Abstract
Introduction
Medications with anticholinergic activity (MACs) are used to treat diseases common in older adults. Evidence on the association between anticholinergic burden (AB) and increased risk of fractures and osteoporosis or reduced bone mineral density (BMD) is inconsistent. Our aim was to conduct a systematic review of observational studies on AB with fractures and osteoporosis or reduced BMD and provide methodological appraisal of included studies.
Methods
We searched MEDLINE, EMBASE, Science Citation Index and CENTRAL as well as grey literature from database inception up to August 2020. Eligibility criteria were: observational design, AB-exposure measured through a scale, fracture of any type or osteoporosis or reduced BMD as outcome, and reported measure of association between exposure and outcome. No restrictions related to time, language or type of data were applied. Eligibility and risk of bias assessment as well as data extraction were performed independently by two reviewers. Risk of bias of the included studies was assessed using the Newcastle–Ottawa Scale and the RTI Item Bank.
Results
The majority of the nine included studies had low risk of bias but heterogeneous methodology. No study used a new user design. Seven studies reported an increased risk of fractures associated with AB. In four studies using the Anticholinergic Risk Scale (ARS), adjusted risk of fractures was increased by 2–61% for ARS = 1, by 0–97% for ARS = 2, by 19–84% for ARS = 3, and by 56–96% for ARS ≥ 4; in three studies the ARS was aggregated, risk increased by 39% for ARS = 1–2 and 17% for ARS = 2–3. Two studies reported increased risk of fractures of 14 and 52% in the highest AB-category and one study reported that change in ARS of ≥ 3 during hospitalization was associated with a 321% increased risk in fractures. Two studies did not find an association between AB and fractures. The association between AB and osteoporosis or reduced BMD could only be assessed in two studies, one reporting increased risk of lower BMD at Ward’s triangle, the other reporting no association between AB and BMD T-score change at the femoral neck.
Discussion
Our study suggests an association between AB and increased risk of fractures with possible dose-exposure gradient in studies using the ARS. The low number of studies and heterogeneity of methods calls for the conduct of more studies.
Plain language summary
We conducted a study investigating the risk of fractures associated with anticholinergic burden, which is the result of taking one or more medication with anticholinergic activity. The results of our study suggest that persons who experience anticholinergic burden might have a higher risk of fractures. However, since we were only able to include nine studies, more studies conducted in a similar way are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This systematic review suggests that the risk of fractures is increased in persons with high anticholinergic burden. |
In studies using Anticholinergic Risk Scale (ARS), the risk increases with increasing anticholinergic burden, suggesting a dose exposure gradient. |
We found that one study reported an increased risk of lower BMD at Ward’s triangle in persons with high anticholinergic burden, however a second study did not find an association. |
Overall, the studies used heterogeneous methods and few studies had high quality. This calls for conduct of more high quality studies. |
1 Introduction
Medications with anticholinergic activity (MACs) are used for the treatment of various conditions including Parkinson’s disease, depression, cardiovascular diseases, asthma, chronic obstructive pulmonary disease (COPD), allergies as well as incontinence and overactive bladder [1, 2]. Prevalence of use of MACs differs depending on the study population: in community dwelling or general populations aged 65 and older between 9 and 57%; [3,4,5] among nursing home residents between 55 and 77% [6,7,8,9].
Anticholinergic burden, often the result of concomitant use of multiple MACs [10], has been associated with adverse effects such as cognitive and functional impairment, reduced quality of life, impaired activities of daily living [2] as well as falls [11, 12] and fall-related injuries, particularly fractures [13,14,15,16]. These effects are usually associated with the person’s total anticholinergic burden, rather than specific medications. Several scores have been proposed to summarize the anticholinergic burden of patients. However, they vary in their rationale, intended use and association with outcomes [12].
Fractures, especially in older adults, often result in permanent disability or death and have a high impact on the health care system and informal caregivers [17,18,19,20]. Approximately one in three older adults experience at least one fall each year; as a consequence, 5% of them will sustain a fracture and 1% a hip fracture [19]. Hip fractures are associated with high short- and long-term mortality, reduced life expectancy, increased risk of dependency and high costs for the health care system [17,18,19].
Despite the high public health relevance of fractures, their possible association with anticholinergic burden has not yet been addressed in a systematic review. Therefore, we aimed to conduct a systematic review on the association between anticholinergic burden and the risk of fractures. Moreover, since a recent study suggested an association between anticholinergic burden and reduced bone mineral density (BMD) [21], which along with osteoporosis is a major risk factor for fractures [22], we also aimed to conduct a systematic review on studies investigating this association. A special emphasis was put on the description of the methodological quality of the included studies for both outcomes.
2 Methods
This systematic review was conducted in accordance with the PRISMA [23] and MOOSE [24] guidelines as well as a guideline for the conduct of systematic reviews and meta-analyses in older adults [25]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD42018116737) and published in a peer-reviewed journal [26]. As the protocol has already been published, we will only give a brief description of methods.
2.1 Sources of evidence and search strategy
Search strategies were developed by the project team under the guidance of an experienced medical librarian. To identify papers on the association between anticholinergic burden and risk of fractures, the search strategy included two concepts: anticholinergic (including medication and burden), and fractures. For the association between anticholinergic burden and osteoporosis or reduced BMD, the search strategy included the concepts anticholinergic (including medication and burden), and osteoporosis or reduced BMD. The appropriate controlled vocabulary representing these concepts in each database was used (see Online Resource 1).
The search strategies were applied in the following electronic databases and information resources: MEDLINE (1950 to July 2020), EMBASE (1947 to August 2020) and Science Citation Index (1900 to July 2020). Moreover, we searched in the Cochrane Controlled Register of Trials (CENTRAL), sources dedicated to grey literature (Open Grey, OSFPreprints, GreyLit and Google Scholar) and relevant open access repositories (Open DOAR) until July 2020.
Additionally, references of included studies, prior systematic reviews and meta-analyses and studies citing included studies were screened for eligible articles. Authors who have published in this field were contacted for articles that may have been missed or are unpublished.
2.2 Eligibility
To be eligible, studies had to be observational (i.e., cohort, case–control, case-crossover or self-controlled cohort studies) and conducted in humans without restrictions regarding demographics (i.e., age and sex) or setting (i.e., both population-based studies and studies including persons hospitalized or residents of nursing homes or other types of long-term care facility). They had to evaluate exposure to the anticholinergic burden through a scale (either previously published or newly developed) or cumulative exposure to MACs. Of note, studies evaluating exposure to one or more individual MACs were excluded.
Moreover, studies were eligible either if they addressed the outcome fractures without restriction to a defined site (that is, fractures of any site, e.g., of the hip, of the hip and the femur, of the wrist) or to a defined type (that is, any fractures for whichever reason, e.g., fall-related, fragility-related) or if they addressed the outcome osteoporosis or reduced BMD. A crude or adjusted measure of association between the exposure and the outcome (i.e., relative risk, odds ratio (OR), hazard ratio (HR) or rate ratio), and the corresponding 95% confidence interval (CI), or sufficient data for its calculation had to be reported. Neither time or language restrictions nor restrictions related to type of data (e.g., primary data or secondary data) were applied. Conference abstracts were not considered in the full-text analysis.
2.3 Selection, data extraction and risk of bias assessment
Eligibility assessment of titles, abstracts and full-text articles as well as data extraction were performed independently by two reviewers (OR and JR). Discrepancies were solved by consensus. In case consensus could not be reached, an expert researcher (FEP) resolved the discrepancy.
The risk of bias for each included study was assessed using two quality assessment tools: the Newcastle–Ottawa Quality Assessment Scale (NOS) [27] and the RTI item bank [28]. We chose to use both quality assessment tools since the NOS provides a concise evaluation of study quality and is widely used, and the RTI item bank provides a detailed evaluation of aspects of the studies that are specifically relevant for studies addressing exposure to medications. Each included study was independently assessed by each reviewer (OR and JR) using both tools. For each item of each tool, disagreement between the ratings of reviewers was solved by consensus. Again, if consensus could not be reached, an expert researcher (FEP) resolved the discrepancy.
2.4 Deviations from protocol
Due to the heterogeneity among the included studies and the low number of included studies overall we decided not to conduct quantitative assessment and to do a qualitative assessment instead.
3 Results
3.1 Study selection
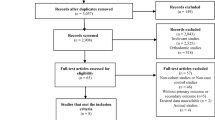
For anticholinergic burden and fractures 1100 articles were identified, leaving 978 potentially eligible articles after duplicates had been removed. Eligibility was assessed based on title and abstract, leading to the exclusion of 929 articles (Fig. 1). Of the 49 articles eligible for full-text assessment, 40 were excluded, as they did not use an anticholinergic burden scale (N = 17), did not assess fractures as an outcome (N = 10), did not report a measure of association (N = 4), or were only published as conference abstracts (N = 9). Nine studies fulfilled all eligibility criteria and were included into the systematic review, corresponding to six cohort [14,15,16, 29,30,31] and three case–control studies [13, 32, 33].
We identified a total of 621 articles on the association between anticholinergic burden and osteoporosis or reduced BMD, leaving 590 articles after removal of duplicates. After screening of title and abstract, 587 were excluded and 3 articles were included into full-text review. One article was excluded as it was only published as a conference abstract. Two full-text articles fulfilled the eligibility criteria and were included in the systematic review [21, 30].
3.2 Anticholinergic burden and fractures
3.2.1 Study population and data source
The characteristics of the included studies are shown in Table 1. The studies included a total of 610,862 persons, 74% (N = 452,659) of which were women [13,14,15,16, 29,30,31,32,33]. Sample sizes ranged from 601 [32] to 202,260 persons [13]. The study population was mainly drawn from North America (N = 363,723; 60%) [13, 29,30,31] and East Asia (N = 175,686; 29%) [14, 16, 32]. The remaining two studies included persons from New Zealand [15] and Colombia [33]. Three studies evaluated persons treated with MACs during the 2010s [15, 32, 33], four studies during the 2000s [13, 14, 16, 29] and two studies during the 1990s [30, 31].
Study participants were mostly older adults. Five studies included persons aged ≥ 65 years [13,14,15,16, 32], one study included persons aged ≥ 60 years [33] and another one persons aged ≥ 50 years [30]. One study was restricted to women between 50 and 79 years [31] and one study included persons with Parkinson’s disease aged ≥ 40 years [29]. The study population was directly drawn from the general population in three studies [14, 16, 33], while three other studies were conducted in cohorts of community dwelling persons [15, 30, 31]. Two studies included only hospitalized patients [29, 32] and another one only nursing home residents [13].
Most studies were based on electronic claims or other administrative data and used prescription or dispensation records to assess the exposure to anticholinergic burden [13, 14, 16, 29, 33]. Two studies were based on primary data and used self-reported use of MACs for exposure assessment [30, 31]. The study of Jamieson et al. [15] was based on both primary and administrative data but used records from a national prescription register for exposure assessment. Kose et al. [32] used inpatient medical records for exposure assessment.
3.2.2 Assessment of Exposure
The most common tool to measure the exposure was the Anticholinergic Risk Scale (ARS), applied in four studies exclusively [16, 29, 32, 33]; Marcum et al. [31] used the Anticholinergic Drug Scale (ADS) and Jamieson et al. [15] used the Drug Burden Index (DBI) [34]. Two studies used more than one scale: the ARS, Anticholinergic Cognitive Burden (ACB) scale and the DBI [14] and the ADS and ACB scale [13]. Finally, Fraser et al. [30] developed a specific tool including the medications with score 2 and 3 from the ARS and those with high anticholinergic effects listed by Ancelin et al. [35].
In four studies, exposure was based on assessment of anticholinergic burden either at baseline or at multiple time points during follow-up. In the study of Crispo et al. [29], anticholinergic burden was assessed using the Anticholinergic Risk Scale (ARS) [36] based on all medication prescribed at the baseline hospital encounter. Fraser et al. [30] assessed exposure to MACs at baseline and at visits after 5 and 10 years. A last-value-carried-forward approach was used and exposure to MACs was assumed to be continuous between visits [30]. Kose et al. [32] evaluated change in ARS scores between hospital admission and discharge and occurrence of hip fracture. Marcum et al. [31] assessed self-reported exposure to MACs during the past two weeks, at baseline and after three years using the Anticholinergic Drug Scale (ADS) [37].
Two studies assessed exposure to MACs during a defined assessment period of 30 days before the occurrence of the outcome among cases and corresponding date controls [13, 33]. Within this assessment period, Chatterjee et al. [13] assessed whether a patient was exposed to at least one level 2 or 3 medication from the ADS. They also conducted sensitivity analyses extending the assessment period to 60 and 90 days and applied the Anticholinergic Cognitive Burden (ACB) scale [38] as a second exposure measurement tool. Conversely, Machado-Duque et al. [33] summed up ARS scores of all prescribed MACs.
The exposure was assessed longitudinally in three studies. In two, cumulative anticholinergic burden scores for each study participant were calculated on a quarterly [16] or monthly [14] basis during the up to 10-year follow-up periods. Jamieson et al. [15] calculated participant’s cumulative anticholinergic burden on a 90-days interval basis, during the up to three-year long follow-up period.
Exposure categories were defined differently across the included studies: While Fraser et al. [30] and Marcum et al. [31] defined exposure simply as use of at least one MAC, levels of anticholinergic burden were distinguished in the studies of Lu et al. (ARS 1–2, ≥ 3) [16], Crispo et al. (ARS 1, 2–3, ≥ 4) [29], Hsu et al. (ARS/ACB 1, 2, 3, ≥ 4; DBI 0 <—≤ 0.5, 0.5 <—≤ 1) [14], Chatterjee et al. (ADS 2, 3 2/3) [13] and Machado-Duque et al. (ARS 1, 2, ≥ 3) [33]. Exposure in the study of Kose et al. was categorized as change of anticholinergic burden of ARS 1, 2 and ≥ 3 [32].
Reference category in eight studies was either non-use of MACs or no anticholinergic burden [13,14,15,16, 29,30,31, 33]. One study used no change in anticholinergic burden during hospitalization as reference category [32]. None of the studies used a new-user design or applied criteria to prevent the inclusion of prevalent users of MACs.
3.2.3 Assessment of outcome
The most commonly assessed outcome was any fracture (4 studies) [14, 16, 29, 30], followed by hip fracture (3 studies) [15, 32, 33], hip/femur fracture (1 study) [13] as well as hip, lower arm/wrist and total fracture (1 study) [31]. The outcome was mostly assessed based on secondary data, that is, diagnostic codes recorded in databases (6 studies) [13,14,15,16, 29, 33], hospital medical records (1 study) [32] or self-reports of fractures adjudicated through medical or radiology records (2 studies) [30, 31]. Three studies excluded patients who had a prior history of fall or fracture [13, 31, 32] and six studies did not [14,15,16, 29, 30, 33].
3.2.4 Baseline prevalence of anticholinergic burden
Baseline prevalence of use of MACs ranged from 8% [30] to 85% [29]. With the exception of Lu et al. [16], baseline use of MACs was lower in studies that were based on primary data [30,31,32] compared to studies that were based on administrative and/or claims data [13,14,15, 29, 33].
Association between anticholinergic burden and fractures.
All nine studies reported adjusted risks [13,14,15,16, 29,30,31,32,33], including known risk factors for fractures. Of these, three studies adjusted for time-varying covariates [14, 15, 32] but one study adjusted only for age and time-varying according to the Charlson Comorbidity Index [14].
Seven studies reported increased risk of fractures associated with anticholinergic burden [13,14,15,16, 29, 32, 33], while two studies did not find an association if factors related to health status and risk factors for fractures were adjusted for [30, 31]. Four studies using the ARS showed a dose-exposure gradient [14, 16, 29, 33] (Fig. 2). In these studies, adjusted risk estimates in the exposure categories of ARS 1 were associated with 2–61% increased risk (compared with ARS = 0) for the respective outcomes [14, 16, 29, 33]. Furthermore, ARS 1–2 was associated with increased risk of 39%, ARS 2 with risks of 0–97%, ARS 2–3 with risks of 17%, ARS 3 with risks of 19–84% and ARS ≥ 4 with risks of 56–96% [14, 16, 29, 33].
3.2.5 Risk of bias assessment
Based on the Newcastle–Ottawa scale, the risk of bias was lowest in Jamieson et al. [15], followed by Lu et al. [16] and Chatterjee et al. [13], while it was highest in Machado-Duque et al. [33] (Table 2). Intermediate risk of bias was found in Hsu et al. [14], Fraser et al. [30], Marcum et al. [31] and Kose et al. [32].
Risk of bias assessments based on the RTI Item Bank showed that the majority of studies had a low risk of bias (Table 3): The risk of bias was low in 88–92% of the items for four studies [13,14,15,16] and in 58–71% of the items for another four studies [29, 31,32,33]. Fraser et al. [30] had a low risk of bias in only 25% of the items. Items 6 “Do the confidence intervals suggest lack of precision?” and 27 “Is the impact of unmeasured confounding important enough to affect the believability of results?” were the items most frequently rated as high risk of bias in the RTI Item Bank (five [15, 29, 30, 32, 33] and four studies, respectively [29, 30, 32, 33]). Item 7 “What is the level of detail in describing the intervention or exposure?” was most frequently rated as unclear risk of bias (five studies [13, 30,31,32,33]).
3.3 Anticholinergic burden and osteoporosis or reduced bone mineral density
The study of Ablett et al. [21], assessed the association between reduced BMD (through dual-energy X-ray absorptiometry) and the anticholinergic burden (ACB scale, based on self-reported use of MACs) among 3,883 UK women aged 45–54 years who participated in the Aberdeen Prospective Osteoporosis Study between 1997 and 2000. In total, 590 (15.2%) women used at least one MAC. Having adjusted for comorbidities (including age), women with ACB score of ≥ 2 had about three times the risk of having reduced BMD in the lowest quintile BMD at Ward’s triangle [OR 2.81 (95% CI 1.16–6.79)], compared with women with ACB = 0, but not at other skeletal sites, such as hip, femur, trochanter or spine.
In addition to the association between anticholinergic burden and falls and fractures, Fraser et al. [30] also assessed change in BMD T-score at the femoral neck for a subgroup of n = 194 participants who reported being treated with MACs at study baseline and at the second assessment five years later. Change of BMD T-score was compared between baseline and the second assessment 10 years later using an independent t test. After adjustment for variables associated with BMD there was no significant association between use of MAC and change in BMD.
Both studies were rated as having an intermediate risk of bias based on the Newcastle–Ottawa scale; Ablett et al. had a low risk of bias in 81% and Fraser et al. in 80% of the items according to RTI Item Bank.
4 Discussion
In this first systematic review of studies assessing the risk of fractures associated with anticholinergic burden, seven out of the nine included studies found a positive association. Four studies that used the ARS showed a dose–response relationship. We also looked at studies that focused on osteoporosis or reduced BMD as an outcome. One of the two included studies reported an association of anticholinergic burden with lower BMD at Ward’s triangle, but not at other skeletal sites [21]. The other study did not find an association between use of MAC and change of BMD T-score at femoral neck [30].
In the included studies that assessed the risk of fractures associated with anticholinergic burden, the increased risk was consistent despite the large heterogeneity in terms of population and study design. Increased risks of fractures were reported in different geographical regions (e.g., North America [13, 29] and East Asia [14, 16, 32]); in the general population [14, 16, 33] as well as in nursing home residents [13], community dwellers [15] and hospitalized persons [29, 32]; in studies with longitudinal [14,15,16] and baseline assessment of anticholinergic burden [13, 29, 32, 33]. Interestingly, the studies that did not find an association between anticholinergic burden and fractures were studies that were based on primary data, assessed anticholinergic burden based on self-reported use of MACs and whose patients were recruited during the 1990s [30, 31].
The included studies differed in regards to the methods used to assess the anticholinergic burden: (i) four different anticholinergic burden scales were used (ARS, ADS, ACB, DBI) and among the five studies that used the ARS scale for the assessment of anticholinergic burden different definitions for MACs were used; (ii) not all studies distinguished between different levels of anticholinergic burden in their exposure assessments, which made dose response assessment difficult (iii) among the studies that used the ARS and distinguished between levels of anticholinergic burden, exposure assessment and categorization of the ARS into exposure categories differed considerably. For example, in their highest exposure category, Fraser et al. [30] included medication with ARS score 2 and 3 and medication with high anticholinergic effects defined by Ancelin et al. [35]. In contrast, the other four studies used the list of MACs from Rudolph et al. [36]. Finally, (iv) exposure assessment was not uniform across studies: Kose et al. [32] defined exposure as the magnitude of change in anticholinergic burden, whereas the other studies that used the ARS measured anticholinergic burden at certain points in time or within time frames.
To our knowledge, this is the first systematic review investigating the association between anticholinergic burden and fractures. Our findings are consistent with some, but not all, systematic reviews on the association between anticholinergic burden and falls [12, 39, 40]. The pathway from falls to fractures is plausible as falls are the main cause of fractures, particularly among older adults [41]. Welsh et al. [12] and Cardwell et al. [11] reported that the majority of studies consistently found an increased risk of falls associated with anticholinergic burden. However, Ruxton et al. [40] concluded that only some MACs (olanzapine and trazodone) were associated with an increased risk of falls while others (amitriptyline, paroxetine and risperidone) were not. In their narrative review, Collamati et al. [39] reported inconclusive evidence regarding increased risk of falls associated with anticholinergic burden.
Strengths of this systematic review include the search for eligible studies in the most relevant literature databases using a comprehensive and reproducible search strategy. Additionally, references of included studies, studies citing included studies as well as grey literature were searched. Evaluation of potentially eligible studies, data extraction as well as the risk of bias assessment were performed by two independent investigators. The review was performed according to the relevant guidelines [23,24,25] and the protocol was first registered in PROSPERO and subsequently published in an open access journal [26].
Limitations of this systematic review include the low number of included studies. We could not quantitatively summarize the risk across the included studies because of their high heterogeneity in particular due to differences in methods for the assessment of anticholinergic burden, specifically the use of different scales and individual modifications to the scale’s lists of MACs. We also could not summarize the results of the subgroup of studies that used the ARS due to different definitions of exposure categories across these studies. Moreover, as prior studies showed only a low concordance between the scales for the assessment of anticholinergic burden [42, 43], we chose not to combine studies in which anticholinergic burden was assessed using different scales. Since none of the included studies used a new user design, the inclusion of prevalent users of MACs may have contributed to depletion of susceptible which may have led to an under ascertainment of fractures occurring early after start of treatment with MACs [44]. Moreover, only three studies used a longitudinal design. Most studies were either conducted in North America or East Asia and two studies were based on data that was collected in the 1990s. Evidence for Europe and other geographical regions is thus lacking.
This systematic review suggests an increased risk of fractures associated with anticholinergic burden and a dose response relationship in studies using the ARS. Physicians should be careful when prescribing MACs and consider all other medications the patient is taking in this regard. Furthermore, medication regimen with potential risk for high anticholinergic burden should be revised and substitutes without anticholinergic activity should be prescribed. If treatment with MACs is necessary, patients should be advised of adverse events including falls and fractures and be closely monitored.
The mixed methodological quality of included studies calls for the conduct of more studies with longitudinal assessment of anticholinergic burden or new user design. Standardization of the method for the assessment of anticholinergic burden would greatly improve the comparability of studies as meta-analysis in this systematic review was not possible due to the differences in use of scales for the assessment and classification of anticholinergic burden. Furthermore, the lack of studies from other geographical areas such as Europe, Africa and South America calls for the conduct of studies in these regions.
We could only include two studies that investigated the association between anticholinergic burden and the risk of osteoporosis or reduced BMD. Therefore, more studies are needed on this outcome before a conclusion can be made.
References
Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751–65.
Nishtala PS, Salahudeen MS, Hilmer SN. Anticholinergics: theoretical and clinical overview. Expert Opin Drug Saf. 2016;15(6):753–68.
Inkeri NM, Karjalainen M, Haanpaa M, Kautiainen H, Saltevo J, Mantyselka P, et al. Anticholinergic drug use and its association with self-reported symptoms among older persons with and without diabetes. J Clin Pharm Ther. 2019;44(2):229–35.
Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23(7):753–8.
van der Meer HG, Taxis K, Teichert M, Griens F, Pont LG, Wouters H. Anticholinergic and sedative medication use in older community-dwelling people: a national population study in the Netherlands. Pharmacoepidemiol Drug Saf. 2019;28(3):315–21.
Chatterjee S, Mehta S, Sherer JT, Aparasu RR. Prevalence and predictors of anticholinergic medication use in elderly nursing home residents with dementia: analysis of data from the 2004 National Nursing Home Survey. Drugs Aging. 2010;27(12):987–97.
Kumpula EK, Bell JS, Soini H, Pitkala KH. Anticholinergic drug use and mortality among residents of long-term care facilities: a prospective cohort study. J Clin Pharmacol. 2011;51(2):256–63.
Palmer JB, Albrecht JS, Park Y, Dutcher S, Rattinger GB, Simoni-Wastila L, et al. Use of drugs with anticholinergic properties among nursing home residents with dementia: a national analysis of Medicare beneficiaries from 2007 to 2008. Drugs Aging. 2015;32(1):79–86.
Sura SD, Carnahan RM, Chen H, Aparasu RR. Prevalence and determinants of anticholinergic medication use in elderly dementia patients. Drugs Aging. 2013;30(10):837–44.
Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001;62(Suppl 21):11–4.
Cardwell K, Hughes CM, Ryan C. The association between anticholinergic medication burden and health related outcomes in the 'Oldest Old': a systematic review of the literature. Drugs Aging. 2015;32(10):835–48.
Welsh TJ, van der Wardt V, Ojo G, Gordon AL, Gladman JRF. Anticholinergic drug burden tools/scales and adverse outcomes in different clinical settings: a systematic review of reviews. Drugs Aging. 2018;35(6):523–38.
Chatterjee S, Bali V, Carnahan RM, Chen H, Johnson ML, Aparasu RR. Anticholinergic medication use and risk of fracture in elderly adults with depression. J Am Geriatr Soc. 2016;64(7):1492–7.
Hsu WH, Wen YW, Chen LK, Hsiao FY. Comparative associations between measures of anti-cholinergic burden and adverse clinical outcomes. Ann Fam Med. 2017;15(6):561–9.
Jamieson HA, Nishtala PS, Scrase R, Deely JM, Abey-Nesbit R, Hilmer SN, et al. Drug burden index and its association with hip fracture among older adults: a national population-based study. J Gerontol A Biol Sci Med Sci. 2019;74(7):1127–33.
Lu WH, Wen YW, Chen LK, Hsiao FY. Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: a retrospective cohort study. CMAJ. 2015;187(4):E130–E137137.
Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–70.
Giannoulis D, Calori GM, Giannoudis PV. Thirty-day mortality after hip fractures: has anything changed? Eur J Orthop Surg Traumatol. 2016;26(4):365–70.
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Archiv Osteoporos. 2013;8:136.
Lin PC, Lu CM. Hip fracture: family caregivers' burden and related factors for older people in Taiwan. J Clin Nurs. 2005;14(6):719–26.
Ablett AD, Wood AD, Barr R, Guillot J, Black AJ, Macdonald HM, et al. A high anticholinergic burden is associated with a history of falls in the previous year in middle-aged women: findings from the Aberdeen Prospective Osteoporosis Screening Study. Ann Epidemiol. 2018; 28(8):557–62e2.
Longo AB, Ward WE. PUFAs, bone mineral density, and fragility fracture: findings from human studies. Adv Nutr. 2016;7(2):299–312.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–122.
Shenkin SD, Harrison JK, Wilkinson T, Dodds RM, Ioannidis JPA. Systematic reviews: guidance relevant for studies of older people. Age Ageing. 2017;46(5):722–8.
Reinold J, Schafer W, Christianson L, Barone-Adesi F, Riedel O, Pisa FE. Anticholinergic burden and fractures: a protocol for a methodological systematic review and meta-analysis. BMJ open. 2019;9(8):e030205.
Wells G, Shea B, Oconnell D. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 22 Aug 2018.
Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65(2):163–78.
Crispo JA, Willis AW, Thibault DP, Fortin Y, Hays HD, McNair DS, et al. Associations between Anticholinergic Burden and Adverse Health Outcomes in Parkinson Disease. PLoS ONE. 2016;11(3):e0150621.
Fraser LA, Adachi JD, Leslie WD, Goltzman D, Josse R, Prior J, et al. Effect of anticholinergic medications on falls, fracture risk, and bone mineral density over a 10-year period. Ann Pharmacother. 2014;48(8):954–61.
Marcum ZA, Wirtz HS, Pettinger M, LaCroix AZ, Carnahan R, Cauley JA, et al. Anticholinergic medication use and fractures in postmenopausal women: findings from the women's health initiative. Drugs Aging. 2015;32(9):755–63.
Kose E, Hirai T, Seki T. Anticholinergic drugs use and risk of hip fracture in geriatric patients. Geriatr Gerontol Int. 2018;18(9):1340–4.
Machado-Duque ME, Castano-Montoya JP, Medina-Morales DA, Castro-Rodriguez A, Gonzalez-Montoya A, Machado-Alba JE. Drugs with anticholinergic potential and risk of falls with hip fracture in the elderly patients: a case-control study. J Geriatr Psychiatry Neurol. 2018;31(2):63–9.
Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781–7.
Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455–9.
Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–13.
Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–6.
Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–20.
Collamati A, Martone AM, Poscia A, Brandi V, Celi M, Marzetti E, et al. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res. 2016;28(1):25–35.
Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209–20.
Ambrose AF, Cruz L, Paul G. Falls and fractures: a systematic approach to screening and prevention. Maturitas. 2015;82(1):85–93.
Naples JG, Marcum ZA, Perera S, Gray SL, Newman AB, Simonsick EM, et al. Concordance between anticholinergic burden scales. J Am Geriatr Soc. 2015;63(10):2120–4.
Pont LG, Nielen JT, McLachlan AJ, Gnjidic D, Chan L, Cumming RG, et al. Measuring anticholinergic drug exposure in older community-dwelling Australian men: a comparison of four different measures. Br J Clin Pharmacol. 2015;80(5):1169–75.
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This systematic review was conducted in accordance with the PRISMA and MOOSE guidelines as well as a guideline for the conduct of systematic reviews and meta-analyses in older adults by Shenkin et al.
Funding
Open Access funding enabled and organized by Projekt DEAL. This systematic review was funded entirely by internal funds of the Leibniz Institute for Prevention Research and Epidemiology—BIPS. The funding institution had no influence on any part of this article.
Conflict of interest
All authors have no conflicts of interest to declare.
Consent for publication
All authors consented to the publication of this study.
Author contributions
JR: Study design, conduct of study, bibliographic research, design of data entry forms, article evaluation and selection, data management, manuscript writing and review. WS: Study design, manuscript review. LC: Bibliographic research design and conduct, manuscript review. FBA: Study design, statistical analysis, scientific guidance/advice, manuscript writing and review. OR: Study conception and design, scientific coordination, article evaluation and selection, manuscript review. FEP: Study conception and design, scientific coordination, article evaluation and selection, manuscript writing and review. All authors contributed to and have approved the final manuscript.
Additional information
At the time of study conception and coordination, FEP worked at the Leibniz Institute for Prevention Research and Epidemiology – BIPS. From 1st March 2020 she is working at Bayer AG. Her current affiliation has no relation with this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Reinold, J., Schäfer, W., Christianson, L. et al. Anticholinergic Burden and Fractures: A Systematic Review with Methodological Appraisal. Drugs Aging 37, 885–897 (2020). https://doi.org/10.1007/s40266-020-00806-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-020-00806-6