Abstract
Difficult-to-treat rheumatoid arthritis is a heterogeneous term in which patients may present with difficulties in their management for different reasons. This can ultimately lead to patients being exposed to multiple treatments because of inefficacy (resulting from mechanisms intrinsic to rheumatoid arthritis or from non-inflammatory causes such as chronic pain syndrome or structural damage, among others), toxicity or adverse effects that may be linked to comorbidities. One particular group in which such characteristics may be more patent is older patients. Increasing life expectancy, an ageing population and the late onset of rheumatoid arthritis have led to an increased interest in the particularities of treating older patients. This may pose a challenge for physicians, as ageing has implications for optimal patient treatment owing to the potential presence of comorbidities, the risk of adverse events and perceptions of disease status by both physicians and patients. All of these factors may have implications for classifying and managing patients aged > 65 years as difficult-to-treat rheumatoid arthritis, as these patients could be misclassified. This can occur when a significant proportion may still exhibit signs of active disease but not necessarily be difficult to treat because the treatment criterion has not been fulfilled. Alternatively, patients may be exposed to multiple biologic/targeted disease-modifying antirheumatic drugs because of contraindications and/or comorbid conditions. Treatment-to-target strategies and an adequate assessment of inflammatory rheumatoid arthritis activity in older patients should be undertaken, taking special care with associated comorbidities, polypharmacy and risk profiles. Such an approach can help to ensure appropriate treatment for older adults and avoid the misclassification of difficult-to-treat patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Treatment of rheumatoid arthritis in older patients should be carried out in the same manner as in younger patients in accordance with the treat-to-target strategy. |
The management of an older rheumatoid arthritis population requires special care in drug selection and dose adjustments to ensure adequate treatment. |
Age-related conditions can lead to patients classified as difficult to treat, owing to the need for frequent treatment changes in response to comorbidities or adverse effects. |
1 Introduction: Defining “difficult-to-treat rheumatoid arthritis”
In recent years, outcomes in rheumatoid arthritis (RA) have been transformed, thanks to a better understanding of the immunological mechanisms involved in the pathophysiology of RA, treatment algorithms that measure disease activity with composite indices, treatment-to-target strategies and therapeutic options based on disease-modifying anti-rheumatic drugs (DMARDs) [conventional, biologic and targeted]. Long-term damage and functional decline have significantly reduced and systemic features diminished [1,2,3,4].
While currently prospects are good for most patients, there remains a percentage who do not achieve the desired therapeutic goals despite receiving multiple DMARDs over the course of their disease. At first, these patients were considered to have refractory RA, and some attempts were made to establish clinical factors that could determine this resistance to disease-modifying drugs [5,6,7,8,9]. Given the variability of the criteria used to classify these patients, and recognising this refractory condition as an emerging issue in the treatment of RA [10], in 2020, the European League Against Rheumatism (EULAR) established a definition of difficult-to-treat RA (D2TRA) [11]. Its goal was to advance a valid definition that could be used in clinical practice and clinical trials and be applicable to future research in order to obtain a better overall understanding of this disease.
This definition consists of three criteria encompassing treatment failures, uncontrolled disease activity and clinical perception of the management of RA signs/symptoms. All three criteria must be present in D2TRA including: (1) treatment according to EULAR recommendations and the failure of two or more biologic and targeted synthetic (b/ts) DMARDs (with different mechanisms of action) after no response to conventional synthetic DMARD therapy (unless contraindicated); (2) signs suggestive of active/progressive disease defined as presenting with one or more of the following: moderate disease activity according to validation composite indexes, signs and/or symptoms suggestive of active disease, inability to taper glucocorticoid treatment below 7.5 mg/day of prednisone or equivalent, rapid radiographic progression or well-controlled disease according to standards but still presenting with RA symptoms; and (3) the management of signs and/or symptoms is perceived as problematic by the rheumatologist and/or the patient [11].
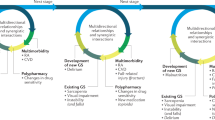
Difficult-to-treat RA ultimately involves the exposure of patients to multiple treatments because of inefficacy (resulting from mechanisms intrinsic to RA or because of non-inflammatory causes such as chronic pain syndrome or structural damage, among others), toxicity or adverse effects that may be linked to comorbidities [12]. A special group of patients in which these characteristics may be more patent is older patients. Mechanisms attributable to immunosenescence may lead to increased chronic inflammation and comorbidities [13], limiting treatment options or necessitating therapeutic changes. In addition, some studies suggest that the prognosis of elderly-onset RA is more severe because these patients may also have higher scores on composite activity indices, which may suggest that the disease is not well controlled [14], leading to changes in disease management. The differences between elderly-onset RA and young-onset RA may be owing to changes in proinflammatory cytokine profiles that occur in old age [15] that lead to typical features of elderly-onset RA (including a more balanced sex distribution, a common abrupt onset with involvement of large proximal joints and increased systemic symptoms) [16]. In addition, these clinical features become more evident as ageing continues [17].
Difficult-to-treat RA in older adults is particularly important because the older RA population is expanding, as a result of an increased life expectancy and an increasing incidence of elderly-onset RA [18]. In the following sections, we break down the implications of ageing on managing older patients with D2TRA.
2 Treatment with b/tDMARDs in Older Population
Treatment of older patients should have exactly the same goals as with younger patients: to control disease activity, prevent structural damage, preserve functionality and decrease the excess mortality conferred by RA. Therefore, the treatment-to-target approach used with older patients with RA should be the same as with younger patients. In addition, patients should be treated early in order to achieve low disease activity/remission [2,3,4, 19]. However, in older patients, this treatment-to-target approach may be limited by the greater prevalence of comorbidities as well as the higher risk of potential adverse effects, which can result in those patients not receiving optimal treatment [2, 4, 20].
Assessments of efficacy in older patients in randomised clinical trials are hampered by the scarcity of data as these patients typically do not meet the established inclusion criteria, or drop out of the studies early, generally because of comorbidities and/or adverse effects. However, there are studies of real-world data showing that the efficacy of RA-specific treatments is similar in older and younger patients [19, 21]. Thus, real-world data suggest that the efficacy of tumour necrosis factor inhibitors (TNFi) in older patients is similar to that in younger patients, especially in terms of clinical response, although functional indexes may not be similar because of associated comorbidities and the presence of osteoarthritis [22,23,24]. Abatacept has a favourable disease control profile in both younger and older patients [25, 26]. Data about the effectiveness of rituximab in older patients are scarce but suggest that this treatment is less effective in comparison with younger patients [27]. In the case of tocilizumab, a recent study carried out in a Japanese population revealed that the retention rates of this drug are higher than those of TNFi in older patients. In addition, the study showed that discontinuation because of inefficacy was lower in the tocilizumab group than in the TNFi group [28]. This could be explained because apparently in older patients there is an overexpression of interleukin-6 [15]. As for JAK inhibitors (JAKi), according to the published data, efficacy in older populations is quite similar to that in younger populations. In addition, these DMARDs seem to offer a valid alternative for those patients who already meet the criteria for D2TRA because of multiple treatment failures, as these drugs possess mechanisms of action against a greater number of cytokines [29].
3 Limitations on the Use of b/tsDMARDs in the Older Population
3.1 Comorbidities and Extra-Articular Manifestations
The exposure of older patients to multiple b/tsDMARDs may have no correlation with a lack of efficacy because as mentioned above, these treatments do not necessarily have to be less effective, per se, in controlling disease activity. However, multiple failures/changes of b/tsDMARDs may be linked to suboptimal treatment because of patient comorbidities or the potential risks of these treatments. Such problems hamper optimal RA management in this group of patients and constitute a contributing factor to the occurrence of D2TRA [30].
When referring to comorbidities in persons aged 65 years and older we must consider both those typically associated with ageing and those that are closely linked to RA, as they share the same pathogenic mechanisms that lead to a chronic inflammatory status and increased risk factors. The most frequent comorbidities associated with RA are cardiovascular, pulmonary, infections, osteoporosis, depression and malignancies [31]. The presence of age-associated or RA-associated comorbidities is difficult to distinguish, but in either case they pose a limitation to the usual application of optimal treatment strategies because of contra-indications in drug prescription strategies [32].
In addition to dramatic increases in the incidence and prevalence of age-related diseases, there has also been a rise in certain extra-articular features of the disease. Rheumatoid arthritis populations have an increased risk of pulmonary disease with respect to the general population, with RA-associated interstitial lung disease being a particular example. Indeed, it has a higher prevalence in older versus younger individuals, which implies a steady worsening in the overall RA management strategies employed in the older population [33,34,35].
In addition to the increased comorbidities that can lead not only to polypharmacy, but also to increases in adverse effects and drug interactions, pharmacokinetics in older patients can vary (e.g. absorption, distribution, metabolism and elimination) in direct relation to age [32, 36]. Renal function may also be impaired, which may necessitate dose adjustments in accordance with creatinine clearance [37]. This is frequent in treatment with methotrexate, in which doses in older adults may be lower than those in younger patients. What this means is that full doses of conventional synthetic DMARDs, or even combinations of DMARDs, cannot be used, thus hampering optimal control of RA [38]. Such problems may lead to the need for a change in treatment, for example, switching from a TNFi to a different b/tsDMARD with another mechanism of action more suitable for use in monotherapy [39, 40]. Not only do conventional DMARDs require such adjustments, but so do other recently introduced agents such as JAKi, filgotinib and baricitinib, which can be administered with a lower daily dose in those aged >75 years compared with younger patients. Baricitinib can be administered at dosages of 2 mg/day and filgotinib at 100 mg/day versus 4 mg/day and 200 mg/day, respectively [41, 42].
Because of the importance of this issue, several studies have taken into account validated comorbidity indices for RA in order to determine the most appropriate drug choice for achieving good clinical control and/or good drug retention rates [43,44,45,46]. Therefore, the impact of comorbidities on the choice of treatment in the older population can lead to the scenario in which such comorbidities may necessitate multiple changes in the prescription of b/tsDMARDs, thereby affecting the D2TRA status [47, 48]. Moreover, these same comorbidities could limit therapeutic options, leaving clinicians unable to optimally control RA. For all these reasons, the risk/benefit factor of switching b/tsDMARDs in older patients must be carefully weighed now more than ever [49,50,51,52].
3.2 Safety of Using b/tsDMARDs in the Older Population
There is increasing evidence concerning the safety of specific RA therapies in the older population. In fact, safety data concerning biologics in older individuals are often similar to those in younger patients, and evidence on TNFi and abatacept has steadily increased. Tumour necrosis factor inhibitors have shown similar safety profiles in both older and younger patients [23, 24]. However, rates of serious adverse events (AEs), severe infections and cancer were slightly higher in the former, although this risk does not appear to increase with age and/or in relation to comorbidities [22, 53]. Abatacept is the biologic with the lowest AE discontinuation rates in the Japanese RA population [25, 26]. Rituximab, however, has been associated with numerically higher infection rates in older patients [27].
One of the AEs of greatest concern in patients receiving biologic therapies is the risk of infection, as infections are among the primary causes of premature mortality in this population. This risk may be higher in the older population because of the worsening of the immune system with age. In fact, a data analysis of the German Biologics Register (RABBIT) revealed an increased risk of infection in those aged older than 60 years with active disease, receiving high doses of corticosteroids and presenting with comorbidities such as pulmonary or renal disease. Thus, not only has age been shown to be an independent risk factor for the development of infections in RA [54, 55], but the presence of comorbidities also then poses a risk factor for the onset of AEs [32, 56].
The case of JAKi seems to be more closely associated with varicella zoster virus risk in older versus younger patients [41, 57]. One concern regarding JAKi is data from a recently published study on the safety of tofacitinib [58]. This study concludes that the use of tofacitinib in patients >50 years of age and/or smokers and with at least one cardiovascular risk factor resulted in an increase of AEs such as major adverse cardiovascular events and malignancies with respect to TNFi. Thus, use of this drug in the older population should be restricted. Ultimately, these findings have also been extended to other JAKi and potentially limit treatment options in older patients.
Malignancies are also important in RA populations, as it has long been recognised that RA is associated with an increased risk of malignancy in general. Indeed, the immunological processes that occur during ageing and RA may contribute to an increased risk of cancer owing to the presence of chronic inflammation and defective DNA repair. Therefore, care must be exercised in treating RA in older patients as the risk of many types of cancer increases with age [59]. Indeed, it would be advisable to perform screening procedures in line with the well-known risk factors and with the health recommendations for cancer prevention for the general population. Examples of such screening in current medical practice include the following: fecal occult blood screening and colonoscopy for colorectal cancer (although incidence of the latter in RA is lower than in the general population); β2-microglobulin for haematological malignancies; determination of prostate-specific antigen for prostate carcinoma; mammography and screening for estrogen-dependent malignancies in postmenopausal women; and chest X-rays especially in patients with a long history of smoking [60]. Therefore, following the clinical context, this would be useful for tailoring treatment but currently there are no screening recommendations to apply more comprehensive cancer screening procedures in patients requiring b/tsDMARDs [61].
Nevertheless, the overall risks associated with biologic DMARDs, primarily TNFi, appear to be low after accounting for confounders. Moreover, as for non-TNF biologic DMARDs, no safety signals of concern have emerged in meta-analyses of clinical trial data, albeit within their limitations. There is a clear need for further investigation of the safety of all biologic DMARD treatments in regard to malignancies [62, 63]. This is also true of new targeted synthetic DMARDs, which require special vigilance owing to recently published data on the incidence of malignancies and cardiovascular events [58].
4 Assessment of Signs of Active/Progressive Disease in the Older Population
In addition to the potential limitations of using different b/tsDMARDs, another issue related to the definition of D2TRA concerns the persistent signs of disease activity and/or progression. In persons aged 65 years and older, patient assessments can prove more challenging and complex versus those conducted on younger patients. Therefore, following the recent publication of the EULAR Task Force on D2TRA, different points must be considered in order to provide holistic management for therapeutic decisions [64].
4.1 Disease Activity
As the erythrocyte sedimentation rate increases with age, it may interfere with interpretations of disease activity indices such as the Disease Activity Score-28, although the former (erythrocyte sedimentation rate) remains adequate for assessing disease activity in moderate-to-severe RA regardless of age or sex. However, remission rates could be underestimated in the older population because of this problem [14]. Baseline disease activity in late-onset RA seems to be higher than in young-onset RA, perhaps owing to the aforementioned elevation of the erythrocyte sedimentation rate; in fact, once corrected for age, there is little difference [65, 66]. However, the duration of disease in terms of therapeutic goal achievement is somewhat controversial, as it appears that a longer disease duration may influence the response to subsequent treatments [40, 67]. In addition, older patients generally score a higher visual analogue scale assessment as they age, which may render assessments of activity indices problematic, as the Disease Activity Score-28 parameter could be overestimated in terms of activity and therapeutic target achievement [68].
4.2 Functional Status
With regard to functional indexes, the health assessment questionnaire may vary with disease duration, presumably because the longer the duration, the worse the functional status, thus leading to a higher disability index. This may correlate with a higher number of painful joints, which ultimately translates into higher scores in the activity indexes as well. This is true not only for long-standing RA, but also for late-onset RA, in which the health assessment questionnaire both at baseline and at follow-up had a higher score [52, 69,70,71].
4.3 Radiographic Progression
Regarding radiographic progression, we found different data in the studies. Mueller et al. noted that late-onset RA may be more erosive at baseline, although there were no significant differences in the variation of radiographic progression between young-onset and late-onset RA during the patient follow-up [65]. However, Krams et al. did find that age at RA onset was associated with greater radiological damage and the occurrence of at least one or more erosions during the 1–2 years of follow-up with respect to younger patients [70].
4.4 Tapering Corticosteroids
Late-onset RA is usually more prevalent in male individuals, with acute systemic onset and large joint involvement, which may mimic polymyalgia rheumatica [72]. In this case, older patients may initially require treatment with higher doses of corticosteroids in order to alleviate symptoms more quickly. Although, as we have seen, DMARDs are generally well tolerated in the older population, these patients may be exposed to a more frequent use of corticosteroids in monotherapy compared with younger individuals. This may lead to a misclassification of such patients as D2TRA because of the inability to taper corticosteroids below 7.5 mg/day of prednisone (or equivalent) [39, 73, 74].
5 Management of Signs or Symptoms is Perceived as Problematic by the Rheumatologist and/or the Patient
Clinical guidelines have played an essential role in improving healthcare for people with RA; however, the application of these guidelines may not take into account the biological and psychosocial changes associated with ageing. Older patients and physicians often have different priorities regarding treatment and health outcomes. Patients may be more focused on current symptom control and, especially older patients, complain of symptoms that might not even been related to the rheumatic disease itself. Therefore, this change in patients’ priorities, in addition to comorbidities, treatment idiosyncrasies and the potential difficulties assessing disease activity assessment in older patients is important in the management of the disease. We now focus on these factors such as psychosocial issues, outcome values, self-efficacy expectancies, patient education and adherence to treatment [75].
While these factors can play various roles in both younger and older patients, in the former (late-onset RA and established RA diagnosed in youth), treatment management can be complicated by the frailty related to ageing and the onset of geriatric syndromes, leading to an overall sense of a lack of control of the disease [76]. The presence of sarcopenia, a syndrome involving a progressive decrease in skeletal muscle mass and strength, which is associated with poor physical condition, fatigue, functional impairment and disability, leads to high morbidity and mortality in these patients [77]. This syndrome is associated with both advanced age and systemic inflammation, while RA per se is a major contributing factor to physical disabilities in patients and is associated with age. Therefore, sarcopenia and inflammation can be regarded as the biological underpinnings of physical frailty. Frailty is associated with older adults who appear more fragile than their age-matched counterparts, despite sharing similar comorbidities, demographics, sex and age [78, 79]. Therefore, it is necessary to take into account not only ageing (understood as age >65 years), but also frailty as an indicator of a state of greater vulnerability.
As we have seen, long-standing RA and the persistence of high disease activity linked to ageing can lead to greater degrees of disability. Approximately 10% of older patients have difficulty performing the basic activities of daily living and experience functional impairment [76]. Patients with RA are associated with a seven-fold greater risk of disability than patients of the same age and sex [80]. This, together with age and the onset of geriatric syndromes such as depression, frailty, cognitive impairment, falls and/or nutritional deficits, contributes to a lower success rate in the treatment of RA and a tendency to depression in these patients [76, 81,82,83].
Depression is a frequent comorbidity in patients with RA, particularly in older patients in which it is coupled with geriatric syndromes. Indeed, cognitive impairment can affect compliance and subsequently the response of older patients to treatment [84, 85]. In fact, some studies have proposed interventions focused on the outpatient management of older patients and multi-morbidities given that frailty and cognitive impairment may be present. These studies have mainly involved comprehensive multidisciplinary reviews by a nurse, pharmacist and physician every 6 months [86, 87] or by primary care teams [88, 89]. In addition, a recent pilot study tried to improve the efficiency of care provided based on patient goals and medication reviews [90]. However, the results of these studies were not entirely promising because of the complexity of implementing these systems.
However, there are data in the literature regarding rheumatology nursing consultations and the resulting quality from a patient standpoint [91, 92]. Thus, in older patients it would be very interesting to attempt this type of intervention in which support is provided to both the patient and his or her close environment (family and/or social) in the management of anti-rheumatic therapies (at least at the beginning of treatment). Education about the disease and the administration route of DMARDs, always tailored to the individual patient and taking into account the burden of disease and concomitant treatments, can be essential to optimising treatment and ensuring compliance to the greatest degree possible.
Therefore, physicians must take into account all of these psychosocial factors. Shared decisions, explaining treatment expectations and providing information on practical aspects can improve adherence. Although the lack of adherence is not directly related to age, a physician’s manner and explanations to older patients, as well as other non-pharmacologic interventions, may improve treatment success in this population [93,94,95].
6 Conclusions
Treatment of RA in the older population should be carried out in the same manner as in younger patients in accordance with the treatment-to-target strategy. However, the associated comorbidities, polypharmacy-related issues and risk profiles of older patients should be carefully assessed in order to treat RA. Care should be taken in drug selection and dose adjustments to ensure adequate treatment. In addition, an inflammatory RA activity assessment is essential prior to any DMARD adjustments. With regard to osteoarthritis, other physiological conditions and geriatric syndromes that may impact the patient’s quality of life, in the older population, it is crucial to better incorporate non-pharmacological actions in order to improve and optimise the treatment of functional disability, pain and fatigue, including physical, physiological, educational exercise and self-management interventions. Older patients present a group of particular concern in D2TRA, as their age-related condition can lead to a misclassification into the D2TRA group, owing to the need for frequent treatment changes in response to comorbidities or adverse effects. In addition, the use of b/tsDMARDs in the older population is constrained by the many issues discussed above and must be undertaken with great caution.
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. https://doi.org/10.1016/S0140-6736(16)30173-8.
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–99. https://doi.org/10.1136/annrheumdis-2019-216655.
Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. https://doi.org/10.1002/art.39480.
Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15. https://doi.org/10.1136/annrheumdis-2015-207524.
Kearsley-Fleet L, Davies R, De Cock D, Watson KD, Lunt M, Buch MH, et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2018;77:1405–12. https://doi.org/10.1136/annrheumdis-2018-213378.
de Hair MJH, Jacobs JWG, Schoneveld JLM, van Laar JM. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology. 2018;57:1135–44. https://doi.org/10.1093/rheumatology/kex349.
Bécède M, Alasti F, Gessl I, Haupt L, Kerschbaumer A, Landesmann U, et al. Risk profiling for a refractory course of rheumatoid arthritis. Semin Arthritis Rheum. 2019;49:30553–5. https://doi.org/10.1016/j.semarthrit.2019.02.004.
Buch MH. Defining refractory rheumatoid arthritis. Ann Rheum Dis. 2018;77:966–9. https://doi.org/10.1136/annrheumdis-2017-212862.
Novella-Navarro M, Plasencia C, Tornero C, Navarro-Compán V, Cabrera-Alarcón JL, Peiteado-López D, et al. Clinical predictors of multiple failure to biological therapy in patients with rheumatoid arthritis. Arthritis Res Ther. 2020;22(1):284. https://doi.org/10.1186/s13075-020-02354-1.
Roodenrijs NMT, de Hair MJH, van der Goes MC, Jacobs JWG, Welsing PMJ, van der Heijde D, et al. Characteristics of difficultto-treat rheumatoid arthritis: results of an international survey. Ann Rheum Dis. 2018;77:1705–9. https://doi.org/10.1136/annrheumdis-2018-213687.
Nagy G, Roodenrijs NM, Welsing PM, Kedves M, Hamar A, van der Goes MC, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(1):31–5. https://doi.org/10.1136/annrheumdis-2020-217344.
Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(1):17–33. https://doi.org/10.1038/s41584-020-00541-7.
Chalan P, van den Berg A, Kroesen BJ, Brouwer L, Boots A. Rheumatoid arthritis, immunosenescence and the hallmarks of aging. Curr Aging Sci. 2015;8(2):131–46. https://doi.org/10.2174/1874609808666150727110744.
Radovits BJ, Fransen J, van Riel PL, Laan RF. Influence of age and gender on the 28-joint Disease Activity Score (DAS28) in rheumatoid arthritis. Ann Rheum Dis. 2008;67(8):1127–31. https://doi.org/10.1136/ard.2007.079913.
Chen DY, Hsieh TY, Chen YM, Hsieh CW, Lan JL, Lin FJ. Proinflammatory cytokine profiles of patients with elderly-onset rheumatoid arthritis: a comparison with younger-onset disease. Gerontology. 2009;55(3):250–8. https://doi.org/10.1159/000164393.
Romão VC, Humby F, Kelly S, Di Cicco M, Mahto A, Lazarou I, et al. Treatment-resistant synovitis and radiographic progression are increased in elderly-onset rheumatoid arthritis patients: findings from a prospective observational longitudinal early arthritis cohort study. Semin Arthritis Rheum. 2020;50:735–43. https://doi.org/10.1016/j.semarthrit.2020.03.018.
Uchiyama S, Takanashi S, Takeno M, Gono T, Kaneko Y, Takeuchi T, et al. Should we reconsider the definition of elderly-onset rheumatoid arthritis in an ageing society? Mod Rheumatol. 2022;32(2):323–9.
Kumagai K, Okumura N, Amano Y, Yayama T, Mimura T, Maeda T, et al. Consideration of differences in drug usage between young-onset and elderly-onset rheumatoid arthritis with target of low disease activity. Mod Rheumatol. 2021;31(6):1094–9. https://doi.org/10.1080/14397595.2021.1883251.
Díaz-Borjón A. Guidelines for the use of conventional and newer disease-modifying antirheumatic drugs in elderly patients with rheumatoid arthritis. Drugs Aging. 2009;26(4):273–93. https://doi.org/10.2165/00002512-200926040-00001.
Soubrier M, Tatar Z, Couderc M, Mathieu S, Dubost JJ. Rheumatoid arthritis in the elderly in the era of tight control. Drugs Aging. 2013;30(11):863–9. https://doi.org/10.1007/s40266-013-0122-8.
Sugihara T, Ishizaki T, Hosoya T, Iga S, Yokoyama W, Hirano F, et al. Structural and functional outcomes of a therapeutic strategy targeting low disease activity in patients with elderly-onset rheumatoid arthritis: a prospective cohort study (CRANE). Rheumatology (Oxford). 2015;54(5):798–807. https://doi.org/10.1093/rheumatology/keu395.
Hyrich KL, Watson KD, Silman AJ, Symmons DP, British Society for Rheumatology Biologics Register. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford). 2006;45(12):1558–65. https://doi.org/10.1093/rheumatology/kel149.
Genevay S, Finckh A, Ciurea A, Chamot AM, Kyburz D, Gabay C, Physicians of the Swiss Clinical Quality Management Program for Rheumatoid Arthritis. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;57(4):679–85. https://doi.org/10.1002/art.22688.
Radovits BJ, Kievit W, Fransen J, van de Laar MA, Jansen TL, van Riel PL, et al. Influence of age on the outcome of antitumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(9):1470–3. https://doi.org/10.1136/ard.2008.094730.
Ebina K, Hashimoto M, Yamamoto W, Hirano T, Hara R, Katayama M, et al. Drug tolerability and reasons for discontinuation of seven biologics in elderly patients with rheumatoid arthritis: the ANSWER cohort study. PLoS ONE. 2019;14(5): e0216624. https://doi.org/10.1371/journal.pone.0216624.
Lahaye C, Soubrier M, Mulliez A, Bardin T, Cantagrel A, Combe B, et al. French Society of Rheumatology. Effectiveness and safety of abatacept in elderly patients with rheumatoid arthritis enrolled in the French Society of Rheumatology’s ORA registry. Rheumatology (Oxford). 2016;55(5):874–82. https://doi.org/10.1093/rheumatology/kev437.
Payet S, Soubrier M, Perrodeau E, Bardin T, Cantagrel A, Combe B, et al. Efficacy and safety of rituximab in elderly patients with rheumatoid arthritis enrolled in a French Society of Rheumatology registry. Arthritis Care Res (Hoboken). 2014;66(9):1289–95. https://doi.org/10.1002/acr.22314.
Jinno S, Onishi A, Dubreuil M, Hashimoto M, Yamamoto W, et al. Comparison of the drug retention and reasons for discontinuation of tumor necrosis factor inhibitors and interleukin-6 inhibitors in Japanese patients with elderly-onset rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther. 2021;23(1):116. https://doi.org/10.1186/s13075-021-02496-w.
Ochi S, Sonomoto K, Nakayamada S, Tanaka Y. Preferable outcome of Janus kinase inhibitors for a group of difficult-to-treat rheumatoid arthritis patients: from the FIRST Registry. Arthritis Res Ther. 2022;24(1):61. https://doi.org/10.1186/s13075-022-02744-7.
Roodenrijs NMT, van der Goes MC, Welsing PMJ, Tekstra J, Lafeber FPJG, Jacobs JWG, et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology (Oxford). 2021;60(8):3778. https://doi.org/10.1093/rheumatology/keaa860.
Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73(1):62–8. https://doi.org/10.1136/annrheumdis-2013-204223.
Serhal L, Lwin MN, Holroyd C, Edwards CJ. Rheumatoid arthritis in the elderly: characteristics and treatment considerations. Autoimmun Rev. 2020;19(6): 102528. https://doi.org/10.1016/j.autrev.2020.102528.
Wang D, Zhang J, Lau J, Wang S, Taneja V, Matteson EL, et al. Mechanisms of lung disease development in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15(10):581–96. https://doi.org/10.1038/s41584-019-0275-x.
Messina R, Guggino G, Benfante A, Scichilone N. Interstitial lung disease in elderly rheumatoid arthritis patients. Drugs Aging. 2020;37(1):11–8. https://doi.org/10.1007/s40266-019-00727-z.
Selman M, López-Otín C, Pardo A. Age-driven developmental drift in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J. 2016;48(2):538–52. https://doi.org/10.1183/13993003.00398-2016.
Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis. 2015;74(2):415–21. https://doi.org/10.1136/annrheumdis-2013-204021.
Ranganath VK, Furst DE. Disease-modifying antirheumatic drug use in the elderly rheumatoid arthritis patient. Rheum Dis Clin North Am. 2007;33(1):197–217. https://doi.org/10.1016/j.rdc.2006.12.011.
Soubrier M, Mathieu S, Payet S, Dubost JJ, Ristori JM. Elderly-onset rheumatoid arthritis. Jt Bone Spine. 2010;77(4):290–6. https://doi.org/10.1016/j.jbspin.2010.04.004.
Tutuncu Z, Reed G, Kremer J, Kavanaugh A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65(9):1226–9. https://doi.org/10.1136/ard.2005.051144.
Murata K, Ito H, Hashimoto M, Nishitani K, Murakami K, Tanaka M, et al. Elderly onset of early rheumatoid arthritis is a risk factor for bone erosions, refractory to treatment: KURAMA cohort. Int J Rheum Dis. 2019;22(6):1084–93. https://doi.org/10.1111/1756-185X.13428.
Fleischmann R, Alam J, Arora V, Bradley J, Schlichting DE, Muram D, et al. Safety and efficacy of baricitinib in elderly patients with rheumatoid arthritis. RMD Open. 2017;3(2): e000546. https://doi.org/10.1136/rmdopen-2017-000546.
Genovese MC, Kalunian K, Gottenberg JE, Mozaffarian N, Bartok B, Matzkies F, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322(4):315–25. https://doi.org/10.1001/jama.2019.9055.
England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken). 2015;67(6):865–72. https://doi.org/10.1002/acr.22456.
Radner H, Yoshida K, Mjaavatten MD, Aletaha D, Frits M, Lu B, et al. Development of a multimorbidity index: impact on quality of life using a rheumatoid arthritis cohort. Semin Arthritis Rheum. 2015;45(2):167–73. https://doi.org/10.1016/j.semarthrit.2015.06.010.
Biggioggero M, Mesina F, Favalli EG. The use of Rheumatic Disease Comorbidity Index for predicting clinical response and retention rate in a cohort of rheumatoid arthritis patients receiving tumor necrosis factor alpha inhibitors. Biomed Res Int. 2019;2019:6107217. https://doi.org/10.1155/2019/6107217.
Monti S, Klersy C, Gorla R, Sarzi-Puttini P, Atzeni F, Pellerito R, et al. Factors influencing the choice of first- and second-line biologic therapy for the treatment of rheumatoid arthritis: real-life data from the Italian LORHEN Registry. Clin Rheumatol. 2017;36(4):753–61. https://doi.org/10.1007/s10067-016-3528-y.
Takanashi S, Kaneko Y, Takeuchi T. Elderly patients with comorbidities in the definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(11):1491–3.
Takanashi S, Kaneko Y, Takeuchi T. Characteristics of patients with difficult-to-treat rheumatoid arthritis in clinical practice. Rheumatology (Oxford). 2021;60(11):5247–56.
Batko B, Urbański K, Świerkot J, Wiland P, Raciborski F, Jędrzejewski M, et al. Comorbidity burden and clinical characteristics of patients with difficult-to-control rheumatoid arthritis. Clin Rheumatol. 2019;38(9):2473–81. https://doi.org/10.1007/s10067-019-04579-1.
Radner H, Yoshida K, Frits M, Iannaccone C, Shadick NA, Weinblatt M, et al. The impact of multimorbidity status on treatment response in rheumatoid arthritis patients initiating disease-modifying anti-rheumatic drugs. Rheumatology (Oxford). 2015;54(11):2076–84. https://doi.org/10.1093/rheumatology/kev239.
Frisell T, Baecklund E, Bengtsson K, Di Giuseppe D, Forsblad-d’Elia H, Askling J, ARTIS Study Group. Patient characteristics influence the choice of biological drug in RA, and will make non-TNFi biologics appear more harmful than TNFi biologics. Ann Rheum Dis. 2018;77(5):650–7. https://doi.org/10.1136/annrheumdis-2017-212395.
Innala L, Berglin E, Möller B, Ljung L, Smedby T, Södergren A, et al. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2014;16(2):R94. https://doi.org/10.1186/ar4540.
Bathon J, Fleischmann R, Van der Heijde D, Tesser J, Peloso P, White Y, et al. Safety and efficacy of etanercept treatment in elderly subjects with rheumatoid arthritis. J Rheumatol. 2006;33:234–43.
Strangfeld A, Eveslage M, Schneider M, Bergerhausen HJ, Klopsch T, Zink A, et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70(11):1914–20. https://doi.org/10.1136/ard.2011.151043.
Zink A, Manger B, Kaufmann J, Eisterhues C, Krause A, Listing J, et al. Evaluation of the RABBIT risk score for serious infections. Ann Rheum Dis. 2014;73(9):1673–6. https://doi.org/10.1136/annrheumdis-2013-203341.
Vela P, Sanchez-Piedra C, Perez-Garcia C, Castro-Villegas MC, Freire M, Mateo L, et al. Influence of age on the occurrence of adverse events in rheumatic patients at the onset of biological treatment: data from the BIOBADASER III register. Arthritis Res Ther. 2020;22(1):143. https://doi.org/10.1186/s13075-020-02231-x.
Curtis J, Schulze-Koops H, Takiya L, Mebus CA, Terry KK, Biswas P, et al. Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin Exp Rheumatol. 2017;35:390–400.
Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. ORAL Surveillance Investigators. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–26. https://doi.org/10.1056/NEJMoa2109927.
Pedersen JK, Engholm G, Skytthe A, Christensen K, Academy of Geriatric Cancer Research (AgeCare). Cancer and aging: epidemiology and methodological challenges. Acta Oncol. 2016;55(Suppl. 1):7–12. https://doi.org/10.3109/0284186X.2015.1114670.
Nannini C, Cantini F, Niccoli L, Cassarà E, Salvarani C, Olivieri I, et al. Single-center series and systematic review of randomized controlled trials of malignancies in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis receiving anti-tumor necrosis factor alpha therapy: is there a need for more comprehensive screening procedures? Arthritis Rheum. 2009;61(6):801–12. https://doi.org/10.1002/art.24506.
Turesson C, Matteson EL. Malignancy as a comorbidity in rheumatic diseases. Rheumatology (Oxford). 2013;52(1):5–14. https://doi.org/10.1093/rheumatology/kes189.
De Cock D, Hyrich K. Malignancy and rheumatoid arthritis: epidemiology, risk factors and management. Best Pract Res Clin Rheumatol. 2018;32(6):869–86. https://doi.org/10.1016/j.berh.2019.03.011.
Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, van der Heijde D, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1101–36. https://doi.org/10.1136/annrheumdis-2016-210708.
Nagy G, Roodenrijs NMT, Welsing PMJ, Kedves M, Hamar A, van der Goes MC, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2022;81(1):20–33. https://doi.org/10.1136/annrheumdis-2021-220973.
Mueller RB, Kaegi T, Finckh A, Haile SR, Schulze-Koops H, von Kempis J, SCQM Physicians. Is radiographic progression of late-onset rheumatoid arthritis different from young-onset rheumatoid arthritis? Results from the Swiss prospective observational cohort. Rheumatology (Oxford). 2014;53(4):671–7. https://doi.org/10.1093/rheumatology/ket399.
Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2000;27(10):2351–9.
Aletaha D, Maa JF, Chen S, Park SH, Nicholls D, Florentinus S, et al. Effect of disease duration and prior disease-modifying antirheumatic drug use on treatment outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2019;78(12):1609–15. https://doi.org/10.1136/annrheumdis-2018-214918.
Sokka T, Mäkinen H, Hannonen P, Pincus T. Most people over age 50 in the general population do not meet ACR remission criteria or OMERACT minimal disease activity criteria for rheumatoid arthritis. Rheumatology (Oxford). 2007;46(6):1020–3. https://doi.org/10.1093/rheumatology/kem051.
Aletaha D, Ward MM. Duration of rheumatoid arthritis influences the degree of functional improvement in clinical trials. Ann Rheum Dis. 2006;65(2):227–33. https://doi.org/10.1136/ard.2005.038513.
Krams T, Ruyssen-Witrand A, Nigon D, Degboe Y, Tobon G, Fautrel B, et al. Effect of age at rheumatoid arthritis onset on clinical, radiographic, and functional outcomes: the ESPOIR cohort. Jt Bone Spine. 2016;83(5):511–5. https://doi.org/10.1016/j.jbspin.2015.09.010.
Camacho EM, Verstappen SM, Lunt M, Bunn DK, Symmons DP. Influence of age and sex on functional outcome over time in a cohort of patients with recent-onset inflammatory polyarthritis: results from the Norfolk Arthritis Register. Arthritis Care Res (Hoboken). 2011;63(12):1745–52. https://doi.org/10.1002/acr.20609.
Yazici Y, Paget SA. Elderly-onset rheumatoid arthritis. Rheum Dis Clin N Am. 2000;26(3):517–26. https://doi.org/10.1016/s0889-857x(05)70154-x.
Villa-Blanco JI, Calvo-Alén J. Elderly onset rheumatoid arthritis: differential diagnosis and choice of first-line and subsequent therapy. Drugs Aging. 2009;26:739–50. https://doi.org/10.2165/11316740-000000000-00000.
Tutuncu Z, Kavanaugh A. Rheumatic disease in the elderly: rheumatoid arthritis. Rheum Dis Clin N Am. 2007;33:57–70. https://doi.org/10.1016/j.rdc.2006.12.006.
van Onna M, Boonen A. Challenges in the management of older patients with inflammatory rheumatic diseases. Nat Rev Rheumatol. 2022;18(6):326–34. https://doi.org/10.1038/s41584-022-00768-6.
Chen YM, Chen LK, Lan JL, Chen DY. Geriatric syndromes in elderly patients with rheumatoid arthritis. Rheumatology (Oxford). 2009;48(10):1261–4. https://doi.org/10.1093/rheumatology/kep195.
Li TH, Chang YS, Liu CW, Su CF, Tsai HC, Tsao YP, et al. The prevalence and risk factors of sarcopenia in rheumatoid arthritis patients: a systematic review and meta-regression analysis. Semin Arthritis Rheum. 2021;51(1):236–45.
Salaffi F, Di Matteo A, Farah S, Di Carlo M. Inflammaging and frailty in immune-mediated rheumatic diseases: how to address and score the issue. Clin Rev Allergy Immunol. 2022. https://doi.org/10.1007/s12016-022-08943-z.
Hanlon P, Morton F, Siebert S, Jani BD, Nicholl BI, Lewsey J, et al. Frailty in rheumatoid arthritis and its relationship with disease activity, hospitalisation and mortality: a longitudinal analysis of the Scottish Early Rheumatoid Arthritis cohort and UK Biobank. RMD Open. 2022;8(1): e002111.
Sokka T, Krishnan E, Häkkinen A, Hannonen P. Functional disability in rheumatoid arthritis patients compared with a community population in Finland. Arthritis Rheum. 2003;48:59–63.
Dunlop DD, Semanik P, Song J, Manheim LM, Shih V, Chang RW. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005;52(4):1274–82. https://doi.org/10.1002/art.20968.
Shin SY, Julian L, Katz P. The relationship between cognitive function and physical function in rheumatoid arthritis. J Rheumatol. 2013;40(3):236–43. https://doi.org/10.3899/jrheum.120871.
Matcham F, Norton S, Scott DL, Steer S, Hotopf M. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology. 2016;55:268–78. https://doi.org/10.1093/rheumatology/kev306.
Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology. 2013;52:2136–48. https://doi.org/10.1093/rheumatology/ket169.
Michelsen B, Kristianslund EK, Sexton J, Hammer HB, Fagerli KM, Lie E, et al. Do depression and anxiety reduce the likelihood of remission in rheumatoid arthritis and psoriatic arthritis? Data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis. 2017;76(11):1906–10. https://doi.org/10.1136/annrheumdis-2017-211284.
Pannick S, Davis R, Ashrafian H, Byrne BE, Beveridge I, Athanasiou T, et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–98. https://doi.org/10.1001/jamainternmed.2015.2421.
Webster JR, Chew RB, Mailliard L, Moran MB. Improving clinical and cost outcomes in delirium: use of practice guidelines and a delirium care team. Ann Long-Term Care. 1999;7:128–34.
Salisbury C, Man MS, Bower P, Guthrie B, Chaplin K, Gaunt DM, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet. 2018;392:41–50.
Boult C, Leff B, Boyd CM, Wolff JL, Marsteller JA, Frick KD, et al. A matched-pair cluster-randomized trial of guided care for high-risk older patients. J Gen Intern Med. 2013;28:612–21.
Van Onna M, Öztürk B, Starmans M, Peeters R, Boonen A. Disease and management beliefs of elderly patients with rheumatoid arthritis and comorbidity: a qualitative study. Clin Rheumatol. 2018;37:2367–72.
Muñoz-Fernández S, Aguilar MD, Rodríguez A, Almodóvar R, Cano-García L, Gracia LA, et al. SCORE Working Group. Evaluation of the impact of nursing clinics in the rheumatology services. Rheumatol Int. 2016;36(9):1309–17.
Calvo-Aranda E, Sánchez-Aranda FM, Cebrián Méndez L, Matías de la Mano MLÁ, Lojo Oliveira L, Navío Marco MT. Perceived quality in patients with gout treated in a rheumatology clinic with a clinical nurse specialist. Rheumatol Clin (Engl Ed). 2021. https://doi.org/10.1016/j.reumae.2021.07.001.
Pasma A, van’t Spijker A, Hazes JM, Busschbach JJ, Luime JJ. Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum. 2013;43(1):18–28. https://doi.org/10.1016/j.semarthrit.2012.12.001.
Balsa A, García de Yébenes MJ, Carmona L, ADHIERA Study Group. Multilevel factors predict medication adherence in rheumatoid arthritis: a 6-month cohort study. Ann Rheum Dis. 2022;81(3):327–34. https://doi.org/10.1136/annrheumdis-2021-221163.
Roodenrijs NMT, Hamar A, Kedves M, Nagy G, van Laar JM, van der Heijde D, et al. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open. 2021;7(1): e001512. https://doi.org/10.1136/rmdopen-2020-001512.
Acknowledgements
The authors thank Dr. Chamaida Plasencia for her critical reading of the draft manuscript. The authors also thank the Spanish Foundation of Rheumatology for providing medical writing/editorial assistance during the preparation of the manuscript (FERBT2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of interest/competing interests
Marta Novella-Navarro reports grants from UCB, Galapagos, Lilly and Janssen outside the submitted work. Alejandro Balsa reports grants form Abbvie, Amgen, Pfizer, Galapagos, Novartis, Gilead, BMS, Nordic, Sanofi, Sandoz, Lilly, UCB and Roche outside the submitted work.
Ethics approval
This review article did not require approval by an ethics committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
MN-N and AB designed and drafted the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Novella-Navarro, M., Balsa, A. Difficult-to-Treat Rheumatoid Arthritis in Older Adults: Implications of Ageing for Managing Patients. Drugs Aging 39, 841–849 (2022). https://doi.org/10.1007/s40266-022-00976-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-022-00976-5