Abstract
A growing number of clinical trials are documenting that adding a long-acting muscarinic antagonist (LAMA) to established asthma treatment with an inhaled corticosteroid (ICS) and a long-acting β2-agonist (LABA) is a treatment option that improves the health of patients with uncontrolled severe asthma even when therapy is optimized. These favorable results are the reason why the leading guidelines recommend triple therapy with ICS + LABA + LAMA in patients with asthma uncontrolled by medium- to high-dose ICS-LABA. However, we suggest adding LAMAs to ICS-LABAs at an earlier clinical stage. Such action could positively influence airflow limitation, exacerbations, and eosinophilic inflammation, conditions that are associated with acetylcholine (ACh) activity. It could also interrupt the vicious cycle related to a continuous release of ACh leading to the progressive expansion of neuronal plasticity resulting in small airway dysfunction. The utility of an earlier use of triple therapy in asthma should, in any case, be confirmed by statistically powered trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cholinergic tone that facilitates pathophysiological processes such as bronchoconstriction, inflammation, and remodeling is increased in asthma. |
The National Heart, Lung and Blood Institute (NHLBI)/National Asthma Education and Prevention Program (NAEPP) and Global Initiative for Asthma (GINA) recommendations advocate using an LAMA before starting oral corticosteroids or biologic drugs in patients with severe asthma uncontrolled despite usual therapies. |
We speculate that, if initiated as a GINA step 3 treatment, triple therapy might optimize bronchodilation, reduce pulmonary hyperinflation, and influence neuronal plasticity. |
1 Introduction
The vagus nerve, with its tonically active parasympathetic nerves, is the dominant neuronal pathway in the control of airway smooth muscle (ASM) tone [1,2,3]. Acetylcholine (ACh) is the neurotransmitter of the parasympathetic nervous system. After being released from the vagus, and therefore referred to as neuronal ACh, it activates muscarinic ACh receptors (mAChRs) located post-synaptically on ASM. The contraction of ASM is mediated mainly by the activation of Gq-coupled M3 mAChRs [1,2,3].
Despite the importance of cholinergic innervation of the lungs, for a long time mAChR antagonists were not considered helpful for the regular treatment of asthma. They were thought to be less effective than β2-agonists [4] because it was believed that cholinergic reflex mechanisms did not play a major role in asthma [5]. In 2004, the Cochrane Airways Group published a systematic review and meta-analysis on the efficacy of short-acting mAChR antagonists (SAMAs) in chronic asthma [6]. It showed that, when administered by inhalation, these agents produce inhibition of bronchoconstriction. However, adding an mAChR antagonist to regular treatment with short-acting β2-agonists did not demonstrate significant benefits to justify its routine use. Nevertheless, it was not excluded that there were sub-groups of patients who gained some symptomatic benefit.
At the time, older generation mAChR antagonists such as ipratropium bromide and oxitropium bromide were used, although none of them had been approved for treating asthma. Their use was reserved for older asthma patients, those with intrinsic asthma or asthma of psychogenic origin, in the presence of bronchospasm induced by the unintentional use of a β-blocker, and in subjects who could not tolerate the adverse effects triggered by a β2-agonist [7].
In the last two decades, expressly designed basic studies and the progressive availability of long-acting mAChR antagonists (LAMAs) have made it possible to progressively change the negative view about using mAChR antagonists in asthma.
In this article, we aim to illustrate our views on the role of LAMAs in the treatment of asthma and why we suggest starting their use at an earlier stage than proposed by the Global Initiative for Asthma (GINA) strategy.
2 Pharmacological Rationale for the Use of LAMAs in Asthma
Cholinergic tone increases in asthma [8], facilitating pathophysiological processes such as bronchoconstriction, inflammation, remodeling, mucus hypersecretion, and chronic cough [9]. Increased neural activity may result from neuronal remodeling and plasticity brought on by immune cells/inflammation, including mast cells and eosinophils, or cells that stimulate and support nerve development and dysfunction, such as neurotrophins [3, 9,10,11]. ACh is released more often when the density of the parasympathetic ganglia increases, which is a sign of plasticity [3, 11]. The enhanced signal generated by ACh produces a rise in the density of cholinergic nerves and a subsequent increase in ACh release that further amplifies the downstream effects of ACh, resulting in a vicious circle [3, 11]. Facilitated ganglionic and postganglionic ACh release by inflammatory mediators influences airway hyperresponsiveness [10].
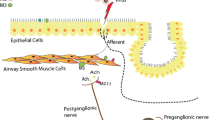
The role of ACh produced by non-neuronal cells [12] through the activation of choline acetyltransferase by inflammatory mediators in epithelial cells and other structural cells, including ASM cells, fibroblasts, and mast cells, as well as in inflammatory cells such as macrophages, lymphocytes, and granulocytes is equally critical [9] (Fig. 1). High concentrations of immunoglobulin E also cause an increase in non-neuronal ACh release [12]. Airway epithelial cells and other structural cells produce and release pro-inflammatory cytokines in response to mAChR activation [13]. It is assumed that inhibiting cholinergic neurotransmission could play a role by reducing neuromodulator levels [14].
Potential effects of ACh on asthmatic small airways. ACh acetylcholine, ChAT choline acetyltransferase, ECM extracellular matrix (reproduced from Cazzola et al. [17])
Due to their ability to block the effects of ACh on ASM M3 mAChRs, LAMAs prevent or at least reduce ACh-induced ASM contraction [3, 14, 15]. In addition, they also avoid the pro-inflammatory, proliferative, and pro-fibrotic effects that ACh induces by activating mAChRs [14]. Neuronal and non-neuronal ACh can potentiate airway remodeling and inflammation in asthma [16], mainly through M3 mAChRs [14]. M3 mAChRs are also expressed in fibroblasts, epithelial cells, and inflammatory cells [17]. In addition, increased ASM mass and excessive extracellular matrix deposition depend on the activation of mAChRs that cause collagen synthesis [18] and induce the transition from fibroblasts to myofibroblasts [19].
It has been suggested that the greater utility of LAMAs in asthma compared with SAMAs would reside in the well-known variations in kinetic selectivity (pKi) between SAMAs and LAMAs for M3 mAChRs compared with M2 mAChRs, with LAMAs having longer residence times on M3 mAChRs and shorter residence times on M2 mAChRs [20]. It is known that the blocking of M2 mAChRs results in increased ACh release [1].
There is documentation that mAChR antagonists can also interact reciprocally with inhaled corticosteroids (ICSs), causing a synergistic relaxation of passively sensitized human middle and small airways linked to an increase in cyclic adenosine monophosphate (cAMP) concentration [21]. Furthermore, when combined at low doses, mAChR antagonists and β2-agonists have synergistic interactions at the ASM level and reduce ACh release from human bronchi [22]. Non-neuronal ACh production by the epithelium decreases and cAMP concentrations increase in the ASM and bronchial epithelium due to this interaction [23].
Therefore, the addition of an LAMA to ICS/LABA would allow advantage to be taken not only of the well-known bidirectional molecular interactions between corticosteroids and β2-agonists for the reciprocal enhancement of the pharmacological effects of ICS and LABA but also of the anti-remodeling activities in addition to the known bronchodilator and anti-inflammatory activities induced by the interaction between an LAMA and an ICS [2, 3] (Fig. 2). Furthermore, pharmacological intervention based on the combination of an LABA with an LAMA would shift the relaxation–contraction imbalance toward a relaxed ASM profile [2, 3].
The rationale for triple therapy. The exact nature of the interactions between LAMAs, LABAs, and ICSs is not completely understood, but there is cross-talk at many levels in ASM cells that is also regulated by the activity of calcium-activated potassium channels and protein tyrosine kinases. AC Adenylyl cyclase, β2-AR β2-adrenoceptor, cAMP cyclic adenosine monophosphate, GR glucocorticoid receptor, KCa++ calcium-activated potassium channel, nAChR nicotinic acetylcholine receptor, PKC protein kinase C, PTK protein tyrosine kinase (reproduced from Cazzola et al. [3])
Pharmacological characterization of the potential beneficial effect of adding an LAMA to an ICS and a long-acting β2-agonist (LABA) was carried out using passively sensitized human airways. ICS + LABA + LAMA combinations induced synergistic bronchorelaxant effects in medium and small human airways, at least in ex vivo experiments [24, 25]. This synergy was related to significant reduction in the release of interleukin (IL)-4, IL-5, IL-6, IL-9, IL-13, tumor necrosis factor-α, thymic stromal lymphopoietin, neurokinin A, substance P, and non-neuronal ACh and a massive increase in intracellular cAMP concentration [25].
3 Clinical Evidence Supporting the Use of LAMAs in Asthma
The clinical impact of adding an LAMA to standard asthma therapy has been evaluated in many randomized clinical trials (RCTs). However, these RCTs had different drug combination components, inhaler devices, and frequency of administration. Quantitative synthesis of the currently available data by meta-analysis can be used to overcome this bias and obtain consistent and homogeneous estimates [26]. The GINA strategy, when referring to approaches to the management and treatment of asthma patients, ranks systematic reviews with meta-analyses at the top of evidence [27].
A network meta-analysis used data from 9535 asthmatic patients selected from five phase III RCTs performed in symptomatic patients suffering from uncontrolled asthma [28]. The addition of an LAMA to high-dose ICS + LABA was significantly more effective in preventing the risk of moderate to severe asthma exacerbations than the medium-dose ICS + LABA combinations and also the addition of an LAMA to medium-dose ICS + LABA. It also showed a trend toward significance compared with high-dose ICS + LABA combinations. Furthermore, high-dose ICS + LABA + LAMA was the most effective treatment in improving trough forced expiratory volume in 1 s (FEV1), followed by medium-dose ICS + LABA + LAMA combinations, high-dose ICS + LABA combinations, and medium-dose ICS + LABA combinations.
Adding an LAMA to medium- or high-dose ICS + LABA or increasing the dose of ICS in the context of medium-dose ICS + LABA + LAMA reduced the risk of moderate to severe asthma exacerbation and increased trough FEV1. Adding an LAMA and increasing the dose of ICS further prevented the risk of moderate to severe asthma exacerbation and improved the trough FEV1. ICS + LABA + LAMA combinations effectively improved Asthma Control Questionnaire score, although a trend toward significance was detected for high-dose ICS + LABA + LAMA versus medium-dose ICS + LABA + LAMA.
There was no substantial difference between the examined combinations regarding the risk of severe adverse events, pneumonia, and major cardiovascular events.
A systematic review and meta-analysis, which included 20 RCTs and 11,894 patients with moderate to severe persistent asthma, compared triple therapy (ICS + LABA + LAMA) with dual therapy (ICS + LABA) [29]. High-certainty evidence showed that triple therapy compared with dual therapy was significantly associated with a reduced risk of severe exacerbations and improved asthma control. Triple therapy was significantly associated with more patients achieving a 200 mL increase in trough FEV1 from baseline compared with dual therapy. There were no significant differences in asthma-related quality of life or mortality. The frequency of previous exacerbations (< 1 exacerbation versus ≥ 1 exacerbation), age (< 18 years versus ≥ 18 years), smoking history, ICS dose and type of intervention or comparator, type and dose of LAMA, or inflammatory phenotype [type-2 (T2)-high versus T2-low asthma defined by peripheral blood eosinophil count] were not credible effect modifiers in subgroup analyses for asthma severity. Treatment-related severe adverse events were not significantly different between groups.
Another systematic review and network meta-analysis evaluated the efficacy and safety of dual (ICS + LABA) and triple (ICS + LABA + LAMA) therapies compared with each other and with different doses of ICS in adolescents and adults with uncontrolled asthma [30]. A total of 17,161 patients from 17 studies were included. The results indicated that, compared to medium-dose ICS + LABA, medium- and high-dose ICS + LABA + LAMA treatments reduced asthma exacerbations requiring corticosteroid treatment (high-certainty evidence) but not asthma-related hospitalizations. Regarding the reduction of asthma attacks requiring corticosteroids, treatment with high-dose ICS + LABA + LAMA appeared superior to therapy with medium-dose ICS + LABA + LAMA (moderate-certainty evidence). However, triple therapy did not significantly improve symptoms or quality of life compared with dual therapy. According to subgroup studies, people with a history of asthma exacerbation in the previous year seemed most likely to benefit from the reduction in exacerbations requiring corticosteroids associated with ICS + LABA + LAMA. Compared with medium-dose ICS + LABA, high-dose ICS + LABA + LAMA reduced all-cause adverse events and probably also dropout rates, a reduction not seen with medium-dose ICS + LABA + LAMA (evidence of moderate certainty). Furthermore, there was no difference between triple therapy and dual therapy regarding significant all-cause or asthma-related adverse events (evidence of high certainty).
4 Current Positioning of LAMAs in the Treatment of Asthma
Current National Heart, Lung and Blood Institute (NHLBI)/National Asthma Education and Prevention Program (NAEPP) guidelines [31] and GINA therapeutic strategy [27] exclude the possibility of using an LAMA in monotherapy or replacing LABA with an LAMA in combination with an ICS unless LABAs are unavailable to the patient or are not acceptable due to efficacy or safety issues. The exclusion of the possibility of using an LAMA in monotherapy does not consider the results of the SIENA study in which the clinical response to ICS or tiotropium favored ICS when the eosinophil level was ≥ 2% in patients with mild persistent asthma, as expected [32]. However, when the sputum eosinophil count was < 2%, no drug superiority was evident, although patients improved with mometasone and tiotropium compared with placebo. NHLBI/NAEPP recommends the addition of an LAMA to low-dose ICS at step 3 and medium-dose ICS at step 4 as an alternate controller treatment [31]. Adding an LAMA to ICS controller therapy is more successful than using ICS controller therapy alone, but it is not more effective than adding an LABA [31]. It should be noted that the NHLBI/NAEPP guidelines were limited to tiotropium.
The European Respiratory Society/American Thoracic Society guideline for management of severe asthma recommends the addition of an LAMA (tiotropium) for children, adolescents, and adults with severe asthma uncontrolled despite GINA steps 4–5 or NHLBI/NAEPP step 5 therapies [33]. The NHLBI/NAEPP [31] and GINA [27] recommendations advocate using an LAMA before starting oral corticosteroids or biologic drugs. LAMAs are indicated as add-on therapy to high-dose ICS + LABA as a single inhaler or a combination (triple) inhaler in GINA stage 5 (i.e., uncontrolled severe asthma despite proper ICS + LABA administration). Also, GINA step 4 includes the addition of an LAMA as an alternate controller treatment [27].
5 Use of LAMAs Considering Treatable Traits
Asthma is currently recognized as a collection of distinct clinical and pathophysiological syndromes that may overlap [34]. Nonetheless, both NHLBI/NAEPP and GINA suggest a treatment strategy for asthma that ignores the underlying disease processes and instead focuses on so-called “severity phenotypes” based on lung function, symptoms, and quality of life that allows a “one-size-fits-all” approach [35]. However, escalation to phenotype-specific personalized biological therapies should be understood with the first, albeit limited, attempt at personalized therapy in asthma [36].
A new approach acknowledges the complexity and heterogeneity of asthma and focuses on targeted interventions against identifiable and treatable mechanistic pathways or treatable traits [37]. This approach completely embraces the notion of precision medicine, which contrasts with the conventional “one-size-fits-all” approach to asthma therapy [38]. It entails breaking down obstructive airway diseases, including asthma, into their constituent elements to precisely identify and then target/treat the relevant features in an individual [39]. There are many pulmonary, extrapulmonary, or lifestyle/environmental traits that are treatable. The most critical treatable traits in asthma are symptoms due to airflow limitation and the risk of asthma attacks/exacerbations secondary to T2 airway inflammation [37].
The already quoted evidence shows that triple therapy is more effective than ICS + LABA combinations in preventing asthma exacerbations [28,29,30], and this also in the absence of T2 airway inflammation [29], which is responsive to standard treatment with ICSs [36], and in the presence of fixed airflow limitation [40].
The therapeutic approach to airflow limitation is more complex because it can be caused by several pathogenic processes that we can still not fully recognize. This makes it impossible to make reliable predictions about prognosis and response to treatment [41]. Mechanical compression establishes an asthma-like molecular signature in human bronchial epithelial cells obtained from non-asthmatic donors, even without any inflammatory stimuli [37]. Airway distortion lasts for a considerable time following the onset of airway constriction. It may cause structural changes in the airways, including remodeling, that promote the development of asthma [42, 43].
Therefore, bronchoconstriction as a disease modifier might be targeted to improve patient outcomes [38]. Solid evidence has shown that combining an LAMA with an LABA results in a synergistic relaxing effect in medium-caliber bronchi, which correlates with an improvement in FEV1, and in small airways with a positive influence on compressive air trapping [44], which is a feature of the natural history of asthma [42] and an essential target for asthma therapy [45]. However, an LABA + LAMA combination must always be administered in conjunction with an ICS for treating asthma [27]. It is not trivial that asthmatics with fixed airflow limitation can have elevated eosinophil blood counts [46]. On the other hand, it also cannot be overlooked that tiotropium appeared to be as effective as mometasone in patients with asthma who have low levels of eosinophils in their sputum [32].
Airway remodeling can affect both large and small airways [47]. Ach’s potential to facilitate remodeling suggests that it is appropriate to target the damaging actions of ACh on ASM and the persistent inflammatory airway insult caused by immune/inflammatory cells, which means using an LAMA and an ICS + LABA combination at the same time [48]. Noteably, this therapeutic approach also benefits from the decreased ACh release that comes with LABA + LAMA combination [23]. This might prevent, or at least reduce, neuronal functional changes (plasticity) that could sustain and expand inflammation and worsen airway structure and function through excessive ACh release. For this reason, we believe that neuronal plasticity should be considered a treatable trait [3].
6 The Possible Value of an Earlier Use of LAMAs in Asthma
The use of triple therapy in asthma directed at treatable traits, independent of the pharmacological options for asthma recommended by the NHLBI/NAEPP guidelines [31] and the GINA strategy [27], might be an intriguing possibility that needs to be supported by statistically powered studies [48].
We know that there are many treatable traits in an asthma patient, and all deserve attention. However, we should focus on those at the lung level that have appeared to be the most frequent in fundamental studies that attempted to identify treatable asthma traits [49,50,51]. Eosinophilic airway inflammation, airflow limitation, and tendency to exacerbate are the most common treatable traits. Still, mucus hypersecretion, neutrophilic airway inflammation, small airway dysfunction, and, most importantly, neuronal plasticity must also be considered.
Awareness that a given patient can present multiple treatable traits, which indicates that the presence of a trait does not exclude that of others [52] and that only the use of a combination of ICS + LABA + LAMA can treat the aforementioned treatable traits with a high probability of success (Fig. 3), suggests the need to test the clinical validity of extending the use of triple therapy to the whole spectrum of asthma [48], not just severe asthma, at least as recommended by the GINA strategy [27].
The triple therapy targeting treatable traits (5T) approach in asthma. Tracts that can be treated with ICSs, ICSs + LABAs, and ICSs + LABAs + LAMAs, respectively (reproduced from Cazzola et al. [48])
Experimental data and preclinical studies [24, 25] allow us to speculate that, if initiated as a GINA step 3 treatment (i.e., in patients with moderate persistent asthma) option, offered at least as an alternative by NHLBI/NAEPP guidelines [31], triple therapy might optimize bronchodilation, reduce pulmonary hyperinflation, and influence neuronal plasticity. The likely consequence of this may be better control of asthma and, thus, a reduction in the need for frequent and problematic step-ups and step-downs [34], with benefits for patients unwilling to discontinue a therapy that they consider effective [53]. Improved quality of life, reduced hospitalization, and hopefully, a slower decline in lung function and reduced mortality are other imaginable outcomes of an earlier start of triple therapy, which means anticipating the use of LAMAs in asthma patients, at least in those with moderate persistent asthma.
References
Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. https://doi.org/10.1124/pr.111.004580.
Matera MG, Page CP, Calzetta L, Rogliani P, Cazzola M. Pharmacology and therapeutics of bronchodilators revisited. Pharmacol Rev. 2020;72(1):218–52. https://doi.org/10.1124/pr.119.018150.
Cazzola M, Rogliani P, Matera MG. The latest on the role of LAMAs in asthma. J Allergy Clin Immunol. 2020;146(6):1288–91. https://doi.org/10.1016/j.jaci.2020.06.014.
Cazzola M, Centanni S, Donner CF. Anticholinergic agents. Pulm Pharmacol Ther. 1998;11(5–6):381–92.
Barnes PJ. Airway inflammation and autonomic control. Eur J Respir Dis Suppl. 1986;147:80–7.
Westby M, Benson M, Gibson P. Anticholinergic agents for chronic asthma in adults. Cochrane Database Syst Rev. 2004;2004(3):CD003269. https://doi.org/10.1002/14651858.CD003269.pub2.
Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol. 2006;533(1–3):36–9. https://doi.org/10.1016/j.ejphar.2005.12.072.
Kageyama N, Igarashi A, Ichinose M, et al. Chronic allergen exposure enhances cholinergic neurotransmission in sensitized guinea-pigs. Eur Respir J. 1995;8(5):752–4.
Pieper MP. The non-neuronal cholinergic system as novel drug target in the airways. Life Sci. 2012;91(21–22):1113–8. https://doi.org/10.1016/j.lfs.2012.08.030.
Verbout NG, Lorton JK, Jacoby DB, Fryer AD. Atropine pretreatment enhances airway hyperreactivity in antigen-challenged guinea pigs through an eosinophil-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1126–35. https://doi.org/10.1152/ajplung.00455.2006.
Kistemaker LEM, Prakash YS. Airway innervation and plasticity in asthma. Physiology (Bethesda). 2019;34(4):283–98. https://doi.org/10.1152/physiol.00050.2018.
Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther. 2003;98(1):59–69. https://doi.org/10.1016/s0163-7258(03)00004-4.
Kistemaker LE, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci. 2015;36(3):164–71. https://doi.org/10.1016/j.tips.2014.11.005.
Gosens R, Gross N. The mode of action of anticholinergics in asthma. Eur Respir J. 2018;52(4):1701247. https://doi.org/10.1183/13993003.01247-2017.
Cazzola M, Ora J, Rogliani P, Matera MG. Role of muscarinic antagonists in asthma therapy. Expert Rev Respir Med. 2017;11(3):239–53. https://doi.org/10.1080/17476348.2017.1289844.
Elliot JG, Jones RL, Abramson MJ, Green FH, Mauad T, McKay KO, et al. Distribution of airway smooth muscle remodelling in asthma: relation to airway inflammation. Respirology. 2015;20(1):66–72. https://doi.org/10.1111/resp.12384.
Cazzola M, Calzetta L, Matera MG. Long-acting muscarinic antagonists and small airways in asthma: which link? Allergy. 2021;76(7):1990–2001. https://doi.org/10.1111/all.14766.
Haag S, Matthiesen S, Juergens UR, Racké K. Muscarinic receptors mediate stimulation of collagen synthesis in human lung fibroblasts. Eur Respir J. 2008;32(3):555–62. https://doi.org/10.1183/09031936.00129307.
Milara J, Serrano A, Peiró T, et al. Aclidinium inhibits cigarette smoke-induced lung fibroblast-to-myofibroblast transition. Eur Respir J. 2013;41(6):1264–74. https://doi.org/10.1183/09031936.00017712.
Matera MG, Rinaldi B, Berardo C, Rinaldi M, Cazzola M. A review of the pharmacokinetics of M3 muscarinic receptor antagonists used for the treatment of asthma. Expert Opin Drug Metab Toxicol. 2020;16(2):143–8. https://doi.org/10.1080/17425255.2020.1716730.
Cazzola M, Calzetta L, Rogliani P, Puxeddu E, Facciolo F, Matera MG. Interaction between corticosteroids and muscarinic antagonists in human airways. Pulm Pharmacol Ther. 2016;36:1–9. https://doi.org/10.1016/j.pupt.2015.11.004.
Calzetta L, Matera MG, Cazzola M. Pharmacological mechanisms leading to synergy in fixed-dose dual bronchodilator therapy. Curr Opin Pharmacol. 2018;40:95–103. https://doi.org/10.1016/j.coph.2018.03.011.
Cazzola M, Calzetta L, Puxeddu E, et al. Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respir Res. 2016;17(1):70. https://doi.org/10.1186/s12931-016-0386-8.
Rogliani P, Matera MG, Facciolo F, Page C, Cazzola M, Calzetta L. Beclomethasone dipropionate, formoterol fumarate and glycopyrronium bromide: synergy of triple combination therapy on human airway smooth muscle ex vivo. Br J Pharmacol. 2020;177(5):1150–63. https://doi.org/10.1111/bph.14909.
Rogliani P, Ritondo BL, Facciolo F, Matera MG, Nikolaev I, Calzetta L. Indacaterol, glycopyrronium, and mometasone: pharmacological interaction and anti-inflammatory profile in hyperresponsive airways. Pharmacol Res. 2021;172: 105801. https://doi.org/10.1016/j.phrs.2021.105801.
Pare’ G, Trudel M-C, Jaana M, Kitsiou S. Synthesizing information systems knowledge: a typology of literature reviews. Inf Manag. 2015;52(2):183–99. https://doi.org/10.1016/j.im.2014.08.008.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2022 update). https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf.
Rogliani P, Ritondo BL, Calzetta L. Triple therapy in uncontrolled asthma: a network meta-analysis of phase III studies. Eur Respir J. 2021;58(3):2004233. https://doi.org/10.1183/13993003.04233-2020.
Kim LHY, Saleh C, Whalen-Browne A, O’Byrne PM, Chu DK. Triple vs dual inhaler therapy and asthma outcomes in moderate to severe asthma: a systematic review and meta-analysis. JAMA. 2021;325(24):2466–79. https://doi.org/10.1001/jama.2021.7872.
Oba Y, Anwer S, Maduke T, Patel T, Dias S. Effectiveness and tolerability of dual and triple combination inhaler therapies compared with each other and varying doses of inhaled corticosteroids in adolescents and adults with asthma: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2022;12(12):CD013799. https://doi.org/10.1002/14651858.CD013799.pub2.
Expert Panel Working Group of the National Heart, Lung and Blood Institute. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. https://www.nhlbi.nih.gov/resources/2020-focused-updates-asthma-management-guidelines.
Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380(21):2009–19. https://doi.org/10.1056/NEJMoa1814917.
Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. https://doi.org/10.1183/13993003.00588-2019.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25. https://doi.org/10.1038/nm.2678.
Cazzola M, Matera MG, Rogliani P, Calzetta L, Ora J. Step-up and step-down approaches in the treatment of asthma. Expert Rev Respir Med. 2021;15(9):1159–68. https://doi.org/10.1080/17476348.2021.1935245.
Cazzola M, Ora J, Cavalli F, Rogliani P, Matera MG. Treatable mechanisms in asthma. Mol Diagn Ther. 2021;25(2):111–21. https://doi.org/10.1007/s40291-021-00514-w.
Pavord ID, Barnes PJ, Lemière C, Gibson PG. Diagnosis and assessment of the asthmas. J Allergy Clin Immunol Pract. 2023;1(1):1–8. https://doi.org/10.1016/j.jaip.2022.09.034.
Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400. https://doi.org/10.1016/S0140-6736(17)30879-6.
Fuhlbrigge AL, Castro M. Precision medicine in asthma-using phenotypes to understand endotypes that lead us to new therapeutic options. J Allergy Clin Immunol Pract. 2020;8(2):496–7. https://doi.org/10.1016/j.jaip.2019.12.001.
Singh D, Virchow JC, Canonica GW, et al. Extrafine triple therapy in patients with asthma and persistent airflow limitation. Eur Respir J. 2020;56(3):2000476. https://doi.org/10.1183/13993003.00476-2020.
Kılıç A, Ameli A, Park JA, et al. Mechanical forces induce an asthma gene signature in healthy airway epithelial cells. Sci Rep. 2020;10(1):966. https://doi.org/10.1038/s41598-020-57755-8.
Veerati PC, Mitchel JA, Reid AT, et al. Airway mechanical compression: its role in asthma pathogenesis and progression. Eur Respir Rev. 2020;29(157): 190123. https://doi.org/10.1183/16000617.0123-2019.
Pascoe CD, Green FHY, Elliot JG, James AL, Noble PB, Donovan GM. Airway remodelling with spatial correlations: implications for asthma pathogenesis. Respir Physiol Neurobiol. 2020;279: 103469. https://doi.org/10.1016/j.resp.2020.103469.
Calzetta L, Matera MG, Cazzola M, Rogliani P. Optimizing the development strategy of combination therapy in respiratory medicine: from isolated airways to patients. Adv Ther. 2019;36(12):3291–8. https://doi.org/10.1007/s12325-019-01119-w.
Braido F, Scichilone N, Lavorini F, et al. Manifesto on small airway involvement and management in asthma and chronic obstructive pulmonary disease: an Interasma (Global Asthma Association—GAA) and World Allergy Organization (WAO) document endorsed by Allergic Rhinitis and its Impact on Asthma (ARIA) and Global Allergy and Asthma European Network (GA2LEN). Asthma Res Pract. 2016;2:12. https://doi.org/10.1186/s40733-016-0027-5.
Mogensen I, Jacinto T, Alving K, Fonseca JA, Janson C, Malinovschi A. Inflammatory patterns in fixed airflow obstruction are dependent on the presence of asthma. PLoS ONE. 2020;15(12): e0243109. https://doi.org/10.1371/journal.pone.0243109.
Elliot JG, Jones RL, Abramson MJ, et al. Distribution of airway smooth muscle remodelling in asthma: relation to airway inflammation. Respirology. 2015;20(1):66–72. https://doi.org/10.1111/resp.12384.
Cazzola M, Braido F, Calzetta L, et al. The 5T approach in asthma: triple therapy targeting treatable traits. Respir Med. 2022;200: 106915. https://doi.org/10.1016/j.rmed.2022.106915.
McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24(1):37–47. https://doi.org/10.1111/resp.13389.
Simpson AJ, Hekking PP, Shaw DE, et al. Treatable traits in the European U-BIOPRED adult asthma cohorts. Allergy. 2019;74(2):406–11. https://doi.org/10.1111/all.13629.
Wu WW, Zhang X, Li M, et al. Treatable traits in elderly asthmatics from the Australasian severe asthma network: a prospective cohort study. J Allergy Clin Immunol Pract. 2021;9(7):2770–82. https://doi.org/10.1016/j.jaip.2021.03.042.
McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: treatable traits down under International Workshop report. Eur Respir J. 2019;53(5):1802058. https://doi.org/10.1183/13993003.02058-2018.
Cazzola M, Puxeddu E, Matera MG, Rogliani P. A potential role of triple therapy for asthma patients. Expert Rev Respir Med. 2019;13(11):1079–85. https://doi.org/10.1080/17476348.2019.1657408.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement.
Conflict of interest
The authors have no financial or non-financial relationships or activities concerning this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed equally to this review with the conception and design of the study, literature review and analysis, drafting and critical revision and editing, and approval of the final version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cazzola, M., Rogliani, P. & Matera, M.G. Might It Be Appropriate to Anticipate the Use of Long-Acting Muscarinic Antagonists in Asthma?. Drugs 83, 957–965 (2023). https://doi.org/10.1007/s40265-023-01897-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01897-2