Abstract
Purpose of the review
There are numerous causes of chronic pruritus (itch) which is associated with a variety of inflammatory skin diseases, including many primarily extracutaneous disorders. This review provides an overview of typical skin and systemic diseases involving pruritus. We focus on relevant molecular mechanisms of pruritus and the current therapeutic strategies.
Recent findings
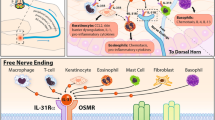
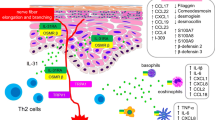
IL-31, released by eosinophil granulocytes, may play an important role in the neuromodulation of pruritus. Consequently, the anti-IL-31 antibody nemolizumab shows promising results in clinical trials. The novel IL-4 receptor antagonist dupilumab is already approved for the treatment of atopic dermatitis and significantly inhibits inflammation and pruritus. Further, novel treatment approaches include the H4-receptor pathway which has been successfully investigated in a recent clinical trial in patients with atopic dermatitis.
Summary
The emergence, transmission, and perception of pruritus are complex processes that greatly depend on the underlying condition. As more and more specific molecular pathways of these processes are understood, the development of effective treatment strategies for pruritus is becoming increasingly available. Future research should thus focus on the less well-understood forms of pruritus and their underlying mechanisms.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stander S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87(4):291–4.
Stander S, Stander HF, Steinke S, Bruland P, Dugas M, Augustin M. Chronic pruritus: care in daily practice. Hautarzt. 2016;67(8):640–7.
Jackson EM. Itch: Mechanisms and management of pruritus, edited by J. D. Bernhard, McGraw Hill, New York, 1994. J Toxicol Cutan Ocul Toxicol. 2008;14(4):309–10.
Stander S, Zeidler C, Augustin M, Bayer G, Kremer AE, Legat FJ, et al. S2k-Leitlinie zur Diagnostik und Therapie des chronischen Pruritus - Update - Kurzversion. J Dtsch Dermatol Ges. 2017;15(8):860–73.
Marron SE, Tomas-Aragones L, Boira S, Campos-Rodenas R. Quality of life, emotional wellbeing and family repercussions in dermatological patients experiencing chronic itching: a pilot study. Acta Derm Venereol. 2016;96(3):331–5.
Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: disease burden, measurement, and treatment benefit. Am J Clin Dermatol. 2016;17(2):163–9.
Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26(2):84–91.
Greaves MW, Khalifa N. Itch: more than skin deep. Int Arch Allergy Immunol. 2004;135(2):166–72.
Yosipovitch G, Greaves MW, Schmelz M. Itch Lancet. 2003;361(9358):690–4.
Krause L, Shuster S. Mechanism of action of antipruritic drugs. Br Med J (Clin Res Ed). 1983;287(6400):1199–200.
Nakagawa H, Hiura A. Four possible itching pathways related to the TRPV1 channel, histamine, PAR-2 and serotonin. Malay J Med Sci. 2013;20(4):5.
Cataldi M, Borriello F, Granata F, Annunziato L, Marone G. Histamine receptors and antihistamines: from discovery to clinical applications. Chem Immunol Allergy. 2014;100:214-26.
Hill S, Ganellin C, Timmerman H, Schwartz J, Shankley N, Young J, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49(3):253–78.
•• Werfel T, Layton G, Yeadon M, Whitlock L, Osterloh I, Jimenez P, et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(5):1830-7. This is a novel and excellently performed clinical trial showing the efficacy of targeting H4 receptor in AD with reduction of eczema and pruritus.
Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33(12):550–8.
Stander S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol. 2003;139(11):1463–70.
Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8.
Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89(5):2441–8.
Reddy VB, Iuga AO, Shimada SG, RH LM, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28(17):4331–5.
Namer B, Reeh P. Scratching an itch. Nat Neurosci. 2013;16(2):117–8.
Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64(1):129–38.
Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7(7):535–47.
Cevikbas F, Braz JM, Wang X, Solorzano C, Sulk M, Buhl T, et al. Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J Allergy Clin Immunol. 2017;140(2):454–64 e2.
Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142(2):374–80.
Sugimoto Y, Iba Y, Nakamura Y, Kayasuga R, Kamei C. Pruritus-associated response mediated by cutaneous histamine H3 receptors. Clin Exp Allergy. 2004;34(3):456–9.
Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6(2):151–8.
Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56(4):1391–400.
Schmelz M, Hilliges M, Schmidt R, Orstavik K, Vahlquist C, Weidner C, et al. Active “itch fibers” in chronic pruritus. Neurology. 2003;61(4):564–6.
Schmelz M. Itch and pain differences and commonalities. Handb Exp Pharmacol. 2015;227:285–301.
Rosenberg HF, Dyer KD. Eosinophil cationic protein and eosinophil-derived neurotoxin. Evolution of novel function in a primate ribonuclease gene family. J Biol Chem. 1995;270(50):30234.
Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011;6(7):e22029.
Noga O, Englmann C, Hanf G, Grutzkau A, Guhl S, Kunkel G. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin Exp Allergy. 2002;32(9):1348–54.
Hon KL, Lam MC, Wong KY, Leung TF, Ng PC. Pathophysiology of nocturnal scratching in childhood atopic dermatitis: the role of brain-derived neurotrophic factor and substance P. Br J Dermatol. 2007;157(5):922–5.
Raap U, Goltz C, Deneka N, Bruder M, Renz H, Kapp A, et al. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol. 2005;115(6):1268–75.
Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99(6):2214–20.
Takano N, Sakurai T, Kurachi M. Effects of anti-nerve growth factor antibody on symptoms in the NC/Nga mouse, an atopic dermatitis model. J Pharmacol Sci. 2005;99(3):277–86.
Raap U, Deneka N, Bruder M, Kapp A, Wedi B. Differential up-regulation of neurotrophin receptors and functional activity of neurotrophins on peripheral blood eosinophils of patients with allergic rhinitis, atopic dermatitis and nonatopic subjects. Clin Exp Allergy. 2008;38(9):1493–8.
O'Reilly M, Alpert R, Jenkinson S, Gladue RP, Foo S, Trim S, et al. Identification of a histamine H4 receptor on human eosinophils--role in eosinophil chemotaxis. J Recept Signal Transduct Res. 2002;22(1–4):431–48.
Tedeschi A, Asero R, Marzano AV, Lorini M, Fanoni D, Berti E, et al. Plasma levels and skin-eosinophil-expression of vascular endothelial growth factor in patients with chronic urticaria. Allergy. 2009;64(11):1616–22.
Krause K, Krull C, Kessler B, Lange-Asschenfeldt B, Maurer M, Metz M. Effective control of recalcitrant pruritus by bevacizumab: a possible role for vascular endothelial growth factor in chronic itch? Acta Derm Venereol. 2013;93(2):175–9.
Sakamoto M, Miyagaki T, Kamijo H, Oka T, Takahashi N, Suga H, et al. Serum vascular endothelial growth factor A levels reflect itch severity in mycosis fungoides and Sezary syndrome. J Dermatol. 2018;45(1):95–9.
Kunsleben N, Rudrich U, Gehring M, Novak N, Kapp A, Raap U. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J Investig Dermatol. 2015;135(7):1908–11.
Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117(2):418–25.
Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010;184(7):3526–34.
Raap U, Gehring M, Kleiner S, Rudrich U, Eiz-Vesper B, Haas H, et al. Human basophils are a source of - and are differentially activated by - IL-31. Clin Exp Allergy. 2017;47(4):499–508.
Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Gronhoj-Larsen C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18(1):35–43.
•• Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138(2):500–8 e24 In this study, primary sensory neuron cultures were stimulated with IL-31 which leads to elongation and branching of sensory nerves. Further, using wild-type, IL-31-transgenic, and IL-31 pump-equipped mice, the authors could confirm their in vitro findings in vivo.
Arai I, Tsuji M, Miyagawa K, Takeda H, Akiyama N, Saito S. Repeated administration of IL-31 upregulates IL-31 receptor A (IL-31RA) in dorsal root ganglia and causes severe itch-associated scratching behaviour in mice. Exp Dermatol. 2015;24(1):75–8.
Arai I, Tsuji M, Takeda H, Akiyama N, Saito S. A single dose of interleukin-31 (IL-31) causes continuous itch-associated scratching behaviour in mice. Exp Dermatol. 2013;22(10):669–71.
Cedeno-Laurent F, Singer EM, Wysocka M, Benoit BM, Vittorio CC, Kim EJ, et al. Improved pruritus correlates with lower levels of IL-31 in CTCL patients under different therapeutic modalities. Clin Immunol. 2015;158(1):1–7.
Raap U, Weissmantel S, Gehring M, Eisenberg AM, Kapp A, Folster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol. 2012;23(3):285–8.
Raap U, Wichmann K, Bruder M, Stander S, Wedi B, Kapp A, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122(2):421–3.
Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2016;174(2):296–304.
Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol. 2018;142(4):1121–30 e7.
• Ruzicka T, Mihara R. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376(21):2093 This phase 2 clinical trial showed a significant reduction of pruritus after targeting the interleukin-31 receptor A in patients with AD.
Rudrich U, Gehring M, Papakonstantinou E, Illerhaus A, Engmann J, Kapp A, et al. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Derm Venereol. 2018;98(8):766–71.
Raap U, Wieczorek D, Gehring M, Pauls I, Stander S, Kapp A, et al. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp Dermatol. 2010;19(5):464–6.
Hartmann K, Wagner N, Rabenhorst A, Pflanz L, Leja S, Forster A, et al. Serum IL-31 levels are increased in a subset of patients with mastocytosis and correlate with disease severity in adult patients. J Allergy Clin Immunol. 2013;132(1):232–5.
Nattkemper LA, Martinez-Escala ME, Gelman AB, Singer EM, Rook AH, Guitart J, et al. Cutaneous T-cell lymphoma and pruritus: the expression of IL-31 and its receptors in the skin. Acta Derm Venereol. 2016;96(7):894–8.
Jackson S, Gilchrist H, Nesbitt LT Jr. Update on the dermatologic use of systemic glucocorticosteroids. Dermatol Ther. 2007;20(4):187–205.
Wahlgren CF, Scheynius A, Hagermark O. Antipruritic effect of oral cyclosporin A in atopic dermatitis. Acta Derm Venereol. 1990;70(4):323–9.
Viegas L, Ferreira M, Kaplan A. The maddening itch: an approach to chronic urticaria. J Investig Allergol Clin Immunol. 2014;24(1):1–5.
Kim J-M, Lim K-M, Kim H-S, Ko H-C, Kim M-B, Kim B-S. Urticarial dermatitis: clinical characteristics of itch and therapeutic response to cyclosporine. Ann Dermatol. 2017;29(2):143–8.
Nakahara T, Morimoto H, Murakami N, Furue M. Mechanistic insights into topical tacrolimus for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 2018;29(3):233–8.
Martins JC, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015;7.
Şavk E. Neurologic itch management. Itch-management in clinical practice.Curr Probl Dermatol. 2016;50:116-23.
Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60(5):693–6.
Farah CS, Badal T, Reed N, Rogers PG, King GG, Thamrin C, et al. Mepolizumab improves small airway function in severe eosinophilic asthma. Respir Med. 2019;148:49–53.
Blakely K, Gooderham M, Papp K. Dupilumab, a monoclonal antibody for atopic dermatitis: a review of current literature. Skin Therapy Lett. 2016;21(2):1–5.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48.
Kraft M, Worm M. Dupilumab in the treatment of moderate-to-severe atopic dermatitis. Expert Rev Clin Immunol. 2017;13(4):301–10.
Vangipuram R, Tyring SK. Dupilumab for moderate-to-severe atopic dermatitis. Center Clin Stud. 2017;1795.
Mollanazar NK, Elgash M, Weaver L, Valdes-Rodriguez R, Hsu S. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155(1):121–2.
Duval A, Dubertret L. Aprepitant as an antipruritic agent? N Engl J Med. 2009;361(14):1415–6:4790810.
He A, Alhariri JM, Sweren RJ, Kwatra MM, Kwatra SG. Aprepitant for the treatment of chronic refractory pruritus. Biomed Res Int. 2017;2017.
Maronas-Jimenez L, Estrach T, Gallardo F, Perez A, Andres Borja H, Servitje O, et al. Aprepitant improves refractory pruritus in primary cutaneous T-cell lymphomas: experience of the Spanish Working Group on Cutaneous Lymphomas. Br J Dermatol. 2018;178(4):e273–e4.
Jimenez Gallo D, Albarran Planelles C, Linares Barrios M, Fernandez Anguita MJ, Marquez Enriquez J, Rodriguez Mateos ME. Treatment of pruritus in early-stage hypopigmented mycosis fungoides with aprepitant. Dermatol Ther. 2014;27(3):178–82.
Tsianakas A, Zeidler C, Riepe C, Borowski M, Forner C, Gerss J, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99(4):379-85.
Huang K, Hu DD, Bai D, Wu ZY, Chen YY, Zhang YJ, et al. Persistent extracellular signal-regulated kinase activation by the histamine H4 receptor in spinal neurons underlies chronic itch. J Investig Dermatol. 2018;138(8):1843–50.
Solak Y, Biyik Z, Atalay H, Gaipov A, Guney F, Turk S, et al. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: a prospective, crossover study. Nephrology (Carlton). 2012;17(8):710–7.
Wallengren J. Treatment of notalgia paresthetica with topical capsaicin. J Am Acad Dermatol. 1991;24(2 Pt 1):286–8.
Pereira MP, Luling H, Dieckhofer A, Steinke S, Zeidler C, Agelopoulos K, et al. Application of an 8% capsaicin patch normalizes epidermal TRPV1 expression but not the decreased intraepidermal nerve fibre density in patients with brachioradial pruritus. J Eur Acad Dermatol Venereol. 2018;32(9):1535–41.
Pereira MP, Stander S. Chronic pruritus: current and emerging treatment options. Drugs. 2017;77(9):999–1007.
Funding Sources
Ulrike Raap is supported by a research grant of the DFG RA 1026/3-1 for the Research Group Translational Pruritus Research. Nika Kotnik is supported by a PhD studentship funded by the University of Oldenburg (Oldenburg-Groningen Joint PhD Programme)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
N. Helge Meyer declares that he has no conflict of interest. Nika Kotnik declares that she has no conflict of interest. Volker Meyer declares that he has no conflict of interest. Bernhard F. Gibbs declares that he has no conflict of interest. Ulrike Raap declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Urticaria and Atopic Dermatitis
Rights and permissions
About this article
Cite this article
Meyer, N.H., Kotnik, N., Meyer, V. et al. The Complexity of Pruritus Requires a Variety of Treatment Strategies. Curr Treat Options Allergy 6, 189–199 (2019). https://doi.org/10.1007/s40521-019-00217-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-019-00217-y