospitals [11, 23,24,25,26,27,28,29,30,31], outpatient clinics [11, 33, 36, 37], long-term care facilities/rehabilitation wards [32, 34, 38,39,40], and community pharmacies [35]. For two studies, the intervention was started during hospitalization and was continued when patients were discharged to home [29] or PAC facility [28]. Furthermore, for studies that initiated interventions in the hospital, follow-up for outcomes occurred during the hospital stay for six studies, whereas four studies had follow-up from 1 to 12 months after discharge [25, 27,28,29]. Follow-up for inpatient studies was < 2 weeks, and ranged from 2 weeks to 12 months for the remaining included studies [11, 25, 27,28,29, 32,33,34,35,36,37,38,39,40].
Most studies (n = 18) utilized interventions to improve overall prescribing rather than focusing specifically on reducing ADRs, and one study focused on improving the safety of warfarin [38]. Details of the interventions are provided in Table 2. The most common type of intervention was pharmacist-led (10 studies, 11 comparisons), with a core component of medication review that involved a number of implicit structured methods (i.e., judgment-based) to perform medication review and identify drug-related problems [24, 25, 29, 33, 34, 36, 37, 40] or a combination of explicit (e.g., criterion-based, Screening Tool of Older People’s Prescriptions/Screening Tool to Alert to Right Treatment [STOPP/START criteria]) and implicit approaches [31, 35]. One study used clinical decision support software (CDSS) to support the pharmacists’ medication review process [31]. Different modes of communication were used to relay recommendations to the prescriber. In most pharmacist-led studies, the recommendations were made in-person to the prescriber [25, 29, 34,35,36, 40]; however, other studies primarily communicated recommendations through the medical record or facsimile [24, 31, 33, 37, 41]. Pharmacists also provided education directly to patients in some studies [29, 33, 34, 36, 37].
Intensity of interventions varied across the studies, with most studies delivering a one-time intervention, but in six studies, the intervention was delivered at two or more times during the study [11, 27,28,29, 34, 38]. The intervention involving the most contact was conducted by Vasilevskis et al., where the pharmacist/nurse practitioner-led intervention spanned transitions of care from hospital to PAC facility and eventually home [28]. The study team called the PAC nurse to review medications within 48 h of transfer to the facility from the hospital; called weekly to review medications and monitor deprescribing actions; and at PAC discharge, sent a discharge list and ongoing deprescribing recommendations to outpatient prescribers.
Nine studies used other interventions to optimize medication use to reduce ADRs: other health professional-led interventions [11, 23, 28, 30, 38]; use of technology for clinical decision support or patient-specific reports to guide deprescribing [26, 27, 39], and a one-time educational session for physicians [32]. The three studies that used CDSS technology to generate recommendations for prescribers did not find a reduction in ADRs [26, 27, 39], whereas the pharmacist-led study that was supported by CDSS to identify potential drug-related problems did lead to a significant reduction in ADRs [31].
ADR was the primary outcome in 11 studies [11, 24,25,26, 29,30,31,32, 37,38,39], part of a general drug-related problem outcome in three studies [33,34,35], and a secondary outcome in four studies [23, 28, 36, 40]. Two studies evaluated interventions to reduce drug-related hospital readmissions [25, 29]. Most studies used medical record review to detect potential ADRs [11, 24,25,26, 28,29,30,31,32, 34, 35, 38,39,40], with four studies using more than one method [11, 23, 25, 34]. Eleven studies used two or more reviewers to assess ADRs [11, 24,25,26,27, 29, 31, 32, 35, 38, 39], and eight studies used an explicit ADR causality algorithm to evaluate probability, including Naranjo [11, 28, 37], WHO–UMC [26, 30, 31], and others [25, 27].
3.2 Methodological Quality of Studies
Assessment of the risk of bias for new studies is summarized in electronic supplementary material (ESM) Fig. 1. Overall, two studies had low bias, whereas four studies had some concerns about bias.
3.3 Meta-Analysis
Of the 19 studies included in the meta-analysis, one study reported the intervention for two different settings and one study examined two interventions compared with usual care [11, 37]. Thus, 21 between-intervention comparisons were included in the meta-analysis. In the pooled analysis, the intervention group participants were 19% less likely to experience an ADR (OR 0.81, 95% confidence interval [CI] 0.68–0.96) (Fig. 2). Pharmacist-led interventions were common (n = 11) and reduced the risk by 35% (OR 0.65, 95% CI 0.48–0.89). Other types of interventions (other professional-led, technology, education) reduced the risk by 8% (OR 0.92, 95% CI 0.76–1.12) in others (Fig. 3). However, test of a difference in effect by whether the intervention was led by a pharmacist was only marginally significant (z = 1.87; p = 0.061). In the 9 studies (10 interventions) that examined serious ADRs, a reduction of 32% was noted (OR 0.68, 95% CI 0.48–0.96) (Fig. 4). Within each healthcare setting, ADR rates were not significantly different but the pooled point estimates descriptively favored intervention (pooled ORs 0.53–0.93; p = 0.145–0.411) (Fig. 5).
Forest plot of the intervention effects on the likelihood of experiencing any adverse drug reaction (Q = 58.2, p < 0.001, I2 = 65.6%). Pooled estimates (diamond) calculated by the random effects model for pooling the individual log odds ratios and obtaining an overall estimate that incorporates between-study heterogeneity.
Forest plot of the intervention effects on the likelihood of experiencing any adverse drug reaction (not pharmacist-led: Q = 28.0, p = 0.001, I2 = 67.8%; pharmacist-led: Q = 23.4, p = 0.009, I2 = 57.2%). Pooled estimates (diamond) calculated by the random effects model for pooling the individual log odds ratios and obtaining an overall estimate that incorporates between-study heterogeneity.
Egger’s test for publication bias was marginally significant (p = 0.0842) and the funnel plot (ESM Fig. S2) also indicated an asymmetry, suggesting substantial publication bias. Leave-one-out analysis did not materially change the pooled OR, but the study by Gillespie et al. continues to be the most influential, whose removal attenuated the risk reduction to 14% (OR 0.86, 95% CI 0.74–1.00; p = 0.046) [29]. Within pharmacist-led interventions, removal of the study by Gillespie et al. attenuated the risk reduction to 24% (OR 0.76, 95% CI 0.62–0.94; p = 0.012). The cumulative meta-analysis showed that the magnitude of the pooled OR started favoring intervention in 2009 with the study by Gillespie et al., its statistical significance was attained in 2016 with the study by O’Connor et al., and results continue to be stable with the newer studies included in the present update.
4 Discussion
We added six new randomized controlled trials to the present update examining the effectiveness of interventions to reduce ADRs in older adults. Importantly, the results of this update confirm our original report, with a significant 19% reduction for any ADRs and a 32% reduction in serious ADRs in the intervention groups compared with usual care. We also found that pharmacist-led interventions reduced the risk of any ADR by 35%, compared with 8% for other types of interventions. Given the range of settings in which trials were conducted, we also sought to examine whether effectiveness varies across settings. However, the number of studies in each setting was relatively small, precluding definitive conclusions. To our knowledge, other systematic reviews on interventions to optimize medication use to reduce ADRs have not been published.
ADR reduction is a public health priority because ADRs are common, often preventable, and can have a substantial negative impact on health outcomes and health care costs [4]. Regarding how common ADRs are, estimates vary, with ADRs occurring in 16% of hospitalized older adults [42], 10% of hospital admissions [3], and 5–15% of hospitalized patients in the month following a hospital discharge [27, 43, 44]. An ideal intervention to reduce ADRs would focus on older adults at the highest risk and settings, where interventions can be implemented within the existing workflow. Of included studies, the hospital was the most common study setting for the implementation of interventions, with some studies focused on inpatient ADRs while others focused on ADRs in the post-discharge period. Hospitalized patients have high comorbidity and acute illness that increases the risk for ADRs. Furthermore, conducting inpatient studies offers a pragmatic benefit in that hospitalized patients can be monitored easily for ADRs, unlike in other settings. Only two studies in this review focused on the outcome of drug-related hospital readmissions [25, 29], a challenging yet important area for future study given the serious nature of these ADRs. We await the completion of an ongoing study that is evaluating the effectiveness of an intervention that incorporates a validated ADR risk score to determine whether ADRs following hospital discharge are reduced [45]. Few studies were conducted in long-term care facilities, despite comparable ADR incidence as in hospitals [46, 47].
The present review demonstrates that a variety of interventions were successful in reducing the risk of ADRs. Half of the studies utilized a pharmacist-led intervention; however, even among them, the interventions varied considerably with regard to intensity, method of communication with providers, provision of patient education, and setting. The use of information technology to support medication reviews is becoming common practice, but few studies in our review incorporated information technology to assist with ADR reduction [26, 27, 30, 38]. The use of technology to support medication review and deprescribing that integrates into workflow has the potential to lead to cost-effective interventions. Four studies in our review included technology to assist with prioritizing medications for deprescribing, of which one study supported a pharmacist-led intervention [26, 27, 31, 39]. These findings suggest that technology interventions may be most successful when the recommendations are implemented by a trained health professional.
Studies used different definitions and strategies for identifying ADRs, which may result in considerable heterogeneity between studies. Studies that measured only ADEs could not be included in this review as therapeutic failures and ADWEs could not be subtracted. Identifying ADRs is subjective and some ADRs can be misinterpreted as symptoms or signs of another medical condition, rather than effects of medications. Thus, it is important to assess the causal link between an observed adverse event and a suspected drug using a validated probability scale, such as the Naranjo or WHO–UMC algorithms, by at least two reviewers. Only eight studies in this review assessed causality [25,26,27, 30, 31], thus in most studies, it is not clear whether the adverse event was drug-related.
A remaining question is whether interventions to reduce ADRs benefit other downstream health and economics outcomes. While not focused on ADRs, as was the focus for this review, a meta-analysis by Tecklenborg et. al., reported that interventions to reduce ADEs did not did benefit hospitalizations, emergency department visits, mortality, quality of life, mental health, or physical function [48]. This is not surprising as the risks are multifactorial for these health outcomes, with medications being only one aspect. The systematic review was limited by the small number of studies and lack of information regarding the intervention effect on ADE incidence. Furthermore, it is unclear if the studies were statistically powered to examine these downstream effects. This is an important focus for future research.
Strengths of this review include the comprehensive search strategy and verification with authors when necessary to identify ADRs from the broader outcome of ADEs. We used standard methods to reduce bias, including the use of a standardized abstraction form and independent review by two authors, with disagreements discussed through a consensus meeting. However, there are several limitations to our report. It is possible that we missed non-English-language randomized controlled intervention trials. The interventions varied considerably in implementation and duration across studies, even among pharmacist-led interventions. The effectiveness difference between pharmacist-led and other interventions was only marginally statistically significant, precluding stronger conclusions. However, such tests of interaction generally have low statistical power. Regarding generalizability, there were too few studies to conduct analyses specific to country. Most studies were conducted at a single center; therefore, it is unclear how easily the interventions can be replicated in other health care systems. The low number of studies included in each health care setting contributed to uncertainty in point estimates and precision. However, these are common limitations in meta-analyses and are not particularly unique to ours.
5 Conclusion
We conclude that interventions designed to optimize medication use reduce the risk of ADRs, including serious ADRs, in older adults. Pharmacist-led interventions may be particularly effective in improving medication safety in older adults, but stronger evidence is needed. Further research should focus on whether interventions vary according to healthcare setting. Lastly, research is needed to identify how best to disseminate and implement these interventions into varied health care systems.
References
Karch FE, Lasagna L. Adverse drug reactions. A critical review. JAMA. 1975;234(12):1236–41.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9. https://doi.org/10.1016/S0140-6736(00)02799-9.
Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. 2017;73(6):759–70. https://doi.org/10.1007/s00228-017-2225-3.
Zazzara MB, Palmer K, Vetrano DL, Carfì A, Onder G. Adverse drug reactions in older adults: a narrative review of the literature. Eur Geriatr Med. 2021;12(3):463–73. https://doi.org/10.1007/s41999-021-00481-9.
Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. https://doi.org/10.1517/14740338.2013.827660.
Gray SL, Hart LA, Perera S, Semla TP, Schmader KE, Hanlon JT. Meta-analysis of interventions to reduce adverse drug reactions in older adults. J Am Geriatr Soc. 2018;66(2):282–8. https://doi.org/10.1111/jgs.15195.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9 (W264).
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116(6):394–401. https://doi.org/10.1016/j.amjmed.2003.10.031.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. https://doi.org/10.1002/jrsm.1411.
Gustafsson M, Sjolander M, Pfister B, Jonsson J, Schneede J, Lovheim H. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: a randomized controlled trial. Eur J Clin Pharmacol. 2017;73(7):827–35. https://doi.org/10.1007/s00228-017-2249-8.
Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med. 2018;178(3):375–82. https://doi.org/10.1001/jamainternmed.2017.8274.
Tamblyn R, Abrahamowicz M, Buckeridge DL, Bustillo M, Forster AJ, Girard N, et al. Effect of an Electronic Medication Reconciliation Intervention on Adverse Drug Events: A Cluster Randomized Trial. JAMA Netw Open. 2019;2(9): e1910756. https://doi.org/10.1001/jamanetworkopen.2019.10756.
Gurwitz JH, Kapoor A, Garber L, Mazor KM, Wagner J, Cutrona SL, et al. Effect of a multifaceted clinical pharmacist intervention on medication safety after hospitalization in persons prescribed high-risk medications: a randomized clinical trial. JAMA Intern Med. 2021;181(5):610–8. https://doi.org/10.1001/jamainternmed.2020.9285.
Ceschi A, Noseda R, Pironi M, Lazzeri N, Eberhardt-Gianella O, Imelli S, et al. Effect of Medication Reconciliation at Hospital Admission on 30-Day Returns to Hospital: A Randomized Clinical Trial. JAMA Netw Open. 2021;4(9): e2124672. https://doi.org/10.1001/jamanetworkopen.2021.24672.
Blum MR, Sallevelt B, Spinewine A, O’Mahony D, Moutzouri E, Feller M, et al. Optimizing Therapy to Prevent Avoidable Hospital Admissions in Multimorbid Older Adults (OPERAM): cluster randomised controlled trial. BMJ. 2021;374: n1585. https://doi.org/10.1136/bmj.n1585.
Roughead EE, Pratt NL, Parfitt G, Rowett D, Kalisch-Ellett LM, Bereznicki L, et al. Effect of an ongoing pharmacist service to reduce medicine-induced deterioration and adverse reactions in aged-care facilities (nursing homes): a multicentre, randomised controlled trial (the ReMInDAR trial). Age Ageing. 2022. https://doi.org/10.1093/ageing/afac092.
Wehling M, Burkhardt H, Kuhn-Thiel A, Pazan F, Throm C, Weiss C, et al. VALFORTA: a randomised trial to validate the FORTA (Fit fOR The Aged) classification. Age Ageing. 2016;45(2):262–7. https://doi.org/10.1093/ageing/afv200.
Nielsen TRH, Honore PH, Rasmussen M, Andersen SE. Clinical effects of a pharmacist intervention in acute wards—a randomized controlled trial. Basic Clin Pharmacol Toxicol. 2017;121(4):325–33. https://doi.org/10.1111/bcpt.12802.
Lenssen R, Schmitz K, Griesel C, Heidenreich A, Schulz JB, Trautwein C, et al. Comprehensive pharmaceutical care to prevent drug-related readmissions of dependent-living elderly patients: a randomized controlled trial. BMC Geriatr. 2018;18(1):135. https://doi.org/10.1186/s12877-018-0814-3.
O’Mahony D, Gudmundsson A, Soiza RL, Petrovic M, Jose Cruz-Jentoft A, Cherubini A, et al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR* randomized controlled clinical trial. Age Ageing. 2020;49(4):605–14. https://doi.org/10.1093/ageing/afaa072.
McDonald EG, Wu PE, Rashidi B, Wilson MG, Bortolussi-Courval E, Atique A, et al. The MedSafer study-electronic decision support for deprescribing in hospitalized older adults: a cluster randomized clinical trial. JAMA Intern Med. 2022;182(3):265–73. https://doi.org/10.1001/jamainternmed.2021.7429.
Vasilevskis EE, Shah AS, Hollingsworth EK, Shotwell MS, Kripalani S, Mixon AS, et al. Deprescribing medications among older adults from end of hospitalization through postacute care: a shed-MEDS randomized clinical trial. JAMA Intern Med. 2023;183(3):223–31. https://doi.org/10.1001/jamainternmed.2022.6545.
Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900. https://doi.org/10.1001/archinternmed.2009.71.
O’Connor MN, O’Sullivan D, Gallagher PF, Eustace J, Byrne S, O’Mahony D. Prevention of hospital-acquired adverse drug reactions in older people using screening tool of older persons’ prescriptions and screening tool to alert to right treatment criteria: a cluster randomized controlled trial. J Am Geriatr Soc. 2016;64(8):1558–66. https://doi.org/10.1111/jgs.14312.
O’Sullivan D, O’Mahony D, O’Connor MN, Gallagher P, Gallagher J, Cullinan S, et al. Prevention of adverse drug reactions in hospitalised older patients using a software-supported structured pharmacist intervention: a cluster randomised controlled trial. Drugs Aging. 2016;33(1):63–73. https://doi.org/10.1007/s40266-015-0329-y.
Trivalle C, Cartier T, Verny C, Mathieu AM, Davrinche P, Agostini H, et al. Identifying and preventing adverse drug events in elderly hospitalised patients: a randomised trial of a program to reduce adverse drug effects. J Nutr Health Aging. 2010;14(1):57–61.
Lenander C, Elfsson B, Danielsson B, Midlov P, Hasselstrom J. Effects of a pharmacist-led structured medication review in primary care on drug-related problems and hospital admission rates: a randomized controlled trial. Scand J Prim Health Care. 2014;32(4):180–6. https://doi.org/10.3109/02813432.2014.972062.
Willoch K, Blix HS, Pedersen-Bjergaard AM, Eek AK, Reikvam A. Handling drug-related problems in rehabilitation patients: a randomized study. Int J Clin Pharm. 2012;34(2):382–8. https://doi.org/10.1007/s11096-012-9623-5.
Kwint HF, Faber A, Gussekloo J, Bouvy ML. Effects of medication review on drug-related problems in patients using automated drug-dispensing systems: a pragmatic randomized controlled study. Drugs Aging. 2011;28(4):305–14. https://doi.org/10.2165/11586850-000000000-00000.
Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–37. https://doi.org/10.1016/S0002-9343(97)89519-8.
Touchette DR, Masica AL, Dolor RJ, Schumock GT, Choi YK, Kim Y, et al. Safety-focused medication therapy management: a randomized controlled trial. J Am Pharm Assoc (2003). 2012;52(5):603–12. https://doi.org/10.1331/JAPhA.2012.12036.
Field TS, Tjia J, Mazor KM, Donovan JL, Kanaan AO, Harrold LR, et al. Randomized trial of a warfarin communication protocol for nursing homes: an SBAR-based approach. Am J Med. 2011;124(2):179.e171-177. https://doi.org/10.1016/j.amjmed.2010.09.017.
Gurwitz JH, Field TS, Rochon P, Judge J, Harrold LR, Bell CM, et al. Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. J Am Geriatr Soc. 2008;56(12):2225–33. https://doi.org/10.1111/j.1532-5415.2008.02004.x.
Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother. 2004;2(4):257–64.
Nielsen TR, Andersen SE, Rasmussen M, Honore PH. Clinical pharmacist service in the acute ward. Int J Clin Pharm. 2013;35(6):1137–51. https://doi.org/10.1007/s11096-013-9837-1.
Jennings ELM, Murphy KD, Gallagher P, O’Mahony D. In-hospital adverse drug reactions in older adults; prevalence, presentation and associated drugs-a systematic review and meta-analysis. Age Ageing. 2020;49(6):948–58. https://doi.org/10.1093/ageing/afaa188.
Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20(4):317–23. https://doi.org/10.1111/j.1525-1497.2005.30390.x.
Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–7. https://doi.org/10.7326/0003-4819-138-3-200302040-00007.
Cousins J, Parameswaran Nair N, Curtain C, Bereznicki B, Wilson K, Adamczewski B, et al. Preventing Adverse Drug Reactions After Hospital Discharge (PADR-AD): protocol for a randomised-controlled trial in older people. Res Social Adm Pharm. 2022;18(8):3284–9. https://doi.org/10.1016/j.sapharm.2021.09.007.
Handler SM, Wright RM, Ruby CM, Hanlon JT. Epidemiology of medication-related adverse events in nursing homes. Am J Geriatr Pharmacother. 2006;4(3):264–72. https://doi.org/10.1016/j.amjopharm.2006.09.011.
Field TS, Gurwitz JH, Avorn J, McCormick D, Jain S, Eckler M, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161(13):1629–34. https://doi.org/10.1001/archinte.161.13.1629.
Tecklenborg S, Byrne C, Cahir C, Brown L, Bennett K. Interventions to reduce adverse drug event-related outcomes in older adults: a systematic review and meta-analysis. Drugs Aging. 2020;37(2):91–8. https://doi.org/10.1007/s40266-019-00738-w.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported in part by the National Institute on Aging (P30AG024827), and VA Health Services Research and Development Service Merit Award (IIR 18-228).
Conflicts of interest
Shelly L. Gray, Subashan Perera, Tim Soverns, and Joseph T. Hanlon have no conflicts of interest to disclose.
Data sharing
Not applicable to this article as only publicly available information was used.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to study conception, design and interpretation of the data; SLG and JTH drafted the manuscript; TS extracted data from articles; all authors revised the manuscript for critical intellectual content; and SP conducted statistical analyses. All authors had full access to the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsors
The sponsors did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication; or did not contribute to open access fees.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gray, S.L., Perera, S., Soverns, T. et al. Systematic Review and Meta-analysis of Interventions to Reduce Adverse Drug Reactions in Older Adults: An Update. Drugs Aging 40, 965–979 (2023). https://doi.org/10.1007/s40266-023-01064-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-023-01064-y