Abstract
Due to their potent anti-inflammatory capacity (particularly in predominantly eosinophilic inflammation) and immunosuppressive properties, inhaled corticosteroids (ICSs) are widely used in asthmatic patients and also in individuals with chronic obstructive pulmonary disease (COPD) who suffer multiple exacerbations or have peripheral eosinophilia. However, there is little evidence for their use in non-cystic fibrosis bronchiectasis (hereafter, bronchiectasis). According to data extracted from large databases of bronchiectasis in adults, ICSs are used in more than 50% of patients without any scientific evidence to justify their efficacy and contrary to the recommendations of international guidelines on bronchiectasis that generally advise against their use. Indeed, bronchiectasis is a disease with predominantly neutrophilic inflammation and a high likelihood of chronic bacterial bronchial infection. Furthermore, it is known that due to their immunosuppressive properties, ICSs can induce an increase in bacterial infections. This manuscript aims to review the basic properties of ICSs, how they impact bronchiectasis in adults, the current position of international guidelines on this treatment, and the current indications and future challenges related to ICS use in bronchiectasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inhaled corticosteroids (ICSs) have potent anti-inflammatory and immunosuppressant properties. Their use in treating asthma and some forms of COPD patients is well established; however, there is little evidence for their use in bronchiectasis. |
More than 50% of patients with bronchiectasis not due to asthma, COPD, or eosinophilic diseases take ICSs, although the current international guidelines on bronchiectasis discourage this practice. |
Due to the paucity of information on the effect of ICSs in bronchiectasis without COPD or asthma, no definitive conclusions can be drawn either for or against the use of ICSs in bronchiectasis. |
1 Introduction
A recent global consensus defined bronchiectasis as a radiological finding consisting of dilation of the bronchial lumen accompanied by related symptoms, especially persistent productive cough, and a variable number of exacerbations throughout its natural history [1].
Bronchiectasis due to non-cystic fibrosis (hereafter, bronchiectasis) can be produced by multiple pulmonary and extrapulmonary causes [2, 3]. Although there is often no specific pathogenetic link between these different causes, inflammation of the bronchial wall is the common and necessary pathophysiological basis for the genesis of bronchiectasis [4]. In most cases, this inflammation occurs due to a previous bronchial infection and usually has a manifest neutrophilic component [5]. However, eosinophils can also play an important role in the genesis of bronchiectasis and may even characterize a specific phenotype of patients with bronchiectasis (Fig. 1) [6,7,8].
Boxplot graphic for the comparison of the number and severity of exacerbations in patients exposed to inhaled corticosteroids, with and without peripheral eosinophilia. The blue and green boxes refer to 6-month pre- and post-randomization values, respectively. Reproduced from Martinez-Garcia et al. [6] (free access)
Both proinflammatory molecules (mainly elastases and proteases) from inflammatory cells and the microorganisms causing the bronchial infection can irreversibly degrade the bronchial wall, producing its thickening and then inducing the enlargement of the airway lumen that leads to the characteristic symptoms of patients with bronchiectasis [5, 9]. The disruption of the local defense system existing in the bronchial mucosa against infection closes a vicious circle characterized by infection, inflammation, and airway remodeling (commonly called Cole's pathogenic vicious circle) [10], which allows for the negative evolution of the disease, the presence of systemic inflammation [11, 12], and an increase in the number of exacerbations [13].
Between 20 and 45% of cases of bronchiectasis have an unknown origin and are also called 'idiopathic bronchiectasis' [2, 3]. Moreover, bronchiectasis is often associated with the simultaneous presence of other chronic inflammatory airway diseases, such as chronic obstructive pulmonary disease (COPD) [14] and asthma [15], especially in advanced stages. This association increases bronchial inflammation and negatively impacts the severity and prognosis of these underlying diseases. Consequently, from a therapeutic point of view, treating infection and bronchial inflammation is crucial, and the international guidelines on bronchiectasis emphasize this necessity [16,17,18].
The introduction of inhaled corticosteroids (ICSs) significantly improved the treatment of asthma. ICSs are currently the pharmacological treatment of choice for this disease. From a pathophysiological point of view, this is due to their substantial efficacy against eosinophilic inflammation [19,20,21]. In the case of COPD, which is usually characterized by neutrophilic inflammation, the results have been more controversial. After numerous changes in recent years as a consequence of new information being progressively published [22,23,24,25,26], it is now accepted that ICSs in COPD patients should be prescribed combined with a long-acting β2-agonist (LABA) in patients with an overlap with asthma and, considering the blood eosinophil count that predicts the magnitude of the effect of ICS in reducing the risk of further exacerbations, in exacerbators with moderate to very severe COPD when they are not controlled by proper treatment with long-acting bronchodilators [27,28,29].
In bronchiectasis, there is little evidence of a positive effect of ICSs, probably due to bronchial bacterial infections and neutrophilic inflammation [5, 10]. The limited benefit of ICSs in the presence of predominantly neutrophilic inflammation and the possible risk that they may generate more infections, even serious ones, due to their immunosuppressive properties have been the reason why their indication in patients with bronchiectasis is not recommended, except in a few particular cases, despite their excessive use [16,17,18].
In this review, we attempt to update information on the pathophysiological basis related to the efficacy and risks of ICS in adults with bronchiectasis, the current position of international guidelines on this treatment, and the current indications and future challenges related to ICS use in bronchiectasis.
2 Bronchiectasis is a Chronic Inflammatory Airway Disease
As already mentioned, the essential condition for the genesis of bronchiectasis is the presence in the bronchi of chronic inflammation possibly accompanied by a bacterial infection [5]. The chronicity of this inflammation results from an imbalance in favor of proinflammatory molecules secreted by the inflammatory cells and the infecting bacteria over anti-inflammatory molecules capable of controlling inflammation. This imbalance is perpetuated and even accentuated in exacerbations despite antibiotic and anti-inflammatory treatments and the activation of immune defense mechanisms [4].
Both inflammatory cells and proinflammatory mediators have been observed in different respiratory samples (sputum, bronchoalveolar lavage, or biopsy samples) from individuals with bronchiectasis [30,31,32]. As in COPD, the neutrophil is the typical inflammatory cell found in most patients regardless of the etiology of bronchiectasis [5]. The neutrophil chemoattraction and activation are mainly due to the secretion of different mediators, especially interleukin (IL)-8, IL-1β, IL-17, leukotriene (LB) 4, and tumor necrosis factor (TNF)-α by different proinflammatory cells, and especially by bacterial products such as lipopolysaccharide (LPS), or other chemoattractants (platelet-activating factor and C5a) [33]. In addition, the secretion of elastases, peroxidases, and metalloproteases, among other molecules, by neutrophils is greater than that of antiproteases. This leads to the destruction of the bronchial wall and the disruption of local defense mechanisms against infection [34].
However, the neutrophil is not the only cell involved in inflammation in bronchiectasis. Macrophages play an important role in the infection control and the phagocytosis of microorganisms [5]. Little is known about the role of lymphocytes, of which CD4+ lymphocytes are the most prevalent in bronchiectasis [30], or epithelial cells also capable of generating different types of proinflammatory substances [5].
In recent years, it has been suggested that eosinophils may play a role that is more significant than expected in bronchiectasis inflammation. In fact, they are increased in both respiratory samples (> 3%) and peripheral blood (300 cells/µL) of about 20% of patients despite the absence of a history of asthma or other eosinophilic diseases such as allergic bronchopulmonary aspergillosis [7, 35]. Eosinophils can produce proteases and other proinflammatory molecules in response to infections induced by common microorganisms in patients with bronchiectasis, such as Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, and even Pseudomonas aeruginosa [36].
3 Infective Component of Bronchiectasis
The microbiology of bronchiectasis is complex and changes as the disease progresses [37]. Bronchial infection induced by bacteria, fungi, viruses, or mycobacteria that are all potentially pathogenic microorganisms (PPMs), plays a fundamental role in the genesis and progression of bronchiectasis. It causes an increase in both acute (exacerbations) and chronic (chronic bronchial infection) inflammation [5, 13]. Bronchial infection can be present in other airway diseases such as COPD [38], which, as mentioned, may occur simultaneously with bronchiectasis.
The bacterial load observed in the airways of bronchiectasis patients is related to a concurrent rise in local inflammation and its clinical and prognostic effects [39]. Antibiotic therapy, whether short- or long-term, is therefore necessary for some circumstances under this scenario [16,17,18, 40].
Several studies have shown that the lung microbiome is altered in patients with bronchiectasis, especially towards a decrease in its diversity [41, 42]. H. influenzae, S. pneumoniae, S. aureus, and enterobacteria are the most frequently isolated PPMs. However, infection by P. aeruginosa (especially chronic bronchial infection) is undoubtedly the one that has been shown to have a closer relationship with greater severity, more antibiotic resistance, and a worse prognosis of the disease [43, 44]. P. aeruginosa can appear in 15–50% of patients with bronchiectasis throughout its natural history, especially in the most severe forms of the disease, and with a geographical diversity characterized by a higher prevalence in Southern Europe, Asia, and Latin America [45,46,47,48]. Therefore, it is not surprising that it is the microorganism receiving the most attention and whose infection is being treated most aggressively, particularly in patients who exhibit the greatest symptoms.
It is important not to forget the growing importance of non-tuberculous mycobacteria, which are sometimes challenging to treat, and whose prevalence is also greatly influenced by the geographical area, being very frequent in the US [49].
4 Anti-inflammatory Properties of Inhaled Corticosteroids (ICSs)
Since there is an inflammatory bronchial response even when the patient is not in the acute phase of bronchiectasis [5], reducing inflammation may be a positive effect induced by corticosteroid treatment [50]. The mechanism of action of corticosteroids in lung diseases with a high inflammatory burden is still poorly understood, but their efficacy is reasonably linked to their anti-inflammatory properties [51]. The physiological actions of corticosteroids are carried out by a genomic and non-genomic mechanism [52].
The genomic anti-inflammatory actions of corticosteroids are achieved by synthesizing anti-inflammatory proteins and inhibiting proinflammatory proteins [51,52,53]. Transactivation and transrepression mechanisms make up the mechanism of corticosteroid activity. Pro-inflammatory cytokines are expressed less when there is so-called transrepression, whereas there is enhanced production of anti-inflammatory proteins when transactivation occurs [53].
The bulk of the corticosteroids' advantageous anti-inflammatory effects is thought to be caused by transrepression [52]. The clinical effectiveness of synthetic exogenous corticosteroids as anti-inflammatory drugs is mostly related to their capacity to inhibit the expression of pro-inflammatory genes through activating the glucocorticoid receptor (GR), and, concurrently, they decrease the activity of pro-inflammatory transcription factors such nuclear factor kappa B (NF-kB) and activator protein-1 (AP-1) [54]. This process is known as transrepression. In contrast, transactivation, an increase in gene expression mediated by direct binding of GR homodimers to glucocorticoid response elements (GREs) and recruitment of coactivators, is thought to be more involved in the negative adverse effects of corticosteroids [51, 52]. A few hundred genes from each cell type are assumed to be directly induced by corticosteroids to express themselves [55].
Corticosteroids can also induce non-genomic effects that occur immediately after GR ligation [56]. They are triggered by proteins released from the multiprotein complex after binding of corticosteroids to the cytosolic GR, interactions with membrane-bound receptors, and non-specific effects resulting from the interaction of corticosteroids with cell membranes [56].
Most elements of airway inflammation, including inflammatory cells, chemical mediators, and tissue reactions, appear to be affected by corticosteroids. In bronchiectasis, inflammation in the bronchial wall involves predominantly lymphocytes and macrophages, whereas polymorphonuclear leukocytes are predominant in the bronchial lumen [34]. Corticosteroids have inhibitory effects on many inflammatory and structural cells activated in bronchiectasis. They prevent the recruitment of inflammatory cells into the airways. Therefore, they diminish the number of eosinophils, T-lymphocytes, mast cells, and dendritic cells [51]. Resident structural cells are also suppressed by corticosteroids, which lower mediator release and expression on epithelial and endothelial cells, microvascular leak from blood vessels, angiogenesis, the number of mucus glands, and mucus secretion from these glands. They may also restore a more normal epithelium and airway architecture, allowing for a more typical airway response to infection [57].
The capacity to limit mucus glycoprotein production from airway submucosal glands directly as well as indirectly by downregulation of inflammatory stimuli that drive mucus secretion [51, 58] is another important effect of corticosteroids in bronchiectasis. It improves airway clearance and reduces bacterial nutrient availability. This might lead to a lower bacterial burden in the airways and lower sensitivity to respiratory infections [59].
5 Immunosuppressant Properties of ICSs
Corticosteroids can alter the diverse humoral and cellular components of the innate immunity networks functioning in the lungs, which have an inherent ability to keep the human organism mainly free of severe pulmonary infection [57]. Furthermore, they have a significant impact on lymphocyte immunological responses [60]. These effects are a crucial aspect of the immunosuppressive properties of corticosteroids.
Corticosteroids might imbalance innate and adaptive immune responses by altering macrophage gene expression, decreasing interferon (IFN)-γ expression, and upregulating chemokine production [61]. In addition, using ICSs also increases macrophage efferocytosis, which lowers macrophagic bactericidal capabilities [62].
Unfortunately, ICSs can alter the host-microbe interaction, resulting in alterations in the airway microbiota [63]. Actually, the ability of corticosteroids to suppress inflammatory reactions, preventing the migration of inflammatory cells from the circulation to the areas of disease, suppressing the synthesis of chemokines and cytokines, and inhibiting the immune responses of T cells, causes dysregulation of cellular immunity [57]. This in turn inhibits immune reactions and activates a direct stimulatory effect on the lung microbiome, which results in a prolonged presence of viruses and bacteria at infection sites as well as increased vulnerability to opportunistic infections. Furthermore, corticosteroids suppress antimicrobial compounds, inhibit macrophage phagocytosis, and blunt IFN responses, all of which impair the immunological response [63]. These factors may combine to cause the ‘flowering’ of select microorganisms in the lung microbiome, thus lowering diversity and increasing infection risk [59]. In this context, ICSs may be counterproductive in bronchiectasis, as a persistent bronchial infection has been found in 64% of patients [64].
Nevertheless, it has been suggested that the possibility the ICS-induced immunosuppression causes the development of local damage is very low in some individuals and much higher than what is known in others because of the genetic background that differently affects the effectiveness of the epithelial barrier and multiple aspects of the immune response following harmful stimuli and infections [57]. Furthermore, the lack of response to ICS treatment in terms of exacerbation frequency implies that exacerbation occurrence depends more on other factors, such as infection, than inflammation [65].
6 Differences Between Different ICSs
Several ICSs have been licensed for the treatment of chronic airway diseases. Beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone furoate, fluticasone propionate, mometasone furoate, and triamcinolone acetate are among these ICSs. The pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of ICSs, as well as their interplay, may have an impact on their effectiveness and safety profiles [66, 67]
The GR binding affinity, generally reported compared with the reference compound dexamethasone affinity of 100 [68], is the sole PD parameter that changes among ICSs. All ICSs have a greater binding affinity than dexamethasone. It is usually considered that the more the ICS affinity with the GR, the greater the ICS effectiveness. Fluticasone furoate has the most considerable affinity for the GR, with a relative binding affinity of 2989, followed by mometasone furoate at 2100, fluticasone propionate at 1775, budesonide at 935, triamcinolone acetonide at 233, and flunisolide at 190 [66]. The GR binding affinity of beclomethasone dipropionate is modest (relative receptor affinity = 53); however, this low affinity is a consequence of the limited hydrolysis of beclomethasone dipropionate to its active form, beclomethasone-17-monopropionate, which occurs during receptor binding studies, whereas beclomethasone-17-monopropionate binds to the GR with high affinity (relative receptor affinity = 1345) [69]. Desisobutyryl-ciclesonide has a 100-fold greater GR binding affinity than ciclesonide (relative receptor binding affinities of 1212 vs. 12) [70].
Affinity is a popular method for estimating the relative potencies of ICSs. Although relative ICS binding affinities should not be interpreted as absolute differences in potency because there are compounds with high binding affinity and no efficacy due to other PD and PK factors [71], it is widely accepted that the higher the ICS affinity to the GR, the more potent the ICSs [70]
Highly potent ICSs have potent anti-inflammatory properties, translating into better efficacy and lower frequency of systemic adverse effects [68]. Fluticasone furoate > mometasone > fluticasone propionate > beclomethasone > ciclesonide > budesonide > triamcinolone > flunisolide has been shown to have the highest relative potency (from high to low) [72].
Unfortunately, a high affinity for GRs in the lung would also imply a high affinity for systemic receptors, which has been related to an increased risk of unwanted adverse effects [67].
ICS particles deposited in the lungs must be dissolved in the pulmonary lining fluid to penetrate cells, bind to intracellular receptors, and be absorbed into the systemic/pulmonary circulation [73]. The physicochemical features of ICSs influence their PK at the tissue and cellular levels. Several characteristics of the ICSs, such as their formulation and how it relates to the inhaled drug's surface area, their lipophilicity and/or solubility in airway lining fluids, and the amount and composition of these fluids, impact their dissolution in the lungs [74]. For example, flunisolide dissolves the fastest (2 min), followed by budesonide (6 min). In contrast, beclomethasone dipropionate (> 5 h) and fluticasone propionate (> 8 h) dissolve exceedingly slowly due to their low water solubility [75]. However, beclomethasone dipropionate delivered by hydrofluoroalkane-134a (HFA)-propelled inhalers dissolves quickly (2–3 min) [76].
Lipophilicity, or a compound’s degree of lipid solubility, is the most important physicochemical quality that influences the PKs of ICSs [75]. Lipophilicity is often negatively related to solubility in pulmonary luminal fluid, yet also improves pulmonary absorption. Lipophilic ICSs bind to GRs with greater affinity and have a bigger distribution volume and longer half-life than other ICSs [67]. Lipophilicity impacts ICS lung retention time and can result in a prolonged duration of action [77]. Fluticasone furoate >> mometasone furoate > fluticasone propionate > triamcinolone acetate >> budesonide desisobutyryl-ciclesonide > flunisolide beclomethasone-17-monopropionate [66] are the lipophilicity-ordered lung retention times. On the other hand, lipophilicity may modify the distribution of the ICS after systemic absorption, allowing the medication to accumulate with subsequent doses in various body tissues, thereby increasing the risk of systemic adverse effects [68].
The concentration of unbound medication is critical because only the unbound portion of the ICS can interact with the GR, while the protein-bound fraction is pharmacologically inert [68]. Current ICS protein binding varies from 61.2% (flunisolide) to 99.7% (fluticasone furoate) of the circulating quantity [66]. There is no association between unbound ICS amounts in plasma and ICS concentrations in the lung [78] because only minimal protein binding occurs within the lung [70]. Some ICSs (budesonide and desisobutyryl-ciclesonide) undergo fatty acid esterification, which has been shown in animal and in vitro studies to result in longer lung retention [79, 80], but this does not result in a longer half-life, a longer duration of action, or a higher therapeutic index than non-esterified drugs [71].
The clearance values of ICSs, which express how quickly they leave the body, range from 37 L/h−1 (triamcinolone acetonide) to 84 L/h−1 (budesonide) [66]. The figures for beclomethasone dipropionate (120 L/h−1) and ciclesonide (228 L/h−1) are greater because they include extrahepatic metabolism [66]. One characteristic contributing to the low potential for systemic effects is rapid clearance of the absorbed dosage [66].
A longer elimination half-life after inhalation than after intravenous administration implies a prolonged pulmonary residence period [66]. Fluticasone furoate, fluticasone propionate, and desisobutyryl-ciclesonide have significantly longer inhalation half-lives, with a significant difference between fluticasone furoate and fluticasone propionate, whereas beclomethasone-17-monopropionate and budesonide have inhalation half-lives that are comparable with their intravenous half-lives [81, 82].
7 Pseudomonas Aeruginosa and ICSs
Pseudomonas aeruginosa is probably the most virulent pathogenic microorganism in patients with bronchiectasis [16,17,18]. Several studies demonstrate that ICSs may alter microbiome modifications by favoring the persistence of some bacteria while opposing the persistence of others [63]. For example, in a mouse model of allergic airway disease, there is experimental evidence that budesonide can enhance P. aeruginosa invasion of epithelial cells [83]. On the other hand, fluticasone propionate reduced P. aeruginosa cellular adherence in other animal models and cell lines [84].
Available data on the correlation between ICS use and sputum positivity to P. aeruginosa in bronchiectasis patients are somewhat conflicting. Aggregate data from two studies [85, 86] did not indicate an increased risk of P. aeruginosa colonization with ICS therapy [87]. Nonetheless, in another study, the microbial density (CFU/mL) of P. aeruginosa in sputum was not statistically different between the ICS and placebo groups after 4 weeks of therapy, although the value in the placebo group was lower and, compared with baseline, the density of total commensal bacteria in sputum increased [88]. A more recent study using a cohort of 264 patients with bronchiectasis managed at the respiratory outpatient clinics of two Danish university hospitals found that Pseudomonas-positive sputum cultures were more common in ICS-treated patients (6.5 vs. 20%, p = 0.010) [89].
It must be mentioned that an analysis of the US Bronchiectasis and NTM Research Registry showed that P. aeruginosa isolation was significantly associated with ICS use [90]. This finding was confirmed by a study that used US Medicare administrative data, which found that a history of P. aeruginosa increased the likelihood of extended use of ICSs in bronchiectasis patients [91].
Conversely, an old, small study suggested that ICS treatment benefits patients with bronchiectasis, particularly those with P. aeruginosa infection [65]. Furthermore, an observational investigation found that treatment with the inhaled salmeterol/fluticasone combination was beneficial and well tolerated in bronchiectasis patients, particularly those with low lung function or isolated P. aeruginosa [92].
8 Literature Review on the Use of ICSs in Bronchiectasis in Randomized Controlled Trials
A search of the PubMed, EMBASE, and Web of Science databases was conducted using the following terms: inhaled steroids OR inhaled corticosteroids OR beclomethasone OR ciclesonide OR budesonide OR fluticasone [title] AND bronchiectasis OR suppurative lung disease [title/abstract]. The research was restricted to randomized controlled trials (RCTs; with or without a placebo group) in adults with non-cystic fibrosis bronchiectasis. According to this search, there are currently eight RCTs (two on the effect of ICSs as monotherapy in the treatment group on inflammatory parameters in bronchiectasis, four on the clinical effect and safety of ICSs as monotherapy in the treatment group; and two comparing ICSs with other inhaled medication). The citations of each article identified and of the review articles were also examined.
8.1 ICS Effects on Inflammatory Parameters in Bronchiectasis
A 4-week course of high-dose (1000 µg/day) inhaled fluticasone propionate was shown to reduce specific proinflammatory molecules (IL-1β, IL-8, and LTB4) in sputum when compared with placebo in a small RCT of 24 patients with established steady-state bronchiectasis [88]. However, the difference between fluticasone and placebo was only statistically significant for IL-1β, and there were no significant changes in the clinical or microbiological profile. In 37 patients with bronchiectasis, Loukides et al. found no differences in exhaled hydrogen peroxide levels between groups, regardless of whether they were taking an ICS [93]. In 60 stable non-smoking bronchiectasis patients receiving 1000 µg/day of inhaled fluticasone for 1 year, Tsang et al. did not observe a decline in exhaled nitric oxide (eNO) [94]. However, P. aeruginosa infection was linked to noticeably reduced eNO levels. In a 6-month prospective, double-blind, parallel, placebo-controlled study, 77 patients with bronchiectasis were randomly allocated to receive either 400 µg budesonide twice daily or placebo [95]. Only the number of eosinophils in the sputum decreased significantly in the budesonide group compared with the placebo group. In contrast, no significant changes were found in the remaining inflammatory cell types (neutrophils, lymphocytes, macrophages). The serum levels of IL-8 in the budesonide group also decreased, although not significantly compared with the placebo group. Finally, a recent post hoc analysis of two RCTs found that taking ICS (fluticasone or budesonide) for 6 months had no influence on the overall number of peripheral eosinophils [96].
In summary, the few data available in the literature indicate that the effect of ICSs on various aspects of neutrophilic inflammation in patients with bronchiectasis is extremely modest, even when they are administered at high doses.
8.2 Clinical Effects of ICSs in Bronchiectasis
In 2009, based on the findings of three published RCTs, it was proposed that ICSs may be useful in the long-term management of bronchiectasis [97]. Unfortunately, there are currently only five RCTs in the literature on the clinical effect and safety of ICSs as monotherapy in bronchiectasis lasting 4 weeks to 12 months [65, 95, 98,99,100]. Four of these RCTs were placebo-controlled and used medium- or high-dose ICSs (beclomethasone 800–1500 µg/day [98]; beclomethasone 800 µg/day [100], fluticasone 1000 µg/day [86]; and budesonide 800 µg/day [95]), while the fifth study compared high-dose fluticasone (1000 µg/day) with a more moderate dose (500 µg/day) [88]. The sample size was not calculated in any of the studies except one. Asthmatic subjects were excluded in four studies [65, 95, 99, 100] and those with COPD were excluded in one trial [96]. In all studies, the outcomes analyzed were focused on the impact on symptoms and/or lung function. Sputum production was analyzed in two trials [65, 99]. Furthermore, the microbiological profile and quality of life were investigated in two studies [95, 99]. Only one trial reported adverse events [99].
The main results obtained are summarized in Table 1. Only one of the studies showed a significant improvement in forced expiratory volume at 1 s (FEV1) after 6 weeks of treatment with 1600 µg/day beclomethasone [99]; however, a significant reduction was observed in the two studies in which daily sputum volume was measured [65, 99]. The study by Martinez-Garcia et al. [99], in which high and medium doses of fluticasone were compared, showed the most remarkable improvement in clinical parameters and quality of life; however, this was a non placebo controlled study. Reports on adverse events provided by the studies are scarce. Nevertheless, it is noteworthy that high doses of fluticasone induced significantly more local adverse events than medium doses of the same ICS [99].
8.3 Comparison Between ICSs and Other Inhaled Drugs in Bronchiectasis
A small, double-blind, parallel-group clinical study was conducted to assess whether the addition of formoterol (18 µg/day), a LABA, to medium–high doses of budesonide (640 µg/day) for 12 months could improve the safety profile and efficacy compared with high-dose budesonide (1600 µg/day) [101]. The study included 40 patients with bronchiectasis diagnosed by a high-resolution computed tomography (HRCT) scan of the chest. The combination group significantly improved dyspnea and quality of life, increased the percentage of cough-free days, and reduced the number of rescue β2-agonist inhalations. No changes in lung function, microbiological profile, or number of exacerbations were observed. Moreover, there was a significant reduction in the number of adverse effects in the LABA/ICS group that used medium doses of budesonide compared with the group treated with high doses of budesonide, with statistically significant differences in the reduction of pharyngeal irritation, dry mouth, and dysphonia.
8.4 Potential Risks Associated with the Use of ICSs in Bronchiectasis
In addition to the increase in local adverse effects observed in the aforementioned clinical studies, the use of ICSs was generally associated with an increased likelihood of acquiring an infection with P. aeruginosa or non-tuberculous mycobacteria and developing pneumonia, at least in certain subgroups of COPD patients, especially those with lower eosinophil counts [102,103,104].
In a group of 264 patients with HRCT-verification of bronchiectasis, Håkansson et al. found that the 122 subjects using ICS at enrolment had worse lung function (median FEV1 65.2 vs. 80.9% predicted; p < 0.001), higher symptom burden in terms of cough (p = 0. 028), sputum production (p < 0.001) and dyspnea (p < 0.001), more exacerbations (41 vs. 21%; p < 0.001), and more frequent isolation of P. aeruginosa in sputum (6.5 vs. 20%; p = 0.010) [89]. After controlling for age, sex, smoking status, baseline FEV1, and concurrent asthma/COPD, high-dose ICS therapy was substantially linked with all-cause death, with a hazard ratio (HR) of 4.93 (p = 0.003). However, low-to-moderate doses of ICS were not associated with all-cause mortality.
In a study of 192 patients with bronchiectasis, Lee et al. observed that ICSs increased the risk of clinically relevant hemoptysis (odds ratio [OR] 2.34), especially when combined with LABAs, compared with the use of LABAs alone [105]. The explanation for this phenomenon should be that while ICS monotherapy may have a transient vasoconstrictive effect on the vessels of the bronchial mucosa, its combination with a LABA may potentiate the vasodilatory effect of this bronchodilator, thus favoring the development of hemoptysis.
Moreover, in 50 patients with bronchiectasis, Holme et al. demonstrated that subjects receiving ICS (33 patients) had a significantly higher percentage of adrenal suppression (48.5 vs. 23.5%), which was associated with worse quality of life than subjects not receiving ICS [106]. The authors hypothesized that some of the symptoms related to adrenal suppression might even contribute to poorer control of bronchiectasis.
Thus, although ICSs exhibit significant anti-inflammatory action, their apparent limited clinical effectiveness and the risk for serious adverse effects have prompted the long-term use of macrolides as the anti-inflammatory therapy of choice in patients with bronchiectasis. Indeed, macrolides are currently the class of drugs with the strongest evidence of being able to reduce the number of exacerbations in bronchiectasis patients [107]. Unfortunately, macrolides are not without adverse effects.
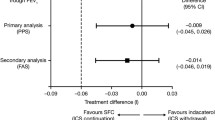
The only study that directly addressed the safety of ICSs and macrolides in patients with bronchiectasis was conducted by Henkle et al., who retrospectively used the 2006–2014 US Medicare Part A (hospital), B (outpatient) and D (prescription drug coverage) datasets [108]. This study retrospectively included 285,043 patients with a pulmonary-associated bronchiectasis claim, of whom 83,589 (29.3%) were new users of chronic ICS and 6500 (2.3%) were new users of macrolides (erythromycin or azithromycin) as monotherapy. New users were defined as the first prescription ≥ 28 days for either drug group after a 12-month clean period. Comparing new users of ICS with macrolide users, the propensity score-adjusted HRs were 1.39 for hospitalized respiratory infection, 1.56 for acute exacerbation, and 1.09 for death (Fig. 2). The ICS group was less likely to have a previous diagnosis of Pseudomonas infection (6.1 vs. 12.5% in the macrolide cohort) and non-tuberculous mycobacteria infection (3.8 vs. 20.1%) but more likely to have COPD/emphysema (84.4 vs. 77.7%). The results favored macrolides when analyzing the subgroup of patients with COPD/bronchiectasis overlap but not when analyzing the subgroup of patients with asthma/bronchiectasis in whom treatment with ICSs had advantages over treatment with macrolides in terms of reducing the number of exacerbations. Finally, no differences in mortality risk were observed when comparing the two treatments (OR 1.09). Compared with ICSs, chronic macrolide use was related to a lower incidence of arrhythmia but not to a statistically significant higher risk of myocardial infarction [109]. Moreover, chronic macrolide monotherapy was linked with a statistically significant, modestly elevated risk of hearing loss. In any case, it is important to emphasize as limitations of the study the fact that it was retrospective, that the diagnosis of bronchiectasis was made using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and that all patients included were at least 65 years of age.
Forest plot of unadjusted (blue) and adjusted (red) HRs (95% CIs) of key outcomes comparing new use of ICS therapy with macrolide monotherapy for bronchiectasis. Adjusted hazard ratios included propensity score decile, oral corticosteroid dose category, and non-tuberculous mycobacteria history. Reproduced from Hencke et al. [108] (free access). HRs hazard ratios, CIs confidence intervals, ICS inhaled corticosteroid
9 What do the Guidelines Say About Using Inhaled Corticosteroids in Bronchiectasis?
International guidelines agree on the lack of indication of ICSs as a routine treatment in patients with bronchiectasis, considering the information previously presented in this review; however, the guidelines recognize that the existing scientific evidence supporting this recommendation is limited (Table 2) [16,17,18, 110, 111].
Nonetheless, depending on the guideline considered, there are some exceptions for which ICSs must be prescribed, may be used, or at least must not be withdrawn (Table 3).
-
1.
Asthma/bronchiectasis overlap This is probably the most well-established indication. Studies have observed that up to 25% of patients with severe forms of asthma may develop bronchiectasis [112, 113]. In this condition, ICSs should still be prescribed because they are the treatment of choice in patients with asthma [19, 20]. However, it is essential to consider de-escalation of ICS therapy following international asthma guidelines when the clinical status allows it due to the potential risk of infectious complications [19, 20]. Unfortunately, the rate of chronic bronchial infection due to PPMs in patients with asthma/bronchiectasis overlap is unknown, but it is expected to be lower than in other associated diseases, such as COPD.
-
2.
COPD/bronchiectasis overlap The prevalence of bronchiectasis in patients with severe COPD is even higher than in severe asthma, reaching 50% [114]. In most of these overlapping patients, a bronchial infection with PPMs, including P. aeruginosa, is also present and is sometimes chronic. Studies show that ICSs in COPD patients increase the risk of pneumonia, atypical mycobacteriosis, and bronchial infection with P. aeruginosa [102,103,104]. ICS users had suppressed IFN expression with increased exacerbation severity through greater viral loads and mucus hypersecretion. Mucus hypersecretion was associated with a more remarkable acute fall in lung function than observed in ICS non-users in a cohort of patients with COPD presenting with virus-associated exacerbation (Fig. 3) [115]. In theory, ICSs could increase de novo pneumonic episodes by stimulating the proliferation of bacteria within the current lung microbiota or aiding in acquiring additional bacteria from the environment. There is evidence that ICSs impair bacterial lung control by suppressing the epithelial synthesis of the antibacterial peptide cathelicidin because they amplify the protease cathepsin D [116]. This steroid-inducible gene may cleave and inactivate cathelicidin. COPD causes an increase in the expression of cathepsin D in the airways [117]. Consequently, ICSs are discouraged or should be limited to the lowest possible dose while optimizing bronchodilation. Nonetheless, ICSs are indicated in COPD patients when there is an overlap with asthma or frequent exacerbations with high blood eosinophil count values [118]. However, the blood eosinophil count above which using an ICS can be beneficial has not yet been established [118]. Furthermore, the impact of eosinophilia on the effect of ICSs in COPD with bronchiectasis when a chronic bronchial infection is present is still unknown [119].
-
3.
Allergic bronchopulmonary aspergillosis (ABPA) ABPA is a recognized etiology of bronchiectasis that mainly causes eosinophilic inflammation. Some guidelines recommend using ICSs even in the presence of bronchiectasis, a common radiological finding of this disease [18, 110].
-
4.
Bronchiectasis with bronchial or peripheral eosinophilic component Up to 20% of patients with bronchiectasis have a local or systemic eosinophilic component (> 3% eosinophils in sputum or > 300 peripheral eosinophils/µL) without an underlying known eosinophilic disease [7]. Some studies have suggested that these patients may respond positively to ICSs (and some biological treatments) with a reduction in the number of exacerbations [6, 98, 120]; however, the type and dose of ICS may be important variables in influencing the response [121].
-
5.
Uncontrollable bronchorrhea Based on the results obtained in some clinical studies, some authors suggest that a test with ICSs might be justified in patients with a significant excess of bronchial secretion that cannot be controlled with other pharmacological or non-pharmacological interventions [17, 111]; however, clinical outcomes must be carefully evaluated.
Long-term impact of inhaled corticosteroid use in COPD/bronchiectasis overlap: review of mechanisms that underlie risks. Reproduced from Singanayagam and Johnston [116] (free access). COPD chronic obstructive pulmonary disease, IFN interferon
10 Why are ICSs Used so Frequently in Bronchiectasis Without a Specific Indication?
Table 4 shows that all available registries indicate that using ICSs in patients with bronchiectasis is excessive. The percentage of patients treated with ICSs sometimes exceeds 65% and is much higher than that of subjects with asthma, COPD, or ABPA [3, 47, 49, 122,123,124].
The possible causes of this overuse of ICSs in individuals with bronchiectasis have not been investigated sufficiently, even though all international guidelines on bronchiectasis state that they should be used with caution. However, some hypotheses can be formulated:
-
1.
There is common diagnostic confusion between COPD and bronchiectasis. For example, O'Brien et al. observed that almost one-third of patients sent to a specialist with a COPD diagnosis did not have airflow obstruction but bronchiectasis in the tomographic study, which could explain their symptoms [125]. Despite this, treatment with ICSs was not discontinued in most cases.
-
2.
COPD and asthma are the best-known chronic inflammatory airway diseases. It cannot therefore be ruled out that in the absence of scientific evidence, and also due to the lack of knowledge of the recommendations of the bronchiectasis guidelines on the use of ICSs, patients with bronchiectasis are treated by extrapolation similarly to those with COPD and asthma.
-
3.
Although scientific evidence on long-acting bronchodilators in bronchiectasis is lacking, these agents are often prescribed to patients with symptomatic bronchiectasis or airflow obstruction [126]. Moreover, in a significant proportion of inhaler devices on the market, bronchodilators are combined with ICSs [127,128,129], which could lead to misuse of ICSs.
-
4.
Knowing the importance of bronchial inflammation in bronchiectasis, physicians may consider ICSs indicated in this disease because of their potent anti-inflammatory activity.
-
5.
As the clinical studies conducted on the effect of ICSs in bronchiectasis without COPD or asthma have been few and always small, no definitive conclusions can be drawn either for or against the use of ICSs in bronchiectasis.
11 Future Challenges and Unmet Knowledge
The limited scientific evidence on the efficacy and safety of ICSs in bronchiectasis makes it urgent and mandatory to perform well designed and powered RCTs in bronchiectasis patients who are naïve to ICS therapy. In addition, it will be worthwhile to verify through a dedicated, pragmatic study involving bronchiectasis subjects treated with ICS without a clear history of asthma or COPD whether discontinuation of ICS is associated with a significant worsening of bronchiectasis. As shown in Table 5, numerous features of ICSs in bronchiectasis must be defined and studied thoroughly.
References
Aliberti S, Goeminne PC, O’Donnell AE, Aksamit TR, Al-Jahdali H, Barker AF, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10(3):298–306.
Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, et al. Etiology of non-cystic fbrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc. 2015;12(12):1764–70.
Martinez-García MA, Villa C, Dobarganes Y, Girón R, Maíz L, García-Clemente M, Sibila O, et al. RIBRON: The Spanish Online Bronchiectasis Registry. Characterization of the first 1912 patients. Arch Bronconeumol. 2021;57(1):28–35.
Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med. 2018;6(9):715–26.
Fuschillo S, de Felice A, Balzano G. Mucosal infammation in idiopathic bronchiectasis: cellular and molecular mechanisms. Eur Respir J. 2008;31(2):396–406.
Martinez-Garcia MA, Posadas T, Sotgiu G, Blasi F, Saderi L, Aliberti S. Repeatability of circulating eosinophil measures and inhaled corticosteroids effect in bronchiectasis. A post hoc analysis of a randomized clinical trial. Arch Bronconeumol (Engl Ed). 2020;56(10):681–3.
Shoemark A, Shteinberg M, De Soyza A, Haworth C, Richardson H, Gao Y, et al. Characterisation of eosinophilic bronchiectasis: a European multicohort study. Am J Respir Crit Care Med. 2022;205(8):894–902.
Martinez-Garcia MA. Bronchiectasis and eosinophils. Arch Bronconeumol. 2021;57:671–2.
Monsó E. Look at the wood and not at the tree: the microbiome in chronic obstructive lung disease and cystic fibrosis. Arch Bronconeumol. 2020;56(1):5–6.
Cole PJ. Inflammation: a two-edged sword—the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6–15.
Posadas T, Oscullo G, Zaldivar E, Villa C, Dobarganes Y, Girón R, et al. C-reactive protein concentration in steady-state bronchiectasis: prognostic value of future severe exacerbations. Data from the Spanish Registry of Bronchiectasis (RIBRON). Arch Bronconeumol. 2021;57(1):21–7.
Saleh AD, Chalmers JD, De Soyza A, Fardon TC, Koustas SO, Scott J. The heterogeneity of systemic inflammation in bronchiectasis. Respir Med. 2017;127:33–9.
Chen CL, Huang Y, Yuan JJ, Li HM, Han XR, Martinez-Garcia MA, et al. The Roles of bacteria and viruses in bronchiectasis exacerbation: a prospective study. Arch Bronconeumol. 2020;56(10):621–9.
Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–11.
Crimi C, Ferri S, Campisi R, Crimi N. The link between asthma and bronchiectasis: state of the art. Respiration. 2020;99:463–76.
Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629.
Martínez-García MÁ, Máiz L, Olveira C, Girón RM, de la Rosa D, Blanco M, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):88–98.
Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn JS, Floto RA, et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69.
Reddel HK, Bacharier LB, Bateman ED, Brighting CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma strategy 2021. Executive summary and rationale for key changes. Arch Bronconeumol. 2022;2022(58):35–51.
Plaza V, Blanco M, García G, Korta J, Molina J, Quirce S. Highlights of the Spanish Asthma Guidelines (GEMA), version 5.0. Arch Bronconeumol. 2022;57(1):11–2.
Plaza V, Gómez-Outes A, Quirce S, Alobid I, Álvarez C, Blanco M, et al. Discrepancies between GEMA and GINA in the classification of inhaled corticosteroids. Arch Bronconeumol. 2020;56:472–3.
Pizzichini E, Pizzichini MM, Gibson P, Parameswaran K, Gleich GJ, Berman L, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1511–7.
Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356(9240):1480–5.
Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60(3):193–8.
Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–71.
Bafadhel M, McCormick M, Saha S, McKenna S, Shelley M, Hargadon B, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83(1):36–44.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2022. Available at: https://goldcopd.org/. Accessed 30 May 2022.
Miravitlles M, Calle M, Molina J, Almagro P, Tomás Gómez J, Trigueros JA, et al. Spanish COPD guidelines (GesEPOC) 2021: Updated pharmacological treatment of stable COPD. Arch Bronconeumol. 2022;58(1):69–81.
Miravitlles M, Calle M, Soler-Cataluña JJ. GesEPOC 2021: One more step towards personalized treatment of COPD. Arch Bronconeumol. 2021;57(1):9–10.
Gaga M, Bentley AM, Humbert M, Barkans J, O’Brien F, Wathen CG, et al. Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax. 1998;53:685–91.
Angrill J, Agustí C, de Celis R, Rañó A, Gonzalez J, Solé T. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2022;57:15–9.
Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Gu YY. Sputum matrix metalloproteinase-8 and -9 and tissue inhibitor of metalloproteinase-1 in bronchiectasis: clinical correlates and prognostic implications. Respirology. 2015;20:1073–81.
Boyton RJ, Altmann DM. Bronchiectasis: current concepts in pathogenesis, immunology, and microbiology. Annu Rev Pathol. 2016;11:523–54.
King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis. 2009;4:411–9.
Tsikrika S, Dimakou K, Papaioannou AI, Hillas G, Thanos L, Kostikas K, et al. The role of non-invasive modalities for assessing inflammation in patients with non-cystic fibrosis bronchiectasis. Cytokine. 2017;99:281–6.
Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allerg Immunol. 2016;50:214–27.
King PT, Daviskas E. Pathogenesis and diagnosis of bronchiectasis. Breathe. 2010;6:341–51.
de la Rosa CD, López-Campos JL, Alcázar Navarrete B, Calle Rubio M, Cantón Moreno R, García-Rivero JL, et al. Consensus document on the diagnosis and treatment of chronic bronchial Infection in chronic obstructive pulmonary disease. Arch Bronconeumol. 2020;56(10):651–64.
Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392(10150):880–90.
De la Rosa D, Martinez-Garcia MA, Barreiro E, Tabernero E, Costa R, Garcia-Clemente M, et al. Effectiveness and safety of inhaled antibiotics in patients with chronic obstructive pulmonary disease. A multicentre observational study. Arch Bronconeumol. 2022;58(1):11–21.
Mac Aogáin M, Narayana JK, Tiew PY, Ali NABM, Yong VFL, Jaggi TK, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med. 2021;27(4):688–99.
Richardson H, Dicker AJ, Barclay H, Chalmers JD. The microbiome in bronchiectasis. Eur Respir Rev. 2019;28: 190048.
Araújo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon TC, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. 2018;51(2):1701953.
Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12(11):1602–11.
Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–85.
Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, Román-Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007;132(5):1565–72.
Dhar R, Singh S, Talwar D, Mohan M, Kant Tripathi S, Swarnakar R, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health. 2019;7(9):e1269–79.
Martinez-Garcia MA, Athanazio RA, Girón R, Máiz-Carro L, de la Rosa D, Olveira C, et al. Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J Chron Obstruct Pulmon Dis. 2017;12:275–84.
Aksamit TR, O’Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, et al. Bronchiectasis Research Registry Consortium. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest. 2017;15:982–92.
Lasserson T, Holt K, Greenstone M. Oral steroids for bronchiectasis (stable and acute exacerbations). Cochrane Database Syst Rev. 2001;2001(4):C002162.
Barnes PJ. Glucocorticosteroids. Handb Exp Pharmacol. 2017;237:93–115.
Caramori G, Nucera F, Mumby S, Lo Bello F, Adcock IM. Corticosteroid resistance in asthma: cellular and molecular mechanisms. Mol Aspects Med. 2022;85: 100969.
Strehl C, Buttgereit F. Optimized glucocorticoid therapy: teaching old drugs new tricks. Mol Cell Endocrinol. 2013;380(1–2):32–40.
De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23(3):281–91.
Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134(1):54–67.
Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci. 2019;40(1):38–49.
Sabroe I, Postma D, Heijink I, Dockrell DH. The yin and the yang of immunosuppression with inhaled corticosteroids. Thorax. 2013;68(12):1085–7.
Adcock IM, Mumby S. Glucocorticoids. Handb Exp Pharmacol. 2017;237:171–96.
Singh S, Pragman AA, Segal LN. Balancing benefits and risks: do inhaled corticosteroids modify the lung microbiome? Am J Respir Crit Care Med. 2021;204(10):1117–9.
O’Sullivan S, Cormican L, Burke CM, Poulter LW. Fluticasone induces T cell apoptosis in the bronchial wall of mild to moderate asthmatics. Thorax. 2004;59(8):657–61.
Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7(1):53.
Stolberg VR, McCubbrey AL, Freeman CM, Brown JP, Crudgington SW, Taitano SH, et al. Glucocorticoid-augmented efferocytosis inhibits pulmonary pneumococcal clearance in mice by reducing alveolar macrophage bactericidal function. J Immunol. 2015;195(1):174–84.
Keir HR, Contoli M, Chalmers JD. Inhaled corticosteroids and the lung microbiome in COPD. Biomedicines. 2021;9(10):1312.
Angrill J, Agustí C, de Celis R, Rañó A, Gonzalez J, Solé T, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57(1):15–9.
Tsang KW, Tan KC, Ho PL, Ooi GC, Ho JC, Mak J, et al. Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax. 2005;60(3):239–43.
Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80(3):372–80.
Matera MG, Rinaldi B, Calzetta L, Rogliani P, Cazzola M. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids for asthma treatment. Pulm Pharmacol Ther. 2019;58: 101828.
Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28(5):1042–50.
Winkler J, Hochhaus G, Derendorf H. How the lung handles drugs: pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1(4):356–63.
Rossi GA, Cerasoli F, Cazzola M. Safety of inhaled corticosteroids: room for improvement. Pulm Pharmacol Ther. 2007;20(1):23–35.
Kelly HW. Comparison of inhaled corticosteroids: an update. Ann Pharmacother. 2009;43(3):519–27.
Ye Q, He XO, D’Urzo A. A review on the safety and efficacy of inhaled corticosteroids in the management of asthma. Pulm Ther. 2017;3(1):1–18.
Hübner M, Hochhaus G, Derendorf H. Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids. Immunol Allergy Clin North Am. 2005;25(3):469–88.
Borghardt JM, Weber B, Staab A, Kloft C. Pharmacometric models for characterizing the pharmacokinetics of orally inhaled drugs. AAPS J. 2015;17(4):853–70.
Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. J Allergy Clin Immunol. 1996;97(1 Pt 2):169–76.
Freiwald M, Valotis A, Kirschbaum A, McClellan M, Mürdter T, Fritz P, et al. Monitoring the initial pulmonary absorption of two different beclomethasone dipropionate aerosols employing a human lung reperfusion model. Respir Res. 2005;6(1):21.
Kelly HW. Pharmaceutical characteristics that influence the clinical efficacy of inhaled corticosteroids. Ann Allergy Asthma Immunol. 2003;91(4):326–34.
Bäckman P, Adelmann H, Petersson G, Jones CB. Advances in inhaled technologies: understanding the therapeutic challenge, predicting clinical performance, and designing the optimal inhaled product. Clin Pharmacol Ther. 2014;95(5):509–20.
Miller-Larsson A, Mattsson H, Hjertberg E, Dahlbäck M, Tunek A, Brattsand R. Reversible fatty acid conjugation of budesonide. Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos. 1998;26(7):623–30.
Nave R, Meyer W, Fuhst R, Zech K. Formation of fatty acid conjugates of ciclesonide active metabolite in the rat lung after 4-week inhalation of ciclesonide. Pulm Pharmacol Ther. 2005;18(6):390–6.
Allen DB, Bielory L, Derendorf H, Dluhy R, Colice GL, Szefler SJ. Inhaled corticosteroids: past lessons and future issues. J Allergy Clin Immunol. 2003;112(3 Suppl):S1–40.
Allen A, Bareille PJ, Rousell VM. Fluticasone furoate, a novel inhaled corticosteroid, demonstrates prolonged lung absorption kinetics in man compared with inhaled fluticasone propionate. Clin Pharmacokinet. 2013;52(1):37–42.
Wang P, Wang X, Yang X, Liu Z, Wu M, Li G. Budesonide suppresses pulmonary antibacterial host defense by down-regulating cathelicidin-related antimicrobial peptide in allergic inflammation mice and in lung epithelial cells. BMC Immunol. 2013;14:7.
Dowling RB, Johnson M, Cole PJ, Wilson R. Effect of fluticasone propionate and salmeterol on Pseudomonas aeruginosa infection of the respiratory mucosa in vitro. Eur Respir J. 1999;14(2):363–9.
Martínez-García MA, Perpiñá-Tordera M, Román-Sánchez P, Soler-Cataluña JJ. Inhaled steroids improve quality of life in patients with steady-state bronchiectasis. Respir Med. 2006;100(9):1623–32.
Hernando R, Drobnic ME, Cruz MJ, Ferrer A, Suñé P, Montoro JB, et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. Int J Clin Pharm. 2012;34(4):644–50.
Kapur N, Petsky HL, Bell S, Kolbe J, Chang AB. Inhaled corticosteroids for bronchiectasis. Cochrane Database Syst Rev. 2018;5(5):CD000996.
Tsang KW, Ho PL, Lam WK, Ip MS, Chan KN, Ho CS, et al. Inhaled fluticasone reduces sputum inflammatory indices in severe bronchiectasis. Am J Respir Crit Care Med. 1998;158(3):723–7.
Håkansson KEJ, Fjaellegaard K, Browatzki A, Dönmez Sin M, Ulrik CS. Inhaled corticosteroid therapy in bronchiectasis is associated with all-cause mortality: a prospective cohort study. Int J Chron Obstruct Pulmon Dis. 2021;16:2119–27.
Henkle E, Aksamit TR, Barker AF, Curtis JR, Daley CL, Anne Daniels ML, et al. Pharmacotherapy for non-cystic fibrosis bronchiectasis: results from an NTM Info & Research Patient Survey and the Bronchiectasis and NTM Research Registry. Chest. 2017;152(6):1120–7.
Henkel E, Chan B, Aksamit TR, Curtis JR, Winthrop KL. Factors associated with extended inhaled corticosteroid use in US Medicare bronchiectasis patients. Am J Respir Crit Care Med. 2018;197:A6282.
Wei P, Yang JW, Lu HW, Mao B, Yang WL, Xu JF. Combined inhaled corticosteroid and long-acting β2-adrenergic agonist therapy for noncystic fibrosis bronchiectasis with airflow limitation: an observational study. Medicine (Baltimore). 2016;95(42): e5116.
Loukides S, Horwarth I, Wodehouse T, Coler PJ, Barnes PJ. Elevated levels of expired breath hydrogen peroxide in bronchiectasis. Am J Respir Crit Care Med. 1998;168:991–4.
Tsang KW, Tan KC, Ho PL, Ooi GC, Khong PL, Leung R, et al. Exhaled nitric oxide in bronchiectasis: the effects of inhaled corticosteroid therapy. Int J Tuberc Lung Dis. 2004;8:1301–7.
Hernando R, Drobnic ME, Cruz MJ, Ferrer A, Suñe P, Montoro J, et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. Int J Clin Pharm. 2012;34:644–50.
Martinez-Garcia MA, Posadas T, Sotgiu G, Blasi F, Saderi L, Aliberti A. Role of inhaled corticosteroids in reducing exacerbations in bronchiectasis patients with blood eosinophilia pooled post-hoc analysis of 2 randomized clinical trials. Resp Med. 2020;172: 106127.
Ilowite J, Spiegler P, Kessler H. Pharmacological treatment options for bronchiectasis: focus on antimicrobial and anti-inflammatory agents. Drugs. 2009;69(4):407–19.
Elborn JS, Johnston B, Allen F, Clarke J, McGarry J, Varguese G. Inhaled steroids in patients with bronchiectasis. Respir Med. 1992;86:121–4.
Martinez-Garcia MA, Perpina-Tordera M, Roman-Sanchez P, et al. Inhaled steroids improve quality of life in patients with steady-state bronchiectasis. Respir Med. 2006;100:1623–32.
Joshi JM, Sundaram P. Role of inhaled steroids in stable bronchiectasis. Indian Pract. 2004;57(4):243–5.
Martínez-García MÁ, Soler-Cataluña JJ, Catalán-Serra P, Román-Sánchez P, Tordera MP. Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest. 2012;141:461–8.
Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;168:1029–36.
Andrejak C, Nielsen R, Thomsen VØ, Duhaut P, Sørensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68:256–62.
Eklöf J, Ingebrigtsen TS, Sorensen R, Isam Saeed M, Alispahic IA, Sivapalan P, et al. Use of inhaled corticosteroids and risk of acquiring Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Thorax. 2022;77:573–80.
Lee JK, Lee J, Park SS, Heo EY, Park YS, Lee CH, et al. Effect of inhalers on the development of haemoptysis in patients with non-cystic fibrosis bronchiectasis. Int J Tuberc Lung Dis. 2014;18:363–70.
Holme J, Tomlinson JW, Stockley RA, Steward PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Resp J. 2008;32:1047–52.
Chalmers JD, Boersma W, Lonergan M, Jayaram L, Crichton ML, Karalus N. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med. 2019;7(10):845–54.
Henkle E, Curtis JR, Chen L, Chan B, Aksamit TR, Daley CL, et al. Comparative risks of chronic inhaled corticosteroids and macrolides for bronchiectasis. Eur Respir J. 2019;54(1):1801896.
Henkle E, Daley CL, Curtis JR, Chan B, Aksamit TR, Winthrop KL. Comparative safety of inhaled corticosteroids and macrolides in Medicare enrolees with bronchiectasis. ERJ Open Res. 2022;8(1):00786–2020.
Al-Jahdali H, Alshimemeri A, Mobeireek A, Albanna AS, Al Shirawi NN, Wali S, et al. The Saudi Thoracic Society guidelines for diagnosis and management of noncystic fibrosis bronchiectasis. Ann Thorac Med. 2017;12(3):135–61.
Chang AB, Bell SC, Torzillo PJ, King PT, Maguire GP, Byrnes CA, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. Thoracic Society of Australia and New Zealand guidelines. Med J Aust. 2015;202(1):130.
Padilla A, Olveira C, Fernandez L, Marco I, Plata AJ, Alvarez A, et al. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res. 2018;19(1):43.
Polverino E, Dimakou K, Hurst J, Martinez-Garcia MA, Miravitlles M, Paggiaro P, et al. The overlap between bronchiectasis and chronic airways diseases: state of the art and future directions. Eur Respir J. 2018;52(3):1800328.
Du Q, Jin J, Liu X, Sun Y. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS ONE. 2016;11(3): e0150532.
Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun. 2018;9(1):2229.
Singanayagam A, Johnston SL. Long-term impact of inhaled corticosteroid use in asthma and chronic obstructive pulmonary disease (COPD): Review of mechanisms that underlie risks. J Allergy Clin Immunol. 2020;146(6):1292–4.
Singanayagam A, Glanville N, Cuthbertson L, Bartlett NW, Finney LJ, Turek E, et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci Transl Med. 2019;11(507):eaav3879.
Cazzola M, Ora J, Calzetta L, Rogliani P, Matera MG. Advances in inhaled corticosteroids for the treatment of chronic obstructive pulmonary disease: what is their value today? Expert Opin Pharmacother. 2022;23(8):917–27.
Martinez-Garcia MA, Faner R, Oscullo G, de la Rosa D, Soler JJ, Ballester M, et al. Inhaled steroids, circulating eosinophils, chronic airway infection, and pneumonia risk in chronic obstructive pulmonary disease. A Network Analysis. Am J Respir Crit Care Med. 2020;201:1079–85.
Aliberti S, Sotgiu G, Blasi F, Saderi L, Posadas T, Martinez-Garcia MA. Blood eosinophils predict inhaled fluticasone response in bronchiectasis. Eur Respir J. 2020;56(2):2000453.
Cosio BG, Shafiek H, Martinez-Garcia MA. Is it time to readjust the doses of inhaled corticosteroids in COPD? Arch Bronconeumol. 2022;58(8):593–4. https://doi.org/10.1016/j.arbres.2022.01.014.
Visser SK, Bye PTP, Fox GJ, Burr LD, Chang AB, et al. Australian adults with bronchiectasis: The first report from the Australian Bronchiectasis Registry. Resp Med. 2019;155:97–103.
Lee H, Dhar R, Oh YM. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology. 2021;26:618–20.
Polverino E, Chalmers JD, Aliberti S, Haworth C, Blasi F, Boersma W, et al. Inhaled corticosteroids use in patients with bronchiectasis: Data from the EMBARC registry. Eur Resp J. 2021;58:OA1312.
O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–42.
Martinez-Garcia MA, Oscullo G, Garcia-Ortega A, Matera MG, Rogliani P, Cazzola M. Rationale and clinical use of bronchodilators in adults with bronchiectasis. Drugs. 2022;82(1):1–13.
Monteagudo M, Nuñez A, Solntseva I, Dhalwani N, Booth A, Barrecheguren M, et al. Treatment pathways before and after triple therapy in COPD: a population-based study in primary care in Spain. Arch Bronconeumol. 2021;57:205–13.
López-Campos JL, Carrasco-Hernández L, Román Rodríguez L, Quintana-Gallego E, Carmona Bernal C, Alcázar NB. The clinical implications of triple therapy in fixed-dose combination in COPD: from the trial to the patient. Arch Bronconeumol. 2020;56(4):242–8.
Ruano-Raviña A, Fernández-Villar A, López-Campos JL. Coping with low mortality and exacerbation rate differences between COPD triple therapy studies, and a proposal for upcoming studies. Arch Bronconeumol. 2020;56(5):336–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement.
Conflict of interest
Miguel Ángel Martínez-García, Grace Oscullo, Alberto García-Ortega, Maria Gabriella Matera, Paola Rogliani, and Mario Cazzola have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Author contributions
MÁM-G and MC: Conceptualization, supervision, writing—original draft, and writing—review and editing. GO: Systematic clinical review of the literature, writing—review and editing. AG-O: Systematic clinical review of the literature, writing—review and editing. MGM: Validation, writing—review and editing. PR: Validation, writing—review and editing.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Martínez-García, M.Á., Oscullo, G., García-Ortega, A. et al. Inhaled Corticosteroids in Adults with Non-cystic Fibrosis Bronchiectasis: From Bench to Bedside. A Narrative Review. Drugs 82, 1453–1468 (2022). https://doi.org/10.1007/s40265-022-01785-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01785-1