Abstract
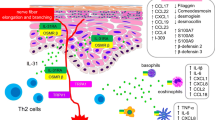
Chronic pruritus is a debilitating symptom with limited treatment options. Identifying molecular targets underlying chronic pruritic dermatoses is essential for the development of novel, targeted therapies. IL-31 is an important mediator of itch by integrating dermatologic, neural, and immune systems. IL-31 helps induce and maintain chronic pruritus via both indirect stimulation of inflammatory cells and through direct neural sensitization. IL-31 is overexpressed in various chronic pruritic skin conditions, and exogenous IL-31 induces itch and scratching behavior. Studies have demonstrated that IL-31R and IL-31 antagonism significantly reduces itch in patients with atopic dermatitis and prurigo nodularis, two extremely pruritic skin conditions. Emerging evidence, including recent phase II clinical trials of IL-31R antagonists, demonstrates that IL-31 plays an important role in itch signaling. Additional studies are ongoing to evaluate IL-31R and IL-31 antagonism as treatments of chronic pruritus.

Similar content being viewed by others
References
Ständer S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87(4):291–4. https://doi.org/10.2340/00015555-0305.
Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23(1):1–11. https://doi.org/10.5021/ad.2011.23.1.1.
Weisshaar E, Matterne U. Frontiers in Neuroscience Epidemiology of Itch. In: Carstens E, Akiyama T, editors. Itch: mechanisms and treatment. Boca Raton (FL): CRC Press/Taylor & Francis © 2014 by Taylor & Francis Group, LLC.; 2014.
Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147(10):1153–6. https://doi.org/10.1001/archdermatol.2011.178.
Whang KA, Khanna R, Williams KA, Mahadevan V, Semenov Y, Kwatra SG. Health-related quality of life and economic burden of chronic pruritus. J Invest Dermatol. 2020. https://doi.org/10.1016/j.jid.2020.08.020.
Jensen P, Zachariae C, Skov L, Zachariae R. Sleep disturbance in psoriasis: a case-controlled study. Br J Dermatol. 2018;179(6):1376–84. https://doi.org/10.1111/bjd.16702.
Patel SP, Khanna R, Choi J, Williams KA, Roh YS, Hong MS, et al. Sleep disturbance in adults with chronic pruritic dermatoses is associated with increased C-reactive protein levels. J Am Acad Dermatol. 2020. https://doi.org/10.1016/j.jaad.2020.08.059.
Chamlin SL, Mattson CL, Frieden IJ, Williams ML, Mancini AJ, Cella D, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159(8):745–50. https://doi.org/10.1001/archpedi.159.8.745.
Yamamoto Y, Yamazaki S, Hayashino Y, Takahashi O, Tokuda Y, Shimbo T, et al. Association between frequency of pruritic symptoms and perceived psychological stress: a Japanese population-based study. Arch Dermatol. 2009;145(12):1384–8. https://doi.org/10.1001/archdermatol.2009.290.
Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17–26. https://doi.org/10.1016/j.neubiorev.2018.01.009.
Dalgard F, Svensson A, Holm J, Sundby J. Self-reported skin morbidity among adults: associations with quality of life and general health in a Norwegian survey. J Investig Dermatol Symp Proc. 2004;9(2):120–5. https://doi.org/10.1046/j.1087-0024.2003.09111.x.
Erturk IE, Arican O, Omurlu IK, Sut N. Effect of the pruritus on the quality of life: a preliminary study. Ann Dermatol. 2012;24(4):406–12. https://doi.org/10.5021/ad.2012.24.4.406.
Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–34. https://doi.org/10.1038/jid.2013.446.
Matterne U, Strassner T, Apfelbacher CJ, Diepgen TL, Weisshaar E. Measuring the prevalence of chronic itch in the general population: development and validation of a questionnaire for use in large-scale studies. Acta Derm Venereol. 2009;89(3):250–6. https://doi.org/10.2340/00015555-0641.
Shive M, Linos E, Berger T, Wehner M, Chren MM. Itch as a patient-reported symptom in ambulatory care visits in the United States. J Am Acad Dermatol. 2013;69(4):550–6. https://doi.org/10.1016/j.jaad.2013.05.029.
Weisshaar E, Szepietowski JC, Darsow U, Misery L, Wallengren J, Mettang T, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563–81. https://doi.org/10.2340/00015555-1400.
Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27(28):7490–7. https://doi.org/10.1523/jneurosci.1249-07.2007.
Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100(4):2062–9. https://doi.org/10.1152/jn.90482.2008.
Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8. https://doi.org/10.1523/jneurosci.17-20-08003.1997.
Andersen HH, Elberling J, Sølvsten H, Yosipovitch G, Arendt-Nielsen L. Nonhistaminergic and mechanical itch sensitization in atopic dermatitis. Pain. 2017;158(9):1780–91. https://doi.org/10.1097/j.pain.0000000000000980.
Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda). 2011;26(4):286–92. https://doi.org/10.1152/physiol.00007.2011.
Yang TB, Kim BS. Pruritus in allergy and immunology. J Allergy Clin Immunol. 2019;144(2):353–60. https://doi.org/10.1016/j.jaci.2019.06.016.
Amin M, Darji K, No DJ, Bhutani T, Wu JJ. Review of IL-17 inhibitors for psoriasis. J Dermatolog Treat. 2018;29(4):347–52. https://doi.org/10.1080/09546634.2017.1395796.
Hao JQ. Targeting interleukin-22 in psoriasis. Inflammation. 2014;37(1):94–9. https://doi.org/10.1007/s10753-013-9715-y.
Wawrzycki B, Pietrzak A, Grywalska E, Krasowska D, Chodorowska G, Roliński J. Interleukin-22 and its correlation with disease activity in Plaque psoriasis. Arch Immunol Ther Exp (Warsz). 2019;67(2):103–8. https://doi.org/10.1007/s00005-018-0527-5.
Fotiadou C, Lazaridou E, Sotiriou E, Ioannides D. Targeting IL-23 in psoriasis: current perspectives. Psoriasis (Auckl). 2018;8:1–5. https://doi.org/10.2147/ptt.S98893.
Mussi A, Bonifati C, Carducci M, D’Agosto G, Pimpinelli F, D’Urso D, et al. Serum TNF-alpha levels correlate with disease severity and are reduced by effective therapy in plaque-type psoriasis. J Biol Regul Homeost Agents. 1997;11(3):115–8.
Bachelez H, van de Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–61. https://doi.org/10.1016/s0140-6736(14)62113-9.
Punwani N, Scherle P, Flores R, Shi J, Liang J, Yeleswaram S, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67(4):658–64. https://doi.org/10.1016/j.jaad.2011.12.018.
Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19(5–6):347–56. https://doi.org/10.1016/j.cytogfr.2008.08.003.
Diveu C, Lelièvre E, Perret D, Lak-Hal AH, Froger J, Guillet C, et al. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J Biol Chem. 2003;278(50):49850–9. https://doi.org/10.1074/jbc.M307286200.
Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5(7):752–60. https://doi.org/10.1038/ni1084.
Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol. 2013;132(2):446-54.e5. https://doi.org/10.1016/j.jaci.2013.03.050.
Maier E, Werner D, Duschl A, Bohle B, Horejs-Hoeck J. Human Th2 but not Th9 cells release IL-31 in a STAT6/NF-κB-dependent way. J Immunol. 2014;193(2):645–54. https://doi.org/10.4049/jimmunol.1301836.
Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117(2):418–25. https://doi.org/10.1016/j.jaci.2005.10.046.
Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. https://doi.org/10.1016/j.jaci.2016.06.033.
Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411–7. https://doi.org/10.1016/j.jaci.2005.10.033.
Ghilardi N, Li J, Hongo JA, Yi S, Gurney A, de Sauvage FJ. A novel type I cytokine receptor is expressed on monocytes, signals proliferation, and activates STAT-3 and STAT-5. J Biol Chem. 2002;277(19):16831–6. https://doi.org/10.1074/jbc.M201140200.
Hermanns HM. Oncostatin M and interleukin-31: cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015;26(5):545–58. https://doi.org/10.1016/j.cytogfr.2015.07.006.
Diveu C, Lak-Hal AH, Froger J, Ravon E, Grimaud L, Barbier F, et al. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. 2004;15(4):291–302.
Maier E, Mittermeir M, Ess S, Neuper T, Schmiedlechner A, Duschl A, et al. Prerequisites for functional interleukin 31 signaling and its feedback regulation by suppressor of cytokine signaling 3 (SOCS3). J Biol Chem. 2015;290(41):24747–59. https://doi.org/10.1074/jbc.M115.661306.
Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006;142(4):1263–71. https://doi.org/10.1016/j.neuroscience.2006.07.009.
Tamura S, Morikawa Y, Miyajima A, Senba E. Expression of oncostatin M receptor beta in a specific subset of nociceptive sensory neurons. Eur J Neurosci. 2003;17(11):2287–98. https://doi.org/10.1046/j.1460-9568.2003.02681.x.
McCandless EE, Rugg CA, Fici GJ, Messamore JE, Aleo MM, Gonzales AJ. Allergen-induced production of IL-31 by canine Th2 cells and identification of immune, skin, and neuronal target cells. Vet Immunol Immunopathol. 2014;157(1–2):42–8. https://doi.org/10.1016/j.vetimm.2013.10.017.
Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448–60. https://doi.org/10.1016/j.jaci.2013.10.048.
Raap U, Gehring M, Kleiner S, Rüdrich U, Eiz-Vesper B, Haas H, et al. Human basophils are a source of—and are differentially activated by—IL-31. Clin Exp Allergy. 2017;47(4):499–508. https://doi.org/10.1111/cea.12875.
Cheung PF, Wong CK, Ho AW, Hu S, Chen DP, Lam CW. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol. 2010;22(6):453–67. https://doi.org/10.1093/intimm/dxq027.
Yamaoka K, Okayama Y, Kaminuma O, Katayama K, Mori A, Tatsumi H, et al. Proteomic approach to FcepsilonRI aggregation-initiated signal transduction cascade in human mast cells. Int Arch Allergy Immunol. 2009;149(Suppl 1):73–6. https://doi.org/10.1159/000211376.
Kasraie S, Niebuhr M, Baumert K, Werfel T. Functional effects of interleukin 31 in human primary keratinocytes. Allergy. 2011;66(7):845–52. https://doi.org/10.1111/j.1398-9995.2011.02545.x.
Kunsleben N, Rüdrich U, Gehring M, Novak N, Kapp A, Raap U. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J Invest Dermatol. 2015;135(7):1908–11. https://doi.org/10.1038/jid.2015.106.
Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol. 2012;91(6–7):552–66. https://doi.org/10.1016/j.ejcb.2011.07.006.
Chattopadhyay S, Tracy E, Liang P, Robledo O, Rose-John S, Baumann H. Interleukin-31 and oncostatin-M mediate distinct signaling reactions and response patterns in lung epithelial cells. J Biol Chem. 2007;282(5):3014–26. https://doi.org/10.1074/jbc.M609655200.
Ip WK, Wong CK, Li ML, Li PW, Cheung PF, Lam CW. Interleukin-31 induces cytokine and chemokine production from human bronchial epithelial cells through activation of mitogen-activated protein kinase signalling pathways: implications for the allergic response. Immunology. 2007;122(4):532–41. https://doi.org/10.1111/j.1365-2567.2007.02668.x.
Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138(2):500-8.e24. https://doi.org/10.1016/j.jaci.2016.02.020.
Sun S, Dong X. Trp channels and itch. Semin Immunopathol. 2016;38(3):293–307. https://doi.org/10.1007/s00281-015-0530-4.
Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7(7):535–47. https://doi.org/10.1038/nrn1950.
Hänel KH, Pfaff CM, Cornelissen C, Amann PM, Marquardt Y, Czaja K, et al. Control of the physical and antimicrobial skin barrier by an IL-31-IL-1 signaling network. J Immunol. 2016;196(8):3233–44. https://doi.org/10.4049/jimmunol.1402943.
Singh B, Jegga AG, Shanmukhappa KS, Edukulla R, Khurana Hershey GH, Medvedovic M, et al. IL-31-driven skin remodeling involves epidermal cell proliferation and thickening that lead to impaired skin-barrier function. PLoS ONE. 2016;11(8):e0161877. https://doi.org/10.1371/journal.pone.0161877.
Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proc Natl Acad Sci USA. 1995;92(25):11874–8. https://doi.org/10.1073/pnas.92.25.11874.
O’Shaughnessy RF, Choudhary I, Harper JI. Interleukin-1 alpha blockade prevents hyperkeratosis in an in vitro model of lamellar ichthyosis. Hum Mol Genet. 2010;19(13):2594–605. https://doi.org/10.1093/hmg/ddq145.
Yagi Y, Andoh A, Nishida A, Shioya M, Nishimura T, Hashimoto T, et al. Interleukin-31 stimulates production of inflammatory mediators from human colonic subepithelial myofibroblasts. Int J Mol Med. 2007;19(6):941–6.
Hanifin JMaR, G. . Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92:44–7.
Arai I, Tsuji M, Takeda H, Akiyama N, Saito S. A single dose of interleukin-31 (IL-31) causes continuous itch-associated scratching behaviour in mice. Exp Dermatol. 2013;22(10):669–71. https://doi.org/10.1111/exd.12222.
Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516(2):180–1. https://doi.org/10.1016/j.ejphar.2005.04.040.
Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Grønhøj-Larsen C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18(1):35–43. https://doi.org/10.1111/j.1600-0625.2008.00766.x.
Gonzales AJ, Humphrey WR, Messamore JE, Fleck TJ, Fici GJ, Shelly JA et al. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24(1):48-53.e11-2. https://doi.org/10.1111/j.1365-3164.2012.01098.x.
Lewis KE, Holdren MS, Maurer MF, Underwood S, Meengs B, Julien SH, et al. Interleukin (IL) 31 induces in cynomolgus monkeys a rapid and intense itch response that can be inhibited by an IL-31 neutralizing antibody. J Eur Acad Dermatol Venereol. 2017;31(1):142–50. https://doi.org/10.1111/jdv.13794.
Hawro T, Saluja R, Weller K, Altrichter S, Metz M, Maurer M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy. 2014;69(1):113–7. https://doi.org/10.1111/all.12316.
Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217-28.e13. https://doi.org/10.1016/j.cell.2017.08.006.
Nygaard U, Hvid M, Johansen C, Buchner M, Fölster-Holst R, Deleuran M, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. 2016;30(11):1930–8. https://doi.org/10.1111/jdv.13679.
Lu J, Wu K, Zeng Q, Xiang Y, Gao L, Huang J. Serum interleukin-31 level and pruritus in atopic dermatitis: a Meta-analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):124–30. https://doi.org/10.11817/j.issn.1672-7347.2018.02.003.
Raap U, Wichmann K, Bruder M, Ständer S, Wedi B, Kapp A, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122(2):421–3. https://doi.org/10.1016/j.jaci.2008.05.047.
Raap U, Weißmantel S, Gehring M, Eisenberg AM, Kapp A, Fölster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol. 2012;23(3):285–8. https://doi.org/10.1111/j.1399-3038.2011.01241.x.
Ezzat MH, Hasan ZE, Shaheen KY. Serum measurement of interleukin-31 (IL-31) in paediatric atopic dermatitis: elevated levels correlate with severity scoring. J Eur Acad Dermatol Venereol. 2011;25(3):334–9. https://doi.org/10.1111/j.1468-3083.2010.03794.x.
Nobbe S, Dziunycz P, Mühleisen B, Bilsborough J, Dillon SR, French LE, et al. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta Derm Venereol. 2012;92(1):24–8. https://doi.org/10.2340/00015555-1191.
Schulz F, Marenholz I, Fölster-Holst R, Chen C, Sternjak A, Baumgrass R, et al. A common haplotype of the IL-31 gene influencing gene expression is associated with nonatopic eczema. J Allergy Clin Immunol. 2007;120(5):1097–102. https://doi.org/10.1016/j.jaci.2007.07.065.
Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118(4):930–7. https://doi.org/10.1016/j.jaci.2006.07.015.
Lin MW, Lee DD, Liu TT, Lin YF, Chen SY, Huang CC, et al. Novel IL31RA gene mutation and ancestral OSMR mutant allele in familial primary cutaneous amyloidosis. Eur J Hum Genet. 2010;18(1):26–32. https://doi.org/10.1038/ejhg.2009.135.
Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26(6):484–97. https://doi.org/10.1016/j.tim.2017.11.008.
Blicharz L, Rudnicka L, Samochocki Z. Staphylococcus aureus: an underestimated factor in the pathogenesis of atopic dermatitis? Postepy Dermatol Alergol. 2019;36(1):11–7. https://doi.org/10.5114/ada.2019.82821.
Kowalski EH, Kneiber D, Valdebran M, Patel U, Amber KT. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163–72. https://doi.org/10.2147/ccid.S188070.
Huang AH, Canner JK, Khanna R, Kang S, Kwatra SG. Real-world prevalence of Prurigo Nodularis and burden of associated Diseases. J Invest Dermatol. 2020;140(2):480-3.e4. https://doi.org/10.1016/j.jid.2019.07.697.
Kwatra SG. Breaking the itch-scratch cycle in Prurigo Nodularis. N Engl J Med. 2020;382(8):757–8. https://doi.org/10.1056/NEJMe1916733.
Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020. https://doi.org/10.1016/j.jaad.2020.04.182.
Whang KA, Kang S, Kwatra SG. Inpatient burden of prurigo nodularis in the United States. Medicines (Basel). 2019. https://doi.org/10.3390/medicines6030088.
Boozalis E, Tang O, Patel S, Semenov YR, Pereira MP, Stander S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79(4):714-9.e3. https://doi.org/10.1016/j.jaad.2018.04.047.
Park K, Mori T, Nakamura M, Tokura Y. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21(1):135–6. https://doi.org/10.1684/ejd.2010.1196.
Hashimoto T, Nattkemper LA, Kim HS, Kursewicz CD, Fowler E, Shah SM, et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol. 2021. https://doi.org/10.1111/exd.14279.
Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.01383.
Bonciani D, Quintarelli L, Del Bianco E, Bianchi B, Caproni M. Serum levels and tissue expression of interleukin-31 in dermatitis herpetiformis and bullous pemphigoid. J Dermatol Sci. 2017;87(2):210–2. https://doi.org/10.1016/j.jdermsci.2017.04.008.
Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Pathophysiologic mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol. 2020;83(1):53–62. https://doi.org/10.1016/j.jaad.2019.07.060.
Huang HT, Chen JM, Guo J, Lan Y, Wei YS. The association of interleukin-31 polymorphisms with interleukin-31 serum levels and risk of systemic lupus erythematosus. Rheumatol Int. 2016;36(6):799–805. https://doi.org/10.1007/s00296-016-3422-6.
Yosipovitch G, Tan A, LoSicco K, Manabat CG, Kannagra A, Carroll C, et al. A comparative study of clinical characteristics, work-up, treatment, and association to malignancy in dermatomyositis between two tertiary skin centers in the USA and Singapore. Int J Dermatol. 2013;52(7):813–9. https://doi.org/10.1111/j.1365-4632.2011.05449.x.
Kim HJ, Zeidi M, Bonciani D, Pena SM, Tiao J, Sahu S, et al. Itch in dermatomyositis: the role of increased skin interleukin-31. Br J Dermatol. 2018;179(3):669–78. https://doi.org/10.1111/bjd.16498.
Guarneri F, Minciullo PL, Mannucci C, Calapai F, Saitta S, Cannavò SP, et al. IL-31 and IL-33 circulating levels in allergic contact dermatitis. Eur Ann Allergy Clin Immunol. 2015;47(5):156–8.
Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Mechanisms of itch in stasis dermatitis: significant role of IL-31 from macrophages. J Invest Dermatol. 2020;140(4):850-9.e3. https://doi.org/10.1016/j.jid.2019.09.012.
Hashimoto T, Satoh T, Yokozeki H. Pruritus in ordinary scabies: IL-31 from macrophages induced by overexpression of thymic stromal lymphopoietin and periostin. Allergy. 2019;74(9):1727–37. https://doi.org/10.1111/all.13870.
Lange M, Gleń J, Zabłotna M, Nedoszytko B, Sokołowska-Wojdyło M, Rębała K, et al. Interleukin-31 polymorphisms and serum IL-31 level in patients with mastocytosis: correlation with Clinical Presen-tation and Pruritus. Acta Derm Venereol. 2017;97(1):47–53. https://doi.org/10.2340/00015555-2474.
Wright A, Wijeratne A, Hung T, Gao W, Whittaker S, Morris S, et al. Prevalence and severity of pruritus and quality of life in patients with cutaneous T-cell lymphoma. J Pain Symptom Manage. 2013;45(1):114–9. https://doi.org/10.1016/j.jpainsymman.2012.01.012.
Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133(12):2783–5. https://doi.org/10.1038/jid.2013.227.
Ohmatsu H, Sugaya M, Suga H, Morimura S, Miyagaki T, Kai H, et al. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm Venereol. 2012;92(3):282–3. https://doi.org/10.2340/00015555-1345.
Nattkemper LA, Martinez-Escala ME, Gelman AB, Singer EM, Rook AH, Guitart J, et al. Cutaneous T-cell lymphoma and pruritus: the expression of IL-31 and its receptors in the skin. Acta Derm Venereol. 2016;96(7):894–8. https://doi.org/10.2340/00015555-2417.
Cedeno-Laurent F, Singer EM, Wysocka M, Benoit BM, Vittorio CC, Kim EJ, et al. Improved pruritus correlates with lower levels of IL-31 in CTCL patients under different therapeutic modalities. Clin Immunol. 2015;158(1):1–7. https://doi.org/10.1016/j.clim.2015.02.014.
Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2016;174(2):296–304. https://doi.org/10.1111/bjd.14207.
Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-Interleukin-31 receptor a antibody for atopic dermatitis. N Engl J Med. 2017;376(9):826–35. https://doi.org/10.1056/NEJMoa1606490.
Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol. 2018;142(4):1121-30.e7. https://doi.org/10.1016/j.jaci.2018.03.018.
Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145(1):173–82. https://doi.org/10.1016/j.jaci.2019.08.013.
Kabashima K, Matsumura T, Komazaki H, Kawashima M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. 2020;383(2):141–50. https://doi.org/10.1056/NEJMoa1917006.
Ständer S, Yosipovitch G, Legat FJ, Lacour JP, Paul C, Narbutt J, et al. Trial of nemolizumab in moderate-to-severe Prurigo Nodularis. N Engl J Med. 2020;382(8):706–16. https://doi.org/10.1056/NEJMoa1908316.
Kiniksa Announces Phase 2 Clinical Trial of Vixarelimab (KPL-716) in Prurigo Nodularis Meets Primary Efficacy Endpoint. kiniksa.com. https://investors.kiniksa.com/news-releases/news-release-details/kiniksa-announces-phase-2-clinical-trial-vixarelimab-kpl-716. Accessed 1 Sep 2020.
Souza CP, Rosychuk RAW, Contreras ET, Schissler JR, Simpson AC. A retrospective analysis of the use of lokivetmab in the management of allergic pruritus in a referral population of 135 dogs in the western USA. Vet Dermatol. 2018;29(6):489-e164. https://doi.org/10.1111/vde.12682.
Michels GM, Ramsey DS, Walsh KF, Martinon OM, Mahabir SP, Hoevers JD, et al. A blinded, randomized, placebo-controlled, dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client owned dogs with atopic dermatitis. Vet Dermatol. 2016;27(6):478-e129. https://doi.org/10.1111/vde.12376.
Acknowledgements
We thank Drs. Sylvie Gabriel and Rajeev Chavda, employees of Galderma SA, for their guidance and input based on prior studies on nemolizumab.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Dr. Kwatra has received grant funding from Pfizer Inc., Kiniksa Pharmaceuticals, and Galderma.
Conflict of Interest
Dr. Shawn G. Kwatra is on the advisory board for Abbvie, Incyte Corporation, Galderma, Menlo Therapeutics, Pfizer Inc., and Regeneron Pharmaceuticals, and has received grant funding from Pfizer Inc., Galderma SA, and Kiniksa Pharmaceuticals. He is also a recipient of a Dermatology Foundation Medical Dermatology Career Development Award. The other author(s) have no conflicts of interest to declare.
Ethics Approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material (Data Transparency)
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code Availability
Not applicable.
Author Contributions
YSR: study design, data collection, manuscript writing. JC, NS, MB, SG, RC, MMK: manuscript editing. SGK: overall supervision, study design, data collection, manuscript editing. All authors read and approved the final version. The content in this manuscript has not been published or submitted for publication elsewhere.
Rights and permissions
About this article
Cite this article
Roh, Y.S., Choi, J., Sutaria, N. et al. IL-31 Inhibition as a Therapeutic Approach for the Management of Chronic Pruritic Dermatoses. Drugs 81, 895–905 (2021). https://doi.org/10.1007/s40265-021-01521-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01521-1