Abstract
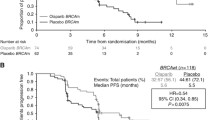
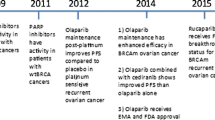
The use of poly (ADP-ribose) polymerase (PARP) inhibitors in the front-line management of advanced ovarian cancer has recently emerged as an exciting strategy with the potential to improve outcomes for patients with advanced ovarian cancer. In this article, we review the results of four recently published Phase III randomised controlled trials evaluating the use of PARP inhibitors in the primary treatment of ovarian cancer (SOLO1, PRIMA, PAOLA-1, and VELIA). Collectively, the studies suggest that PARP maintenance in the upfront setting is most beneficial among patients with BRCA-associated ovarian cancers (hazard ratios range from 0.31 to 0.44), followed by patients with tumours that harbour homologous recombination deficiencies (hazard ratios range from 0.33 to 0.57). All three studies that included an all-comer population were able to demonstrate benefit of PARP inhibitors, regardless of biomarker status. The FDA has approved olaparib for front-line maintenance therapy among patients with BRCA-associated ovarian cancers, and niraparib for all patients, regardless of biomarker status. In determining which patients should be offered front-line maintenance PARP inhibitors, and which agent to use, there are multiple factors to consider, including FDA indication, dosing preference, toxicity, risks versus benefits for each patient population, and cost. There are ongoing studies further exploring the front-line use of PARP inhibitors, including the potential downstream effects of PARP-inhibitor resistance in the recurrent setting, combining PARP-inhibitors with other anti-angiogenic drugs, immunotherapeutic agents, and inhibitors of pathways implicated in PARP inhibitor resistance.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Bell D, Berchuck A, Birrer M, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. https://doi.org/10.1038/nature10166.
Konecny G, Kristeleit R. PARP inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: current practice and future directions. Br J Cancer. 2016;115:1157–73. https://doi.org/10.1038/bjc.2016.311.
McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109–15. https://doi.org/10.1158/0008-5472.can-06-0140.
Lheureux S, Lai Z, Dougherty BA, Runswick S, Hodgson DR, Timms KM, et al. Long-term responders on olaparib maintenance in high-grade serous ovarian cancer: clinical and molecular characterization. Clin Cancer Res. 2017;23(15):4086–94. https://doi.org/10.1158/1078-0432.ccr-16-2615.
Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicenter, open-label, single-arm, Phase 2 trial. Lancet Oncol. 2019;20(5):636–48. https://doi.org/10.1016/S1470-2045(19)30029-4.
Rafii S, Gourley C, Kumar R, Geuna E, Ern Ang J, et al. Baseline clinical predictors of antitumour response to the PARP inhibitor olaparib in germline BRCA1/2 mutated patients with advanced ovarian cancer. Oncotarget. 2017;8(29):47154–60. https://doi.org/10.18632/oncotarget.17005 .
Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, Gonzalez-Martin A, Aghajanian C, Bradley W, Mathews C, Liu J, Lowe ES, Bloomfield R, DiSilvestro P. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505.
Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D, Hoskins P, Freyer G, Baumann K, Jardon K, Redondo A, Moore RG, Vulsteke C, O’Cearbhaill RE, Lund B, Backes F, Barretina-Ginesta P, Haggerty AF, Rubio-Perez MJ, Shahin MS, Mangili G, Bradley WH, Bruchim I, Sun K, Malinowska IA, Li Y, Gupta D, Monk BJ. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402.
Berek JS, Matulonis UA, Peen U, Ghatage P, Mahner S, Redondo A, Lesoin A, Colombo N, Vergote I, Rosengarten O, Ledermann J, Pineda M, Ellard S, Sehouli J, Gonzalez-Martin A, Berton-Rigaud D, Madry R, Reinthaller A, Hazard S, Guo W, Mirza MR. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29(8):1784–92.
Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, Fujiwara K, Vergote I, Colombo N, Maenpaa J, Selle F, Sehouli J, Lorusso D, Guerra Alia EM, Reinthaller A, Nagao S, Lefeuvre-Plesse C, Canzler U, Scambia G, Lortholary A, Marme F, Combe P, de Gregorio N, Rodrigues M, Buderath P, Dubot C, Burges A, You B, Pujade-Lauraine E, Harter P. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28.
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83.
Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL, Cloven NG, Oaknin A, DiSilvestro PA, Morgan MA, Nam JH, Leath CA 3rd, Nicum S, Hagemann AR, Littell RD, Cella D, Baron-Hay S, Garcia-Donas J, Mizuno M, Bell-McGuinn K, Sullivan DM, Bach BA, Bhattacharya S, Ratajczak CK, Ansell PJ, Dinh MH, Aghajanian C, Bookman MA. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–15.
Stronach EA, Paul J, Timms KM, et al. Biomarker assessment of HR deficiency, tumour BRCA1/2 mutations, and CCNE1 copy number in ovarian cancer: associations with clinical outcome following platinum monotherapy. Mol Cancer Res. 2018;16:1103–11.
Hodgson DR, Dougherty BA, Lai Z, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer. 2018;119:1401–9.
Swisher EM, Birrer MJ, Moore KN, Coleman RL, Timms KM, et al. Exploring the relationship between homologous recombination score and progression-free survival in BRCA wildtype ovarian carcinoma: analysis of veliparib plus carboplatin/paclitaxel in the velia study. [abstract] In: Proceedings of the Society of Gynecologic Oncology 2020 Annual Meeting, 2020. Abstract LBA6.
LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15–28.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA 1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84.
Harrison RF, Fu S, Sun CC, Zhao H, Lu KH, Giordano SH, Meyer L. Out-of-pocket costs for PARP inhibitor treatment: are ovarian cancer patients at risk for financial toxicity? [abstract] In: Proceedings of the Society of Gynecologic Oncology 2020 Annual Meeting, 2020. Abstract 38.
Richardson MT, Kapp DS, Chan JE, Liang SY, Mann AK, et al. Upfront maintenance with anti-vascular versus PARP inhibitors in advanced ovarian cancer: a cost-effective analysis. [abstract] In: Proceedings of the Society of Gynecologic Oncology 2020 Annual Meeting, 2020. Abstract 39.
Rottenberg S, Jaspers JE, Kersbergen A, Van der Burg E, Nygren AOH, Zander SAL, et al. High sensitivity of BRCA1-deficient mammary tumours to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci. 2008;105(44):17079–84.
Kondroashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the parp inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7(9):984–98.
Chaudhuri AR, Callen E, Ding X, Gogola E, Duarte AA, Lee JE, et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature. 2016;535:382–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Dr Westin is supported by NIH SPORE in Ovarian Cancer (1P50CA217685-01) and a GOG Foundation Scholar Investigator Award. The funding sources had no involvement in study design, data collection, or writing of the manuscript.
Conflict of interest
SNW is a consultant for AstraZeneca, Circulogene, Clovis Oncology, Eisai, GSK/Tesaro, Merck, Novartis, Pfizer, Roche/Genentech and Zentalis. SNW receives research support from ArQule, AstraZeneca, Bayer, Clovis, Cotinga Pharmaceuticals, Novartis, Roche/Genentech, and GSK/Tesaro. RLC is a consultant for AstraZeneca, Clovis Oncology, GSK/Tesaro, Novartis, Roche/Genentech, Eisai, Merck, Pfizer, Novocure, Genmab, Gamamab, Oncosec, Tarveda. RLC receives research funding from AbbVie, Genmab, Merck, AstraZeneca, Clovis Oncology, Roche/Genentech. MO has no conflicts of interest to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors participated in conception, drafting of manuscript and final approval of manuscript.
Rights and permissions
About this article
Cite this article
Onstad, M., Coleman, R.L. & Westin, S.N. Movement of Poly-ADP Ribose (PARP) Inhibition into Frontline Treatment of Ovarian Cancer. Drugs 80, 1525–1535 (2020). https://doi.org/10.1007/s40265-020-01382-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01382-0