Abstract
Immunosuppressive therapy is mandatory for primary membranous nephropathy with persistent nephrotic proteinuria or anti-phospholipase A2 receptor antibodies, reduced kidney function, or another risk factor for progression. Rituximab has demonstrated efficacy for proteinuria remission compared with renin-angiotensin system blockade or cyclosporine in two well-powered randomized controlled trials. More recently, STARMEN showed that alternating glucocorticoid-cyclophosphamide is superior to sequential tacrolimus-rituximab for proteinuria remission, although it was associated with a higher risk of non-serious adverse events. However, sequential tacrolimus-rituximab involved delayed lower dose rituximab and was the worst-performing rituximab regimen among those tested in randomized clinical trials. The RI-CYCLO pilot study did not demonstrate superiority of glucocorticoid-cyclophosphamide over rituximab and found no difference in adverse events. Overall, STARMEN and RI-CYCLO confirmed the efficacy of glucocorticoid-cyclophosphamide in patients with high-risk membranous nephropathy and the role of rituximab as a valid alternative. However, none of the trials tested an optimized rituximab protocol involving a second rituximab cycle before declaring treatment failure. Calcineurin inhibitors should be considered third-line drugs and sequential use of calcineurin inhibitor rituximab did not add over rituximab-only regimens. We critically review recent randomized controlled trials, propose a research agenda, and call for multinational pragmatic trials that enroll patients at referral centers to address unmet research needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent and relevant multicenter clinical trials have confirmed the efficacy and safety of rituximab in the treatment of membranous nephropathy, and it constitutes an excellent therapeutic option. |
The combined use of cyclophosphamide and corticosteroids has a high efficacy, but with a higher risk of non-serious adverse events. |
Calcineurin inhibitors constitute the third line of treatment because of the risk of relapse and nephrotoxicity. |
This critical and in-depth review highlights unmet needs and unresolved problems, proposing future lines of research. |
1 Current Therapy for Membranous Nephropathy (MN)
Primary membranous nephropathy (MN) is among the main cause of nephrotic syndrome in adults. It is an autoimmune disorder caused in 70–80% of cases by anti-phospholipase A2 receptor (PLA2R) antibodies [1]. Immunosuppressive therapy is mandatory in patients with persistent nephrotic proteinuria, a reduction in renal function, and high titers of anti-PLA2R auto-antibodies [2, 3]. Clinical trials have confirmed the efficacy of rituximab (RTX) on proteinuria remission compared with renin angiotensin system (RAS) blockade or cyclosporine, but until recently, none had compared RTX with alternating cyclophosphamide and corticoids (GC-CYC) [4, 5]. Two recent international, multicenter, randomized controlled trials (RCTs) have formally compared the efficacy of GC-CYC with therapeutic regimens that include RTX. We critically analyze recent clinical trial evidence on the efficacy of RTX regimens, and the potential impact on current clinical practice for MN. The design, baseline clinical characteristics, and outcome definitions of the GEMRITUX, MENTOR, STARMEN, and RI-CYCLO trials are shown in Tables 1 and 2 and Table 1 of the Electronic Supplementary Material (ESM), respectively. Based on this critical review, we highlight unmet needs and unsolved issues and propose future research.
1.1 Standard of Care in the Treatment of MN: Alkylating Agents and Calcineurin Inhibitors (CNIs)
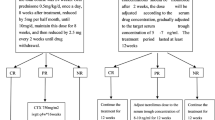
Persons with high or very high-risk MN require immunosuppression [2]. According to the new 2021 KDIGO guidelines, high-risk patients are defined as having an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 and/or proteinuria > 8 g/day for more than 6 months, or normal eGFR with proteinuria > 3.5 g/day and a decrease of < 50% after 6 months of conservative therapy and at least one of the following: serum albumin < 2.5 g/dL, anti-PLA2R > 50 RU/mL, or elevated urinary immunoglobulin G. For high-risk patients, the 2021 KDIGO guidelines recommend starting immunosuppressive treatment with cyclophosphamide associated with steroids, RTX, or calcineurin inhibitors (CNIs) with RTX [2, 3] (Fig. 1).
Therapeutic regimens according to KDIGO 2021 and regimens tested in recent randomized controlled trials. The 2012 KDIGO clinical practice guidelines [2] do not specify the precise or maximum duration of calcineurin inhibitors (CNI, in MENTOR, cyclosporine; in STARMEN, tacrolimus) treatment but recommend at least 6 months. After that, a progressive decrease in dose is recommended (every 4–8 weeks), if remission is maintained and nephrotoxicity does not appear, maintaining CNI for at least 12 months. This is represented by the dotted arrow. The 2021 KDIGO clinical practice guidelines succinctly emphasize immunological monitoring after starting the therapy and for the maintenance of treatment. In MENTOR [5], the second cycle of rituximab (RTX) was only administered in patients whose baseline proteinuria was reduced by at least 25% at 6 months. Other patients were considered treatment failures. The dotted arrow in the CNI arm of MENTOR indicates that cyclosporine was continued between month 6 and month 12 in patients whose baseline proteinuria was reduced by at least 25% at month 6. Other patients in this arm were considered treatment failures. The dotted arrow in the CNI arm of STARMEN [32] corresponds to a period of 3 months (months 6–9) of tacrolimus tapering before withdrawn. Rituximab (1 g × 2) corresponds to two doses of rituximab, administered 2 weeks apart. Rituximab (375 mg/m2 × 2) corresponds to two doses of rituximab, administered 1 week apart. Rituximab (1 g) corresponds to one single dose of rituximab. GC-CYC glucocorticoid-cyclophosphamide, GFR glomerular filtration rate, RASi renin-angiotensin system inhibitors
Very-high risk patients have life-threatening nephrotic syndrome or rapid deterioration of kidney function not otherwise explained. In very-high risk patients, the only recommendation is treatment with cyclophosphamide plus corticosteroids. The basis for recommendations on alkylating agents comes from pioneering studies conducted three decades ago, in which chlorambucil or cyclophosphamide improved nephrotic syndrome and the progression of kidney disease over non-immunosuppressive supportive treatment [6, 7]. However, these trials did not have adequate control groups under current treatment standards, as they only recommended a low-sodium diet, RAS blockers were not used systematically, and hypertension was defined as blood pressure values > 160/90 mmHg. In addition, patients enrolled in these trials were not considered to have very high-risk MN. The apparent long-term benefits of the alkylating regimen [8, 9] have been questioned because of the small number of patients followed long term and with a suboptimal control group [10]. Furthermore, the potential risk of serious infections (higher for chlorambucil), late malignancy, or severe adverse events (SAEs) associated with corticosteroids has led to the search for safer alternatives.
Calcineurin inhibitors predominantly inhibit T-cell proliferation and improve the podocyte cytoarchitecture [11]. They induce remission of nephrotic syndrome in about 70–75% of patients with MN when compared with non-immunosuppressive therapy [12, 13]. In some studies, tacrolimus (TAC) with or without corticosteroids had similar efficacy to cyclophosphamide on overall (complete and partial) proteinuria remission, particularly in Asian patients. The principal limitation is the high rate of relapse after withdrawal (up to 50%) and the fact that they are nephrotoxic [14,15,16,17]. Regarding this nephrotoxicity, a study in 250 Chinese patients with MN showed that serum concentrations of TAC > 5 ng/mL determined higher rates of partial remissions, but with more side effects, without differences in the rates of complete remissions compared to serum TAC concentrations < 5 ng/mL [18]. Furthermore, a recent meta-analysis that included 442 Chinese patients with MN from nine studies (one RCT and eight cohort studies) showed that TAC monotherapy was superior to GC-CYC therapy for complete remission at 6 months (odds ratio 2.2, 95% confidence interval 1.4–3.5), but comparable after 1 year (odds ratio 1.6, 95% confidence interval 0.8–3.2), with fewer drug-related adverse effects. The relapse rate was higher with TAC vs GC-CYC, but without significant differences [19]. In observational studies, the relapse rate decreased when RTX was added at the time of CNI tapering, even in high-risk patients [20]. The combination of RTX (1 g every 15 days initially and a second cycle at least 6 months after the first cycle in patients with a CD19 + B-lymphocyte count > 5 cells/µL regardless of the clinical remission status) with cyclosporine (up to 3 mg/kg/day) for 6 months with slow tapering of the cyclosporine A dose resulted in a high rate of complete and partial remission at 24 months of follow-up (54% and 85%, respectively) [21]. These encouraging data should be confirmed in a formal and well-weighted RCT that should also address whether the high 24-month remission rate was driven by the simultaneous (rather than sequential as in STARMEN) combination of CNI-RTX or by the repeat dosing of RTX based on the impact on B cells and independent from remission status.
2 RTX as a New and Key Player: GEMRITUX and MENTOR Trials
Given the key role of anti-PLA2R antibodies in the pathogenesis of MN and their association with kidney outcomes, it is only logical to test therapies directed at decreasing the production of autoantibodies [1, 22, 23]. Rituximab is a chimeric monoclonal antibody targeted to CD20 + B cells. It induces B-cell apoptosis and decreases the number of B cells and autoantibody production in autoimmune diseases such as rheumatoid arthritis and antineutrophil cytoplasmic antibody-associated vasculitis. Beyond its immunosuppressive action, it also has positive effects on podocyte cytoarchitecture. In experimental models of adriamycin-induced nephrotic syndrome [24] and in patients with post-transplant recurrence focal segmental glomerulosclerosis [25], RTX has been shown to reduce proteinuria by preserving normal regulation of sphingomyelin phosphodiesterase and avoiding a decrease in glomerular expression of nephrin and podocin. These findings would contribute to partially preserving the actin cytoskeleton and reducing apoptosis of podocytes. Preliminary evidence on its efficacy in primary MN came from observational and non-controlled studies and, more recently, from RCTs.
2.1 Observational Studies
In a landmark Italian prospective and observational study in eight idiopathic patients with MN with persistently (≥ 6 months) high urinary protein excretion (≥ 3.5 g/24 h), 4-weekly infusions of RTX (375 mg/m2) induced a significant reduction in proteinuria (8.6 ± 4.2 to 3.0 ± 2.5 g/24 h (− 66%, p < 0.005) at 1 year, including two patients with final proteinuria < 0.5 g/24 h. Kidney function remained stable, and no patient had major drug-related events [26].
In a Mayo Clinic study, 20 patients with MN with proteinuria > 5 g/24 h received four doses of RTX 375 mg/m2, with re-treatment at 6 months regardless of proteinuria response [27]. After 24 months of follow-up, 16 patients (80%) achieved complete or partial remission and one patient had a relapse. Proteinuria decreased from 11.9 to 2.0 g/24 h, and creatinine clearance increased from 72.4 to 88.4 mL/min/1.73 m2 at 24 months. Interestingly, four doses of RTX were more effective on B-cell depletion, but proteinuria reduction was similar to that obtained by two doses of 1 g of RTX separated by 2 weeks [27, 28].
In a subsequent multicenter Italian study, 100 patients with primary MN and persistent nephrotic syndrome were treated with RTX, four 375-mg/m2 weekly doses up to October 2005, and a B-cell targeted approach thereafter consisting of a first dose of RTX and an additional second dose only if the B-cell count was > 5 cells/μL post-first dose [29]. Thirty-two (32%) participants had previously received different immunosuppressors, including alkylating agents, steroids, and CNIs. After a median follow-up of 29 months, 65 patients (65%) achieved complete or partial remission with a median time to remission of 7.1 months. The rates of remission were similar for patients with or without previous immunosuppressive treatment. Four (4%) patients died and four patients (4%) progressed to end-stage kidney disease (ESKD). A lower baseline proteinuria and serum creatinine level, and female sex were independent predictors of better response to therapy. Rituximab was well tolerated and there was no SAE related to treatment.
In summary, in observational studies, RTX induced disease remission and stabilized or improved kidney function in high-risk patients with MN. The advantage of RTX over supportive therapy was confirmed in two meta-analyses of observational studies, with rates of partial or complete remissions > 60% at 24 months, low relapse rate, and mild adverse events (AEs), mostly infusion-related reactions [30, 31]. Despite these positive results, the absence of an adequate control group and the observational nature of these studies prevented drawing solid conclusions.
2.2 GEMRITUX
GEMRITUX was the first formal RCT that compared in 75 patients with primary MN and proteinuria > 3.5 g/24 h the efficacy of RTX (two infusions of 375 mg/m2, separated by 1 week) on top of non-immunosuppressive antiproteinuric treatment (NIAT) with RAS blockade vs NIAT alone with a primary endpoint of complete or partial proteinuria remission at 6 months [4] (Fig. 1) (Tables 1 and 2 and Table 1 of the ESM). No significant differences were observed in the primary endpoint (35% for RTX vs 21% for NIAT), but RTX decreased anti-PLA2R titers and increased serum albumin earlier (month 3) (Figs. 1a and 2a of the ESM). Importantly, after a median post-RCT follow-up period of 17 months, more complete/partial remissions (65% vs 35%), and more complete remission (19% vs 3%) were achieved with RTX compared with NIAT (Fig. 2a) (Table 3). The time to proteinuria remission was 7 months (median). There was a numerical but not statistically significant decrease in eGFR in the RTX arm (change of GFR from randomization to last follow-up: − 5.6 mL/min/1.73 m2 for RTX, and + 0.4 mL/min/1.73 m2 for NIAT) (Table 2). A baseline serum anti-PLA2R of < 275 RU/mL independent of the treatment group was associated with complete or partial remission for the whole cohort. Serious adverse events were uncommon and similar in both groups (Table 4). In this trial, the administration of a single RTX cycle may have underestimated the full potential of RTX therapy.
Frequency of complete or partial remission and of complete remission in four trials in primary membranous nephropathy. a–d Complete or partial remission. e–h Complete remission. Timepoints correspond to endpoint evaluations. Months are counted since randomization. Note that in STARMEN [32] rituximab (RTX) was administered at month 6 since randomization. In the GEMRITUX [4] trial, the median time of extended follow-up was 17 months. Numbers above each bar correspond to the percentage of remissions in each treatment group as determined by the intention-to-treat analysis. CsA cyclosporine A, GC-CYC alternating therapy with glucocorticoid-cyclophosphamide, NA data not available, NIAT non-immunosuppressive antiproteinuric therapy, TAC-RTX sequential therapy with tacrolimus-rituximab
2.3 MENTOR
More recently, MENTOR, a multicenter and non-inferiority RCT from the USA and Canada, compared the efficacy of RTX (1 g × 2 doses separated by 2 weeks) vs cyclosporine (3.5–5.0 mg/kg/day, target blood levels 125–175 ng/mL) in 130 nephrotic patients with MN (5) (Fig. 1) (Tables 1 and 2 and Table 1 of the ESM). Patients with a proteinuria reduction ≥ 25% at 6 months without complete remission received either a second course of two 1-g doses of RTX to complete four doses, or continued cyclosporine for another 6 months. Those with a proteinuria reduction < 25% were considered as treatment failures. The primary endpoint was an intention-to-treat analysis of complete or partial remission at 24 months. At 6 months, patients taking cyclosporine had a numerically higher percentage of complete or partial remissions than RTX (35% RTX vs 49% cyclosporine) (Fig. 2b). However, the immunological remission was almost double for RTX than for cyclosporine (52 vs 28%) (Figs. 1b and 2b of the ESM) (Table 3). At 12 months, efficacy was similar (60% RTX vs 52% cyclosporine) and RTX was not inferior to cyclosporine. However, RTX was superior in achieving complete/partial remission at 18 months (62% vs 23%) and at the primary endpoint of 24 months (60% vs 20%), that is 6–12 months after stopping cyclosporine (Fig. 2b). The decreasing percentage of clinical remissions over time in the cyclosporine arm was associated with a progressive decrease in the percentage of patients with an immunological response (Fig. 1b of the ESM). In this regard, RTX also induced a faster, larger, and more persistent decline in anti-PLA2R titers (Fig. 2b of the ESM). Rituximab was also superior in achieving complete remission at 24 months (35% vs none) (Fig. 2f). At 6 months, the risk of treatment failure was slightly higher in the RTX arm compared with the cyclosporine arm (17% vs 12%), at 12 months was similar (26% vs 32%), but at 24 months, this risk was clearly lower in the RTX arm (40% vs 80%). This last finding was to be expected, given the known high probability of relapse when patients stop taking CNI.
Kidney function was better preserved on RTX, possibly because of the known nephrotoxicity of cyclosporine. Thus, at 24 months, the change of creatinine clearance from baseline was + 10.08 mL/min/1.73 m2/year higher in the RTX arm than in the cyclosporine arm (Table 2 of the ESM). The only case of ESKD occurred in the cyclosporine arm.
The incidence of any AE was similar in both groups (RTX 71% vs cyclosporine 78%) with a non-significant trend towards a higher incidence of SAEs with cyclosporine (17% vs 31%, p = 0.06) (Table 4). The greater efficacy of RTX could be explained, in part, by a more persistent impact on the immunological response. A longer treatment with cyclosporine (18–24 months) and a slower tapering regimen may have improved the proteinuria result at the potential expense of longer exposure to a nephrotoxic drug.
In this trial, classifying cases that did not achieve a proteinuria reduction > 25% at 6 months as treatment failures likely reduced the overall efficacy of therapy because clinical remissions generally follow the immune response and may take longer than 12–18 months. Thus, in the RTX arm, treatment failures may have been overestimated.
3 STARMEN and RI-CYCLO Trials
STARMEN and RI-CYCLO are the most recent RCTs exploring RTX-containing regimens for primary MN. In both, the comparator was conventional immune suppression with GC-CYC.
3.1 STARMEN
STARMEN compared the frequency of complete or partial remission at 24 months (primary outcome) achieved by sequential TAC (0.05 mg/kg/day for 6–9 months) and RTX (single 1-g dose at month 6) [TAC-RTX] vs alternating GC-CYC in 86 patients with nephrotic MN [32] (Fig. 1) (Tables 1 and 2, Table 1 of the ESM). The rationale for sequential therapy was to first reduce proteinuria using the CNI and then administer RTX, as its half-life would be expected to be longer in non-nephrotic patients [33], to decrease the risk of relapse post-TAC withdrawal [20]. Thus, the RTX regimen differed from other RTX regimens tested in RCTs in the lower dose and delayed (6 months after randomization) administration of RTX.
GC-CYC induced more complete/partial remissions (84% vs 58%) and more complete remissions (60% vs 26%) at 24 months than TAC-RTX (Fig. 2c) (Table 3). At 26%, the complete remission rate for TAC-RTX was the lowest of the RTX arms of the RCTs reporting 24-month data, as in MENTOR it was 35% and in RI-CYCLO it was 42% (Fig. 2e–h). Subgroup analyses for different baseline characteristics consistently showed a tendency toward more efficacy for the GC-CYC group, being statistically significant for the subgroups of male patients, serum albumin > 2.6 g/dL, and anti-PLA2R serum levels > 80 RU/mL.
The relapse rate with TAC-RTX was lower than previously reported for TAC alone, likely because of the administration of RTX at the time TAC was tapered. There were no statistically significant differences in relapses post-remission, although these were less frequent in the GC-CYC arm (GC-CYC 2.7% vs TAC-RTX 12%).
The immunological response occurred also significantly earlier with GC-CYC (77% vs 45% at 3 months, and 92% vs 70%, respectively, at 6 months) (Figs. 1c and 2c of the ESM). However, up to 6 months only TAC had been administered in the TAC-RTX arm, thus these immunological responses should be ascribed to TAC, which is remarkable because they were 2.5-fold more frequent than those achieved by cyclosporine in MENTOR and were higher than in any other arm of the four trials at 6 months except for GC-CYC in STARMEN. In this regard, there were no differences in immunological remissions at 12 and 24 months, i.e., 6 and 18 months after RTX administration (88% vs 79%, 88% vs 83%, respectively). The early immunological response was associated with proteinuria remission at 24 months. Patients without proteinuria remission had a slower reduction in anti-PLA2R levels and less frequent immunological responses. These findings support that an early immunological response predicts long-term clinical response.
The number of AEs (239 vs 170) was significantly higher with GC-CYC, but they occurred in a similar percentage of patients (98% vs 91%) (Table 4). There were no statistically significant differences in the number of SAEs (17% vs 12%) or in the number of patients with SAEs (19% vs 14%). As expected, there were more cases of Cushing’s syndrome and leukopenia in the GC-CYC group and more cases of hyperkalemia, increased serum creatinine, distal tremor, and diarrhea in the TAC-RTX group. These TAC-RTX AEs were thought to be mainly related to TAC. Thus, the addition of TAC to the regimen was in part responsible for its AE profile. One (2%) patient in the GC-CYC arm reached ESKD vs none in the TAC-RTX group.
The design of STARMEN was criticized as RTX lagged 6 months with respect to the initiation of cyclophosphamide, thus the impact of RTX would be expected to be delayed 6 months, i.e., from the point of view of RTX efficacy, the timepoint of 24 months for GC-CYC would be comparable to the timepoint of 30 months of the TAC-RTX protocol. Furthermore, only one RTX cycle was administered, and the RTX dose was the lowest of all four RCTs. Overall, addition of TAC did not appear to improve outcomes but was responsible for AEs.
3.2 RI-CYCLO
The RI-CYCLO pilot trial compared the rates of complete remissions at 12 months (primary outcome), and complete or partial remissions (secondary outcomes) for RTX (1 g on days 1 and 15) vs alternating GC-CYC in 75 patients with nephrotic MN [34] (Fig. 1) (Tables 1 and 2, Table 1 of the ESM). There were no statistically significant differences in complete remissions at 12 (GC-CYC 32% vs RTX 16%) or 24 months (n = 57, 35% vs 42%) (Table 3). The same pattern was observed for partial or complete remissions: no significant differences at any timepoint of 6, 12, 18, and 24 months (65% vs 51%, 73% vs 62%, 79% vs 66%, and 81% vs 85%, respectively, Fig. 2d).
In a subgroup analysis, GC-CYC was more effective in achieving complete or partial remissions at 12 months in male individuals (odds ratio 0.15, 95% confidence interval 0.02–1.00) and persons aged older than 55 years, or with more severe baseline characteristics (albuminemia ≤ 2.5 g/dL and proteinuria ≥ 6 g/24 h).
The relapse rate at 24 months with GC-CYC was higher than with RTX (22% vs 13%), and similar to previous GC-CYC studies [7, 9]. Two (5%) patients in the GC-CYC group reached ESKD vs none in the RTX group. Overall, in the two RCTs comparing RTX regimens with GC-CYC, ESKD was only observed in the GC-CYC group.
There were no significant differences in the percentages of immunological responses (GC-CYC vs RTX 50% vs 63% at 6 months, 56% vs 62% at 12 months, and 75% vs 90% at 24 months) (Figs. 1a and 2d of the ESM). The percentage of patients with AEs and SAEs was similar in both arms of the study. There were more patients with drug infusion reactions in the RTX group (24% vs 3%) (Table 4). In this trial, the administration of a single RTX cycle may have underestimated the full potential of RTX therapy.
4 Comparative Analysis of Recent MN Trials
The four well-designed RCTs had adequate clinical follow-up, appropriate control groups, and compared modern and presumably safer treatments with classical and potentially more toxic drugs under current clinical care conditions. In general, the methodological quality and the reporting of the trials were acceptable, and they followed the recommendations of the CONSORT guidelines (Table 1 of the ESM). All trials were open-label because of the complexity of the treatment regimens. Because MN is a rare disease, the trials were multicenter. In all trials, except for RI-CYCLO (complete remissions), the frequency of partial or complete remissions was the primary endpoint, and all except GEMRITUX (up to 18 months) showed results at 24 months, although this was not always the primary endpoint. In this regard, it should be emphasized that in prior studies, with or without immunosuppressive therapy, the proteinuria nadir did not occur until 24–36 months. Only STARMEN included eGFR stability (≥ 45 mL/min/1.73 m2) to the primary outcome criterion (Table 1 of the ESM). The complete remission proteinuria threshold was less strict in GEMRITUX (0.5 g/24 h) than in the other trials (0.3 g/24 h) at similar timepoints (Table 1 of the ESM). The positive cut-off point for anti-PLA2R antibodies was different in the RI-CYCLO and MENTOR studies.
The clinical characteristics of participants according to the treatment arm were similar in all trials (Table 2). There was a non-significant numerically higher proportion of men in the RTX arm in all trials except for MENTOR. Kidney function was determined by different methods (Table 2). As reported in the original manuscripts, it was slightly lower in GEMRITUX participants. However, estimation of GFR by the CKD-EPI equation from baseline serum creatinine values, mean age, and sex distribution in MENTOR (original data presented as creatinine clearance) suggests that eGFR in MENTOR (~58 mL/min/1.73 m2 for the RTX arm and 60 mL/min/1.73 m2 for the cyclosporine arm) was around 20–25 mL/min/1.73 m2 lower than in STARMEN and RI-CYCLO. Baseline albuminemia and proteinuria were similar in the four trials, although a severity gradient could be observed by combining baseline eGFR and proteinuria that ranked participants in MENTOR as those with the more severe disease and participants in RI-CYCLO as having the less severe disease (Fig. 3 of the ESM). Anti-PLA2R antibodies were positive in 59–78% of participants in whom they were assessed in the four trials (Table 2). Serum anti-PLA2R antibody titers were higher in MENTOR, possibly because of a different technique and cut-off points used (Table 1 of the ESM). In the other studies, median values for each treatment group were around 50 RU/mL, except for the TAC-RTX group in STARMEN (median 113 RU/mL), although this did not differ significantly from the control group (Table 2). All studies were powered at 80% except RI-CYCLO, which was a pilot, mainly descriptive trial and focused on confidence intervals.
4.1 Head to Head: MENTOR vs GEMRITUX
Impact measures for all studies, including relative risk, absolute risk reduction, and number-needed-to-treat (benefit) were estimated (Table 3). In MENTOR and GEMRITUX, RTX was clearly superior at month 18, when patients who received RTX were two-fold more likely to achieve complete or partial remission than with supportive treatment (GEMRITUX) and almost three-fold more likely than with cyclosporine (MENTOR) (Table 3, Fig. 2). The absolute risk reduction was > 30% in both studies and the number needed to treat was 3 to achieve partial or complete remission at 18 months. At month 18, differences were more evident for complete remission, which was residual (≤ 3%) in non-RTX arms. In MENTOR, the benefit of RTX was confirmed at 24 months, when the absolute risk reduction was more than 35% and the number needed to treat was 3 for complete remission. Cyclosporine was equally effective in inducing remission at 12 months as RTX. However, it was not effective in maintaining remission once discontinued, a fact previously observed with TAC [13], another CNI. Prolonged use of cyclosporine could have prevented relapses, but that would occur at the expenses of nephrotoxicity. As demonstrated in MENTOR, 12 months of cyclosporine use was sufficient to induce a significant loss of GFR that did not recover even to baseline levels by month 24 (i.e., 10 months after complete discontinuation of cyclosporine). As long-term preservation of kidney function should be the goal of treatment, prolonged use of a CNI, even at low dose is not advisable. Nevertheless, in MENTOR, 25% of the patients were anti-PLA2R negative and had milder proteinuria, and in this group, there was no significant benefit from the use of RTX over cyclosporine.
In summary, RTX was more effective than supportive treatment (GEMRITUX) or than short-term cyclosporine (MENTOR) at 18–24 months of follow-up (Fig. 3, pooled analysis), with adequate tolerance and few AEs, but still 40% of patients did not respond to RTX. The apparent high non-response rate in MENTOR may have been favored by the protocol considering treatment failure those patients who failed to reduce proteinuria by at least 25% at 6 months after starting treatment. This decision was based on the consensus that in patients treated with a CNI, a lack of response at 6 months equals no response at later timepoints and was thus considered unethical to keep patients in the study without switching to alternative therapy, a criterion that necessarily had to be applied also to the RTX arm. In previous non-controlled studies, 80% of patients treated with RTX monotherapy achieved partial or complete response at 24 months, regardless of proteinuria at 6 months [27].
Pooled analysis of MENTOR and GEMRITUX trials comparing treatments based on rituximab vs cyclosporine or non-immunosuppressive therapy at last evaluation. a Complete and partial remission. b Complete remission only (right) at the last trial assessment (MENTOR [5] at 24 months and GEMRITUX [4] at 17 months)
In both studies, the immunological response was clearly higher at 6 months in the RTX group (Table 4). Non-immunosuppressive antiproteinuric treatment barely impacted on immunological responses while the response to cyclosporine was transient. In MENTOR, the difference increased at month 12, as cyclosporine-treated patients lost immunological responses after stopping cyclosporine (Table 4) (Figs. 1b and 2b of the ESM).
4.2 Head to Head: STARMEN vs RI-CYCLO Trials
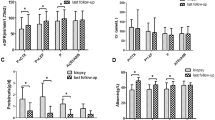
STARMEN and RI-CYCLO had GC-CYC as a reference (control) and participants had similar baseline clinical characteristics. Changes in kidney and immunological parameters from randomization in these studies are shown in Fig. 4 and Fig. 1 of the ESM.
Changes in renal and immunological parameters from randomization to month 24: STARMEN and RI-CYCLO trials. Note that in STARMEN [32] rituximab was administered at month 6 from randomization. RI-CYCLO [34] did not publish exact numerical data for estimated glomerular filtration rate values, only graphically in the ESM. eGFR estimated glomerular filtration rate, GC-CYC alternating therapy with glucocorticoid-cyclophosphamide, PLA2R phospholipase A2 receptor, RTX rituximab
STARMEN showed a clear benefit of GC-CYC over TAC-RTX, which was observed from month 6 to month 24, both for complete and partial remissions, as well as for complete remissions alone (Table 3, Fig. 2, Fig. 2c of the ESM). At month 24, the relative risk of remission was 1.4 favoring GC-CYC, the absolute risk reduction was 26, and the number needed to treat was 4. While complete remissions increased from 12 to 24 months with GC-CYC, the number of complete and partial remissions combined did not increase significantly. On the contrary, RI-CYCLO, a pilot study, did not show significant differences between GC-CYC and RTX at 24 months and in the percentage of complete or partial remissions at months 6 and 18, although these were numerically higher for GC-CYC (Table 3, Fig. 2). End-stage kidney disease was infrequent but only occurred in the GC-CYC arm of both trials, for an overall incidence of 3/80 (3. 8%) (Table 2 of the ESM).
In both studies, a subgroup analysis suggested that in men and in persons with higher baseline proteinuria, GC-CYC achieved more remissions (partial or complete) [STARMEN at 24 months, and RICYCLO at 12 months]. However, these results must be interpreted with caution and should be validated in new trials, stratifying patients during randomization according to these characteristics. The pooled analysis of both trials shows the benefit of GC-CYC over RTX-based regimens at 1 year of follow-up on proteinuria remission, but this difference was not significant at 2 years of follow-up (Fig. 5). Although as already commented, follow-up times shorter than 24 months may be inappropriate, the TAC-RTX arm of STARMEN improved the percentage of partial or complete remissions from 44% at month 6 (representing solely the impact of TAC, as RTX had not yet been administered) to 58% at month 24 (+ 32% increase), that is, 18 months after RTX administration. This is the smallest change of all RTX regimens, suggesting that the TAC-RTX regimen did not perform as others, or that follow-up should have been prolonged to 24 months after RTX (Fig. 4 of the ESM). Complete remissions increased after RTX administration from 0% at month 6 to 20% at month 24 from randomization in STARMEN and from 8% to 42% in RI-CYCLO, supporting again the underperformance of the TAC-RTX regimen (Fig. 2g). TAC-RTX underperformance is also supported by the increase in proteinuria observed at month 12 in the TAC-RTX arm, the only regimen that resulted in median proteinuria values above 3.5 g/day at this stage (Fig. 2 of the ESM). RI-CYCLO did not report on RAS blocker use (Table 2), which may limit conclusions regarding the percentage decrease in proteinuria in each treatment group.
Pooled analysis of STARMEN and RI-CYCLO trials comparing treatments based on rituximab (RTX) vs glucocorticoid-cyclophosphamide (GC-CYC) at 12 and 24 months from randomization. The RTX arm at STARMEN used a sequential regimen of tacrolimus (TAC) for 6 months, followed by a single lower dose of RTX at month 6. Thus, the regimens are not fully comparable and the longest follow-up from the RTX dose in STARMEN was 18 months (i.e., 24 months from randomization). a Complete or partial remission at month 12 since randomization. b Complete remission at month 24 since randomization. c Complete or partial remission at month 6 since randomization. d Complete remission at month 24 since randomization
The immunological response was earlier and more marked in the GC-CYC arm of STARMEN, but in RI-CYCLO, the immune responses were numerically higher in the RTX arm, without reaching statistical significance (Table 4). Possible explanations are the higher dose of RTX used in RI-CYCLO compared with STARMEN (2 g vs 1 g) and the delayed administration of RTX (month 6 of randomization) in STARMEN. Strikingly, the percentage of immunological responses after 6 months of TAC (70%) in STARMEN was higher than the response obtained by any other regimen, with the sole exception of GC-CYC in the same STARMEN trial (Fig. 1 of the ESM).
In STARMEN, there was a higher number of AEs in the GC-CYC arm, but there were no significant differences in SAEs (Table 4). In RI-CYCLO, the occurrence of AEs was similar in both arms. The only death occurred in a patient with lung cancer in the RTX arm of RI-CYCLO (Table 4). The lower cumulative doses of cyclophosphamide used in STARMEN (10 g) and RI-CYCLO (180 mg/kg, approximately 13.6 g) compared with previous studies may have contributed to the low frequency of SAEs, including fatal events. However, 24 months may be too short to adequately assess events such as malignancy or even death. In a large historical cohort from Italy and The Netherlands (100 patients treated with RTX and 103 patients treated with cyclophosphamide), the incidence of AEs (serious, non-serious) at 5 years was three-fold to four-fold lower in participants who received RTX, even after adjusting for confounders, including prior use of immunosuppressants [35].
4.3 Short-Term and Long-Term Efficacy and Safety
Timing of response is important, as it shortens exposure to the risk of infectious, nutrition/metabolic, and prothrombotic complications of nephrotic syndrome.
4.3.1 Short-Term Efficacy
Only GEMRITUX and STARMEN provided information on efficacy at 3 months (Figs. 1a, c and 2a, c of the ESM). This is unfortunate as GEMRITUX did not have an active immunosuppressive comparator and STARMEN had not incorporated RTX at this timepoint. However, these data show that most patients treated with RTX, TAC, or GC-CYC reached an immunological response within 3 months. This information may be incorporated into future decision-making tools. Regarding proteinuria, the delayed low-dose RTX in TAC-RTX appeared to provide the worst result, as stopping TAC was followed by an increase in proteinuria before the therapeutic impact of RTX was noted, and the overall progression over time in terms of complete or partial plus complete remission was suboptimal as compared with other RTX arms (Fig. 4 of the ESM). Thus, only RI-CYCLO allows the comparison of RTX with GC-CYC. Both regimens similarly induced immunological responses, reduced proteinuria (Figs. 1a and 2d of the ESM), and induced complete or complete plus partial remissions at 6 months (Figs. 6a and 7a). Thus, results from RCTs do not allow prioritization of GC-CYC over RTX based on the speed of response. However, it should be noted that the best proteinuria remission and immunological response performance at 6 months of all trials was observed for the GC-CYC arm in STARMEN.
Clinical response at different timepoints in four trials in primary membranous nephropathy. Clinical response was calculated as the sum of complete remissions (CR) and partial remissions (PR) at 6–24 months in the intention-to-treat (ITT) analysis. a Early (6 months) CR or PR. b and c Secondary outcomes: CR or PR at 12 and 18 months by the ITT analysis. d CR or PR at 24 months by the ITT analysis. This is the primary outcome for STARMEN [32] and MENTOR [5]. Numbers above each bar correspond to the percentage of remissions in each treatment group as determined by the ITT analysis. Note that in STARMEN, rituximab (RTX) was administered at month 6. Thus, at 6 months from randomization (shown in the figure), no impact of RTX can be observed, and this timepoint reflects the impact of tacrolimus alone. Note that in RI-CYCLO, the number of patients in months 18 and 24 was lower than at months 6 and 12. CsA cyclosporine A, GC-CYC alternating therapy with glucocorticoid-cyclophosphamide, NIAT non-immunosuppressive antiproteinuric therapy, TAC-RTX sequential therapy with tacrolimus-rituximab
Complete remission (CR) at 24 months by intention-to-treat (ITT) analysis and medications cost for the different therapeutic regimens. a CRs. Numbers above each bar correspond to the percentage of CRs at 24 months in each treatment arm as determined by the ITT analysis. b Estimated medication costs (in euros) for the different regimens. Costs were estimated for a standard 1.8 m2 of body surface area in a patient weighing 70 kg. The second cycle of treatment with rituximab (RTX) or with cyclosporine proposed in MENTOR for patients who reduce baseline proteinuria by at least 25% at 6 months was not considered. If the treatment cycle were repeated, costs would double for both arms. These estimates are based on current costs in Spain and may differ for different countries. In the sequential therapy with tacrolimus-rituximab (TAC-RTX) regimen of STARMEN [32], we also considered the costs of tacrolimus tapering between months 6 and 9. In GEMRITUX [4], the costs of antiproteinuric therapy (NIAT) were not considered. Infusion sets, day hospital facilities and personnel, travel costs, and costs for renin angiotensin system blockade and other concomitant medication not included. CsA cyclosporine A, GC-CYC alternating therapy with glucocorticoid-cyclophosphamide, NA data not available
4.3.2 Long-Term Efficacy
All four recent trials were consistent on the impact of different RTX regimens on outcomes at 18 months, although the TAC-RTX regimen resulted in numerically lower remissions (Fig. 6c). The efficacy of RTX on the primary endpoint of complete or partial remission at 24 months was similar for STARMEN and MENTOR (Fig. 6d). However, the low-dose delayed RTX administration may have penalized STARMEN results while the definition of treatment failure in MENTOR may have penalized the results obtained by the RTX arm. In this regard, in MENTOR, roughly 50% of eventual (partial plus complete) RTX remissions occurred at month 6, and all responders had remitted by month 12 (Fig. 6). By contrast, in RI-CYCLO, the percentage of RTX remissions increased progressively up to month 24. Data from GEMRITUX are insufficient to assess these patterns of remission. The definition of partial or complete remission differed in STARMEN and MENTOR as STARMEN incorporated a second remission criterion for both complete and partial remission, which was an eGFR ≥ 45 mL/min/1.73 m2 (Table 1 of the ESM). This could have penalized the RTX arm if eGFR had been affected by long-term TAC nephrotoxicity (Table 4). However, there were no significant differences in the number of patients with preserved renal function (eGFR ≥ 45 mL/min/1.73 m2) at 24 months between the GC-CYC and TAC-RTX arms (93% vs 86%, p = 0.48).
Regarding complete remissions, all RTX regimens resulted in a progressive increase in the percentage of patients with complete remission up to the last follow-up timepoint, which reached 35–42% at 24 months after RTX administration. However, the response to GC-CYC was heterogeneous: in STARMEN, GC-CYC resulted in a progressive addition of 15% new complete remissions per semester to reach 60%, the best complete remission results of any arm of any trial. However, this was not the case in RI-CYCLO, the trial with better preserved kidney function and lower proteinuria, in which complete remission peaked at 12 months and later stabilized at 35%. We encourage the authors of both trials to perform a combined analysis to try to understand this discrepancy, which would be key to define the relative role of GC-CYC vs RTX in the therapy of MN.
Preservation of kidney function is a key aim of therapy for MN. The information provided on this regard by the RCTs is not conclusive, likely because of low patient numbers and insufficient follow-up (Table 2 of the ESM). A cause of concern is that in RTX vs GC-CYC trials, ESKD was only observed in the GC-CYC arm. Large decreases in eGFR appear to have occurred in the cyclosporine arm of MENTOR and the CG-CYC arm of RI-CYCLO, while the results obtained by the RTX arms appeared to inconsistent between RCTs. For trials providing data at 24 months, eGFR improved in the RTX arm of MENTOR and RI-CYCLO, while STARMEN results are difficult to interpret as they appear to have been marred by TAC nephrotoxicity. Initiation of TAC was associated with a sharp drop in eGFR that only partially recovered after its suspension. Overall, results from RCTs could raise concerns over the negative impact of CNI nephrotoxicity on kidney function in MN. Clinical trial evidence on long-term hard outcomes of therapy for MN under current state-of-the art kidney protection strategies is limited [36]. So far, no RCT has formally evaluated the impact of RTX, TAC, or alkylating agents on the incidence of ESRD in the long term as the primary endpoint compared with other immunosuppressive regimens or with current non-specific kidney protective strategies. A post-hoc 10-year follow-up of an RCT performed in the 1970s or 1980s that compared symptomatic therapy vs 6 months of methylprednisolone and chlorambucil assessed the risk of ESRD but does not mention RAS blockade [8]. An RCT performed in the 1990s compared a 6-month course of GC-CYC with symptomatic therapy that explicitly avoided antiproteinuric therapy with RAS blockade for at least 1 year [9]. Three endpoints are listed (doubling of serum creatinine, development of ESRD, or patient death) but none is explicitly labeled primary, and despite the statement that data were analyzed on an intention-to-treat basis, 13% of patients randomized to immunosuppression were excluded from the analysis. The observational follow-up of a more recent trial in the RAS blockade era by the same group that had as the primary endpoint the 1-year remission did not observe differences in the 6-year incidence of 40% decline in eGFR, death, or end-stage kidney disease between patients randomized to cyclophosphamide plus glucocorticoids or TAC plus glucocorticoids [37]. Notably, the best predictor of long-term outcome is remission of proteinuria [38]. If a patient with MN remains in remission, the long-term outcome is excellent. Considering that RTX induces remission and maintains patients in remission long term (although may require repeated doses), there would be no reason to suppose that patients treated with RTX and in remission would progress to ESKD. Thus, proteinuria remission is a relevant short-term and medium-term outcome that also must be analyzed in any new long-term studies. Regarding long-term complete remission, results are consistent for both RTX alone and GC-CYC. By contrast, a CNI alone or in combination was the less effective approach (Fig. 7a). In any case, in STARMEN, CNI did not appear to add to the efficacy of RTX at 18 months of RTX administration, as compared to other trials or to an increase in remissions after RTX administration.
Results from STARMEN and RI-CYCLO call for a multinational randomized trial, preferably incorporating patients from different continents, that compares RTX with alternating GC-CYC in a population enriched for a higher risk of ESRD (e.g., lower baseline kidney function, < 60 mL/min/1.73 m2) with a longer follow-up (> 3 years) and a combined kidney function endpoint (e.g., doubling of serum creatinine or 40–50% decreased in eGFR, kidney failure, or renal death). Of note, a 10-year observational follow-up should be incorporated with emphasis on long-term safety and efficacy, and non-specific kidney protective agents should be clearly defined and incorporate all recent advances (e.g., they should consist at least of RAS blockade and sodium-glucose transport protein-2 inhibitors).
4.4 Immunological Response
The four trials discussed in this review showed a previous or parallel decrease of anti-PLA2Rs with respect to the proteinuria reduction (Fig. 2 of the ESM), which is consistent with previous results of observational studies. Results from STARMEN suggest that CNIs are at least as effective as GC-CYC and RTX in inducing early (3–6 months) immunological responses (Fig. 1c of the ESM). However, this was not confirmed in MENTOR, in which additionally, the immunological response was transient (Fig. 1b of the ESM). It is unclear whether the difference relates to the drug used or to patient characteristics. However, on top of immune suppression, TAC decreased podocyte PLA2R expression, which may also contribute to the early immunological response [39].
5 Risks and Safety
The combined four trials did not show significant differences in the incidence of AEs or SAEs between RTX-based treatments vs GC-CYC or supportive therapy. However, significant differences were observed in individual trials in the incidence of infections (more likely with GC-CYC or cyclosporine) or of deterioration of kidney function (cyclosporine) (Table 4). In MENTOR, cyclosporine had a numerically higher, although not statistically significant, occurrence of SAEs compared with RTX (Table 4 and pooled analysis, Fig. 8). Interestingly, of four patients who progressed to ESKD, three were taking GC-CYC (STARMEN 1, RI-CYCLO 2) and one was receiving cyclosporine (MENTOR). The only death was in a participant taking RTX in RI-CYCLO. Thus, a post-hoc endpoint of death or ESKD occurred in 3/80 (3.8%) participants taking GC-CYC, 1/65 (1.5%) participants taking cyclosporine, and 1/182 (0.5%) participants taking RTX, with or without TAC. These findings are not aligned with previous studies, where progression to ESKD was less and malignancy more frequent in patients who received alkylating agents [6, 9, 12, 35]. As mentioned above, the relatively short follow-up time (24 months or less) and the lower doses of cyclophosphamide used could explain the lower frequency of SAEs such as cancer or fatal events. We encourage the authors to report longer term (5–10 years) ESKD and survival outcomes of these trials. Using an evidence-based medicine approach and considering estimates of efficacy (number-needed-to-treat [benefit]) and safety (number needed to treat [harm]) of clinical interventions applied in these trials [40], treatment with RTX appears to be clearly more effective and safer than cyclosporine or antiproteinuric therapy alone. Despite the risk of AEs attributed a priori to the GC-CYC, this regimen had a better benefit-to-risk balance than TAC-RTX, but without conclusive differences with respect to RTX monotherapy (Table 5).
Risk of serious adverse events (SAEs) in the STARMEN, RI-CYCLO, GEMRITUX, and MENTOR randomized controlled trials in primary membranous nephropathy. No overall difference in risk was noted. The experimental and control treatment arms were as follows: STARMEN, experimental: tacrolimus-rituximab, control: glucocorticoid-cyclophosphamide; RI-CYCLO, experimental: rituximab, control: glucocorticoid-cyclophosphamide; GEMRITUX, experimental: rituximab, control: non-immunosuppressive antiproteinuric therapy; MENTOR: experimental: rituximab, control: cyclosporine A
5.1 Cost
Cost is a critical variable in resource-limited settings (Fig. 7b). Considering only the cost of medication, alternating GC-CYC was clearly the less costly regimen. However, these basic estimates need to be evaluated in a cost-effectiveness or cost-utility economic analysis that considers direct (immunosuppressive drugs) and indirect costs (pre-medication, concomitant medication, infusion reactions, cost of monitoring white cell counts, AEs, and others) and indicators such as quality of life. Thus, a UK study showed that RTX was cost effective in the short, medium, and long term compared with the alternate GC-CYC treatment [41]. However, this analysis was based on an Italian cohort that used mainly the RTX single dose of 375 mg/m2 [29], which is lower than in the four trials analyzed. STARMEN showed that a lower cost RTX regimen may be effective in a significant proportion of patients. Despite these previous approaches, it is clearly necessary to carry out updated cost-effectiveness or cost-utility studies, where long-term (> 5 years) and clinically relevant outcomes are evaluated. These outcomes should include progression to ESKD, need for renal replacement therapy (including associated costs), hospitalizations, treatments, laboratory tests to monitor side effects, and rehabilitation related to SAEs, life expectancy, and associated death. Moreover, these studies should consider optimal and safe doses of RTX, CNIs, and/or cyclophosphamide. A key issue in long-term trials is cost. A pragmatic trial design may be a cost-effective means of having a long-term follow-up of these trial patients to look at hard outcomes [42].
6 Unmet Needs and Potential Novel Approaches
The four trials analyzed in the present article have shown that RTX is effective and safe for the treatment of patients with nephrotic syndrome due to MN and argue against CNIs as first-line therapy based on efficacy and safety criteria. However, they also identified unmet clinical needs in overall MN management regarding the speed of onset of kidney improvement, effectiveness, safety, the treatment of specific populations (e.g., advanced CKD), and the role of anti-PLA2R monitoring in therapeutic decision making. Up to 20–30% of patients did not respond to GC-CYC, RTX, or CNI, and 65–75% of persons on RTX regimens did not achieve a complete remission (Table 3). Tools for early identification or prediction of non-responders and alternative regimens in these patients are needed. Overall, the following strategies may address current shortcomings (Table 6).
6.1 Optimization of Current Therapeutic Options and Incorporation of Risk-Based and Response-Based Decision Tools
Some of these issues may be addressed through optimization of dosing schedules for current agents and integration of these optimized dosing schemes with risk-based and response-based decision tools. Optimization of RTX timing and dosing (e.g., retreatment at 6 months) could be the key to improving long-term efficacy. Different RTX regimens (dose range from 1 to 4 g of RTX and from one to four infusions distributed over 6 months) were tested and none of the trials administered a second dose of RTX based on B-cell counts or immunological response. Indeed, only one trial (MENTOR) incorporated a second cycle of RTX at 6 months, but penalized patients with an insufficient proteinuric response as treatment failures without offering a second RTX cycle. Thus, available RCTs have failed to fully explore the possibilities of RTX therapy. It is thus open to question what the optimal regimen would be in clinical practice: should an initial lower dose be followed by repeat dosing based on any of the following criteria? a. B-cell counts, b. immunological response, c. proteinuria response, d. other or a combination of the prior criteria. A recent report of two MN cohorts in The Netherlands and in France showed that RTX (cumulative dose up to 2 g) was less effective than cyclophosphamide (dose 1.5 mg/kg/day) in inducing immunological response in patients with high baseline titers of anti-PLA2R [43]. However, in ten patients who did not achieve clinical remission at 6 months, a second course of RTX achieved immunological remission in eight patients (80%), and clinical remission in all ten patients after up of 19.5 months (17.4–30.3 months) [44]. This finding suggests that a single course of RTX might be insufficient in patients with high baseline anti-PLA2R titers, presumably because of a potentially more rapid repopulation of CD19+ B cells, highlighting the importance of the type of lymphocyte populations and baseline serum levels of anti-PLA2R autoantibodies in the response to RTX [45]. Risk stratification protocols should be tested in pragmatic clinical trials.
Potential new strategies include the use of an induction phase with monthly intravenous cyclophosphamide such as the EUROLUPUS study regimen [46], replacement of oral by intravenous cyclophosphamide in the Ponticelli regimen [47, 48], or a combination of low-dose cyclophosphamide and RTX [49]. These have shown encouraging clinical (proteinuria) and immunological remission results, with an excellent safety profile. However, an old and small old RCT in 26 patients with progressive MN did not show differences between 6 months of treatment with intravenous cyclophosphamide plus corticoids vs corticoids alone when a major improvement in kidney function was the primary outcome [50]. A combination of cyclosporine and RTX has also been tested with positive results [21], although the TAC-RTX (single-dose) experience may argue against sequential CNI-RTX to improve over current standards.
6.2 Novel Anti-B-Cell Therapies
New generation anti-CD20 antibodies are available. Obinutuzumab is a fully humanized anti-CD20 monoclonal antibody with reduced fucose content that binds to a CD20 epitope partially overlapping with that recognized by RTX. Obinutuzumab produces a much more intense and long-lasting decline in CD20+ B cells. In refractory MN, two small series showed that obinutuzumab induced early and sustained remission of proteinuria in two of three patients (67%) and nine of ten patients (90%), respectively, as well as a marked decrease in anti-PLA2R antibodies with few AEs [51, 52]. Belimumab is a monoclonal antibody that inhibits BAFF/BLYSS and induces B-cell apoptosis. Its clinical efficacy has been demonstrated in lupus nephritis, including membranous lupus nephritis [53]. A recent open-label prospective study showed that in 14 patients with persistent nephrotic proteinuria due to primary MN (three with prior immunosuppression), belimumab (10 mg/kg/month for 2 years) induced partial or complete remission in nine patients (64%) with significant decreases in anti-PLA2R from week 12 and in proteinuria from week 36 [54], with few AEs and no patient deaths. Another potential alternative is sequential RTX-belimumab therapy, aimed at depleting CD20 + B cells and preventing their BLYSS-driven recovery. An ongoing phase II and double-blind RCT is exploring this novel therapeutic approach in 124 patients with MN with positive anti-PLA2R (NCT03949855).
6.3 Targeting Plasma Cells
Plasma memory cells are responsible for the persistence of autoantibodies, which may condition relapses and refractoriness to conventional immunosuppression, including RTX [55]. Bortezomib is a proteasome inhibitor used in multiple myeloma and non-Hodgkin lymphoma that induces apoptosis of high-turnover plasma cells and has shown efficacy in immunosuppression-refractory lupus nephritis [56]. Four doses of bortezomib (1.6 mg at days 1, 4, 8, and 11) induced complete remission at 12 months in one patient with primary MN with severe nephrotic syndrome that did not respond to RAS blockade or high-dose corticosteroids, and decreased anti-PLA2R titers, with few AEs [57]. Similar results with bortezomib plus dexamethasone have been reported in severe and refractory cases to RTX and/or cyclophosphamide [58, 59]. However, this therapeutic approach has not been formally tested, and RCTs are required to give more robust recommendations. Daratumumab and felzartamab (MOR202) are anti-CD38 antibodies that decrease plasma cell numbers but have not yet been formally studied for refractory MN [60]. However, two ongoing open-label phase II trials are assessing the efficacy, safety, and pharmacokinetics/pharmacodynamics of felzartamab in anti-PLA2R positive patients with MN and are expected to be completed in 2022 and 2023, respectively (NCT04733040, NCT04145440).
6.4 Appropriate Control Groups for New Clinical Trials
For newer agents, GC-CYC should still be considered as the control group for high-risk patients, but for milder cases and considering the risk/benefit of each drug (as recommended in KDIGO 2021), RTX could be considered as the new control group (reference group). However, the issue of ideal vs what can be achieved is a key issue. It is extremely unlikely that industry will ever fund a trial using a combination of cyclophosphamide and corticosteroids as the control arm. That same can be expected from government agencies, for example, the National Institutes of Health refused to fund even a small part of MENTOR. As such, the search for the “ideal” vs the “reality” comes into play. This can be illustrated by current ongoing RCTs, e.g., NCT04629248, A Study Evaluating the Efficacy and Safety of Obinutuzumab in Participants with Primary Membranous Nephropathy, where the comparator arm is TAC.
6.5 Advanced CKD
There are scarce reports of treatment outcomes for patients with advanced CKD (GFR < 30 mL/min/1.73 m2). In this context, alkylating drugs showed better efficacy than CNIs [61]. A small series of 13 patients with CKD stages 4–5 showed that RTX may be a viable alternative [62]. However, these data should be confirmed in RCTs.
6.6 Optimizing Remission Definitions
Anti-PLA2R antibodies play an important role in monitoring the immunological response to treatment in primary MN. In observational studies, baseline anti-PLA2R titers and immunological response (negative anti-PLA2R) predicted the clinical response in the medium term and long term [23, 63,64,65], a finding that was confirmed by STARMEN, RI-CYCLO, and MENTOR. Randomized controlled trials should evaluate the role of anti-PLA2R and other emerging autoantibodies and antigens in therapeutic decision making in primary MN [66]. Furthermore, the definition of disease remission must be modified to include immunological remission (i.e., antibody negative) in addition to proteinuria remission, and should consider the time lag between immune response and clinical remission [67].
6.7 Long-Term Impact on Kidney Function
A key consequence of proteinuric kidney disease is the long-term loss of kidney function. Treatment for MN should preserve kidney function and prevent ESKD. However, the short-term impact of therapy on kidney function may be difficult to interpret, given the hemodynamic impact of some therapies (e.g., CNIs, RAS blockade), the potential for both reversible and chronic nephrotoxicity (CNIs), loss of muscle mass (steroids), and the impact of the presence of large amounts of protein in the urinary space on GFR that may penalize successful therapies. Thus, a long-term follow-up of kidney function should be built into any RCT for MN. This recommendation is consistent with the conclusions of a recent systematic review, where it is observed that immunosuppressive therapy could help prevent progression to ESKD, mainly with alkylating agents, but at the expense of clinically relevant adverse effects associated with these drugs. Importantly, it was emphasized in this review that these conclusions are based on a few high-quality studies and without an adequate long-term follow-up of patients, including with the use of CNIs and new biological agents [68].
7 Conclusions
Recent RCTs have improved our understanding of the role of different therapeutic regimens for primary MN. Overall, RTX was superior to NIAT or cyclosporine (Fig. 2 of the ESM). Information regarding the relative performance of GC-CYC vs RTX is less clear cut, as the TAC-RTX regimen (lower dose, delayed RTX) tested in STARMEN appears to be a suboptimal regimen from the point of view of efficacy and safety and RI-CYCLO was a pilot trial. In any case, these recent RCTs confirmed the efficacy of GC-CYC to induce remission of proteinuria and immunological response in primary MN under current standards of clinical practice in association with a reasonable safety profile (Table 5). However, the occurrence of ESKD only in the GC-CYC arms of both RCTs testing this regimen raises concerns that should be addressed in a long-term follow-up. The available trials do not offer information that allows recommendation of a different regimen for patients at high or very high risk. Interestingly, MENTOR enrolled the most severely affected patients and confirmed the efficacy of RTX. However, none of the RTX regimens tested in RCTs appeared optimal as they did not incorporate retreatment at 6 months or did so only for certain patients, diagnosing treatment failure before a second cycle of RTX. Thus, the therapeutic potential of RTX may have been underestimated in available RCTs. Calcineurin inhibitors, despite their antiproteinuric effect, have a suboptimal AE profile, including persistent nephrotoxicity and a high relapse rate, and cannot be used in patients with advanced CKD. As such, CNIs have been relegated to the third treatment option. In resource-limited settings, GC-CYC provides the lowest cost of therapy, although formal cost-effectiveness analyses would be required. Given the multitude of unanswered questions (Table 6), we suggest that pragmatic RCTs are set up and offered to at least patients cared for at referral centers.
References
Couser W. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–97.
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. Chapter 7: Idiopathic membranous nephropathy. KDIGO clinical practice guidelines for glomerulonephritis. Kidney Int Suppl. 2012;2:186–97.
KDIGO. 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1-276.
Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–58.
Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46.
Ponticelli C, Zucchelli P, Passerini P, et al. A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med. 1989;320:8–13.
Ponticelli C, Altieri P, Scolari F, et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–50.
Ponticelli C, Zucchelli P, Passerini P, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600–4.
Jha V, Ganguli A, Saha TK, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18:1899–904.
Klomjit N, Zand L. Rituximab is preferable to cyclophosphamide for treatment of membranous nephropathy. Kidney360. 2021. https://doi.org/10.34067/KID.0002492021.
Peleg Y, Bomback A, Radhakrishnan J. The evolving role of calcineurin inhibitors in treating lupus nephritis. Clin J Am Soc Nephrol. 2020;15:1066–72.
Cattran DC, Appel GB, Hebert LA, et al. Cyclosporine in patients with steroid resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–90.
Praga M, Barrio V, Juárez GF, Luño J. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71:924–30.
Xu J, Zhang W, Xu Y, Shen P, Ren H, Wang W, et al. Tacrolimus combined with corticosteroids in idiopathic membranous nephropathy: a randomized, prospective, controlled trial. Contrib Nephrol. 2013;181:152–62.
Liang Q, Li H, Xie X, Qu F, Li X, Chen J. The efficacy and safety of tacrolimus monotherapy in adult-onset nephrotic syndrome caused by idiopathic membranous nephropathy. Renal Fail. 2017;39:512–8.
Ramachandran R, Yadav AK, Kumar V, Pinnamaneni VST, Nada R, Ghosh R, et al. Two-year follow-up study of membranous nephropathy treated with tacrolimus and corticosteroids versus cyclical corticosteroids and cyclophosphamide. Kidney Int Rep. 2017;2:610–6.
Qiu TT, Zhang C, Zhao HW, Zhou JW. Calcineurin inhibitors versus cyclophosphamide for idiopathic membranous nephropathy: a systematic review and meta-analysis of 21 clinical trials. Autoimmun Rev. 2017;16:136–45.
Wang Q, Li M, Cheng X, Xu G. Clinical efficacy, and safety of different tacrolimus concentrations for the treatment of patients with idiopathic membranous nephropathy. Sci Rep. 2021;11:1374. https://doi.org/10.1038/s41598-021-92678-y.
Gong L, Xu M, Xu W, Tang W, Lu J, Jiang W, et al. Efficacy and safety of tacrolimus monotherapy versus cyclophosphamide-corticosteroid combination therapy for idiopathic membranous nephropathy: a meta-analysis. Medicine. 2021;100:28.
Segarra A, Praga M, Ramos N, Polanco N, Cargol I, Gutierrez-Solis E, et al. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor-dependent patients. Clin J Am Soc Nephrol. 2009;4:1083–8.
Waldman M, Beck LH Jr, Braun M, Wilkins K, Balow JE, Austin HA III. Membranous nephropathy: pilot study of a novel regimen combining cyclosporine and rituximab. Kidney Int Rep. 2016;1:73–84.
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21.
Bech AP, Hofstra JM, Brenchley PE, Wetzels JFM. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9:1386–92.
Takahashi Y, Ikezumi Y, Saitoh A. Rituximab protects podocyte and exerts anti-proteinuric effects in rat adriamycin-induced nephropathy independent of B lymphocytes. Nephrology (Carlton). 2017;22:49–57.
Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. https://doi.org/10.1126/scitranslmed.3002231.
Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, et al. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol. 2003;14:1851–7.
Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Soc Nephrol. 2010;5:2188–98.
Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cell to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932–7.
Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1416–25.
Zou P, Li H, Cai J, Chen Z, Li C, Li X. Therapy of rituximab in idiopathic membranous nephropathy with nephrotic syndrome: a systematic review and meta-analysis. Chin Med Sci J. 2018;33:9–19.
Zhang J, Bian L, Ma F-Z, Jia Y, Lin P. Efficacy and safety of rituximab therapy for membranous nephropathy: a meta-analysis. Eur Rev Med Pharm Sci. 2018;22:8021–9.
Fernández-Juárez G, Rojas-Rivera J, van de Logt A-E, Justino J, Sevillano A, Caravaca-Fontán F, et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021;99:986–98.
Rojas-Rivera J, Fernández-Juárez G, Ortiz A, Hofstra J, Gesualdo L, Tesar V, et al. A European multicentre and open-label controlled randomized trial to evaluate the efficacy of sequential treatment with tacrolimus-rituximab versus steroids plus cyclophosphamide in patients with primary membranous nephropathy: the STARMEN study. Clin Kidney J. 2015;8:503–10.
Scolari F, Delbarba E, Santoro D, Gesualdo L, Pani A, Dallera N, et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32:972–82.
van den Brand JAJG, Ruggenenti P, Chianca A, Hofstra JM, Perna A, Ruggiero B, et al. Safety of rituximab compared with steroids and cyclophosphamide for idiopathic membranous nephropathy. J Am Soc Nephrol. 2017;28:2729–37.
Rojas-Rivera J, Carriazo S, Ortiz A. Treatment of idiopathic membranous nephropathy in adults: KDIGO 2012, cyclophosphamide and cyclosporine A are out, rituximab is the new normal. Clin Kidney J. 2019;12:629–38.
Ramachandran R, Kumar V, Bharati J, Rovin B, Nada R, Kumar V, et al. Long-term follow-up of cyclical cyclophosphamide and steroids versus tacrolimus and steroids in primary membranous nephropathy. Kidney Int Rep. 2021;6:2653–60.
Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC. Idiopathic membranpus nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66:1199–205.
Cuarental L, Valiño-Rivas L, Mendonça L, Saleem M, Mezzano S, Sanz AB, et al. Tacrolimus prevents TWEAK-induced PLA2R expresión in cultured human podocytes. J Clin Med. 2020;9:2178. https://doi.org/10.3390/jcm9072178.
Altman D. Confidence intervals for the number needed to treat. Br Med J. 1998;317:1309–12.
Hamilton P, Kanigicherla D, Venning M, Brenchley P, Meads D. Rituximab versus the modified Ponticelli regimen in the treatment of primary membranous nephropathy: a health economic model. Nephrol Dial Transplant. 2018;33:2145–55.
Ford I, Norrie J. Pragmatic trials. N Eng J Med. 2016;375:454–63.
Van de Logt A-E, Dahan K, Rousseau A, van der Molen R, Debiec H, Ronco P, Wetzels J. Immunological remission in PLA2R-antibody-associated membranous nephropathy: cyclophosphamide versus rituximab. Kidney Int. 2018;93:1016–7.
Dahan K, Johannet C, Esteve E, Plaisier E, Debiec H, Ronco P. Retreatment with rituximab for membranous nephropathy with persistently elevated titers of anti-phospholipase A2 receptor antibody. Kidney Int. 2019;95:233–4.
Rosenzwajg M, Languille E, Debiec H, Hygino H, Dahan K, Simon T, et al. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 2017;92:227–37.
Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani D, de Ramon GE, Danieli MG, et al. The 10-year follow-up of the Euro-Lupus nephritis trial comparing two low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69:61–4.
Mathrani V, Alejmi A, Griffin S, Roberts G. Intravenous cyclophosphamide and oral prednisolone is a safe and effective treatment option for idiopathic membranous nephropathy. Clin Kidney J. 2017;10:450–4.
Luzardo L, Ottati G, Cabrera J, Trujillo H, Garau M, Bedat CG, et al. Substitution of oral for intravenous cyclophosphamide in membranous nephropathy. Kidney360. 2020;1:943–9.
Zonozi R, Laliberte K, Huizenga NR, Rosenthal JK, Jeyabalan A, Collins AB, et al. Combination of rituximab, low-dose cyclophosphamide, and prednisone for primary membranous nephropathy: a case series with extended follow-up. Am J Kidney Dis. 2021. https://doi.org/10.1053/j.ajkd.2021.04.014.
Falk RJ, Hogan SL, Muller KE, Jennette C, Glomerular Disease Collaborative Network. Treatment of progressive membranous glomerulopathy. Ann Int Med. 1992;116:438–45.
Klomjit N, Fervenza FC, Zand L. Successful treatment of patient with refractory PLA2R-associated membranous nephropathy with obitunuzumab: a report of 3 cases. Am J Kidney Dis. 2020;76:883–8.
Sethi S, Kumar S, Lim K, Jordan SC. Obinutuzumab is effective for the treatment of refractory membranous nephropathy. Kidney Int Rep. 2020;5:1515–8.
Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Eng J Med. 2020;383:1117–28.
Barret C, Willcoks LC, Jones RB, Tarzi RM, Henderson RB, Cai G, et al. Effect of belimumab on proteinuria and anti-phospholipase A2 receptor autoantibody in membranous nephropathy. Nephrol Dial Transplant. 2020;35:599–606.
Hiepe F, Radbruch A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol. 2016;12:232–40.
Segarra A, Arredondo AV, Jaramillo J, Jatem E, Salcedo MT, Agraz I, et al. Efficacy and safety of bortezomib in refractory lupus nephritis: a single-centre experience. Lupus. 2020;29:118–25.
Hartono C, Chung M, Kuo SF, Seshan SV, Muthukumar T. Bortezomib therapy for nephrotic syndrome due to idiopathic membranous nephropathy. J Nephrol. 2014;27:103–6.
Geara AS, Bhoj V, Hogan JJ. Bortezomib treatment for refractory PLA2R-positive membranous nephropathy. Glom Dis. 2021;1:40–3.
Salhi S, Ribes D, Colombat M, Fortenfant F, Faguer S. Bortezomib plus dexamethasone for rituximab-resistant PLA2R + membranous nephropathy. Kidney Int. 2021;100:708–9.
van de Donk NW, Janmaat ML, Mutis T, van Bueren JJL, Ahmadi T, Sasser AK, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270:95–112.
Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, et al. Immunnosupression for progressive membranous nephropathy: a UK randomized controlled trial. Lancet. 2013;381:744–51.
Hanset N, Esteve E, Plaisier E, Johanet C, Michel PA, Boffa J-J, et al. Rituximab in patients with phospholipase A2 receptor-associated membranous nephropathy and severe CKD. Kidney Int Rep. 2020;5:331–8.
Floege J, Barbour SJ, Cattran D, Hogan JJ, Nachman PH, Tang SCW, et al. Management and treatment of glomerular diseases (part 1): conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95:268–80.
Beck LH, Fervenza FC, Beck DM, Bonegio RGB, Malik FA, Erickson SB, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–50.
Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcomes of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–58.
Sethi S. New antigens in membranous nephropathy. J Am Soc Nephrol. 2021;32:268–78.
De Vriese AS, Glassock R, Nath KA, Sethi S, Fervenza FC. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–30.
von Groote TC, Williams G, Au EH, Chen Y, Mathew AT, Hodson EM, et al. Immunosuppressive treatment for primary membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2021;11: CD004293. https://doi.org/10.1002/14651858.CD004293.pub4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Research by the authors was supported by FIS/Fondos FEDER (PI18/01366, PI19/00588, PI19/00815, DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, Fundación Renal Iñigo Álvarez de Toledo (FRIAT), and Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM.
Conflicts of Interest/Competing Interests
Jorge Rojas-Rivera has received consultancy or speaker fees from GlaxoSmithKline, Alexion Pharma Spain S.L., and Otsuka Pharmaceuticals. Alberto Ortiz has received consultancy or speaker fees or travel support from Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Otsuka, and Vifor Fresenius Medical Care Renal Pharma and is a Director of the Catedra Mundipharma-UAM of Diabetic Kidney Disease and the Catedra AstraZeneca-UAM of Chronic Kidney Disease and Electrolytes. F.C. Fervenza reports having consultancy agreements with Alexion Pharmaceuticals, Alnylam, and ByoCrystal; receiving research funding from Chemocentryx, Genentech Inc., Janssen Pharmaceutical, and Questcor/Mallinckrodt; being a scientific advisor for, or member of, Kidney International, Nephrology, Nephrology Dialysis Transplantation, and UpToDate; and receiving honoraria from UpToDate.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
All analysis and graphics for this review were realized with MS Excel for Windows and Stata version 15.0 for Windows.
Authors’ Contributions
Jorge Rojas-Rivera: conception of the study, literature search, data analysis, elaboration of tables and figures, drafting, critical review, and approval of the final manuscript. Alberto Ortiz: rafting, modifications and corrections, elaboration of tables and figures, critical review, and approval of the final manuscript. Fernando C. Fervenza: drafting, modifications, and corrections, critical review, and final approval of the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rojas-Rivera, J., Fervenza, F.C. & Ortiz, A. Recent Clinical Trials Insights into the Treatment of Primary Membranous Nephropathy. Drugs 82, 109–132 (2022). https://doi.org/10.1007/s40265-021-01656-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01656-1