Abstract
The terminal complement protein (C5) inhibitor eculizumab (Soliris®) is the first agent to be specifically approved in the EU, USA, Canada and Japan for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults who are aquaporin-4 water channel autoantibody (AQP4-IgG) seropositive and (in the EU only) for those with a relapsing course of disease. In the phase III PREVENT trial, eculizumab significantly reduced the risk of adjudicated relapse relative to placebo in patients with AQP4-IgG-seropositive NMOSD, approximately a quarter of whom did not receive concomitant immunosuppressive therapies. The beneficial effect of eculizumab was seen across all patient subgroups analysed and was accompanied by improvements in neurological and functional disability assessments, as well as generic health-related quality of life measures; it was sustained through 4 years of treatment, according to combined data from the PREVENT trial and an interim analysis of its ongoing open-label extension study. The safety profile of eculizumab in AQP4-IgG-seropositive NMOSD was consistent with that seen for the drug in other approved indications. Thus, eculizumab provides an effective, generally well tolerated and approved treatment option for this rare, disabling and potentially life-threatening condition.
Video abstract
Video Abstract (MP4 241640 KB)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First agent to be specifically approved for this rare, disabling and potentially life-threatening condition |
Reduces relapse risk, including in patients not receiving other immunosuppressive therapies |
Common adverse events include upper respiratory tract infection, headache, nasopharyngitis and nausea |
Patients must be vaccinated against meningococcal disease (and administered appropriate prophylactic antibiotics if necessary) |
1 Introduction
Neuromyelitis optica spectrum disorder (NMOSD), a well-defined, auto-immune, demyelinating disease of the CNS, is a potentially severely disabling and life-threatening condition (reported mortality rate 7–32%) [1,2,3,4,5,6]. NMOSD is mostly characterized by recurrent attacks of optic neuritis and/or myelitis from which recovery is often incomplete, thus resulting in residual and accumulating impairment (e.g. blindness and paraplegia) [1,2,3,4]. However, variations are seen in clinical course, with some patients (a minority) not accumulating significant disability despite multiple attacks and others only ever experiencing a single attack [7]. NMOSD is a relatively rare disorder that is reported more frequently in non-whites and, in its relapsing form, in women (5- to 11-times more often than in men) [1, 2]. Patients ranging from 3 to 80 years of age have been diagnosed with the disease, although the average age at onset is 40 years [1].
Previously considered a variant of multiple sclerosis, recognition of NMOSD as a separate clinical entity and its differential diagnosis was revolutionized by the discovery of a disease-specific, pathogenic immunoglobulin G (IgG) autoantibody directed against the aquaporin-4 water channel (AQP4) in the serum of ≈ 75–90% of patients. Detection of AQP4-IgG greatly facilitates accurate identification of new cases (less stringent diagnostic criteria apply in AQP4-IgG-seropositive patients) and prompt initiation of treatment [1, 4, 7,8,9]. Interestingly, a sizeable minority of APQ4-IgG seronegative patients presenting with symptoms of NMOSD display an autoantibody against myelin oligodendrocyte glycoprotein (MOG); increasingly, MOG-IgG is being regarded as a potentially pathogenic biomarker for a similar, but separate, disease entity (anti-MOG syndrome), rather than for a subgroup of NMOSD patients [1, 3, 10].
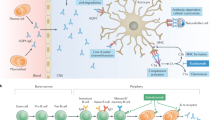
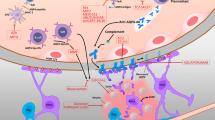
In AQP4-IgG-seropositive patients with NMOSD, AQP4-IgG is thought to enter the CNS through endothelial transcytosis or at areas of increased blood–brain barrier (BBB) permeability and bind to AQP4, which is primarily expressed on astrocyte foot processes that form part of the BBB. This leads to either the injury of astrocytes [predominantly via complement-dependent cytotoxicity (CDC), but potentially also via antibody-dependent cellular cytotoxicity (ADCC) when natural-killer cells are present] or to their activation. The ensuing BBB breakdown and secretion of pro-inflammatory cytokines and chemokines results in the recruitment of granulocytes, macrophages and eosinophils that further disrupt the BBB and secondarily damage oligodendrocytes (which do not express AQP4), causing demyelination, neuron loss and consequential neurological deficit [1, 11,12,13,14]. Additionally, through ‘bystander’ mechanisms, APQ4-IgG-induced CDC and ADCC in astrocytes may also damage oligodendrocytes and neurons directly [15, 16].
Given the disability progression in NMOSD is largely due to the consequences of recurrent attacks, the goals of pharmacotherapy are to aggressively treat acute inflammatory attacks (including the initial episode) and to prevent future relapses, with the overarching aims of minimizing CNS damage and, longer-term, preserving neurological function [1, 8, 17]. The current approach to treating acute attacks usually consists of a short course of high-dose intravenous (IV) corticosteroids (commonly followed by a tapering course of oral corticosteroids), with prompt intervention with plasmapheresis [e.g. plasma exchange (PLEX)] in the case of attacks that do not respond to high-dose IV corticosteroids within the expected time-frame [1, 8, 17]. To date, relapse prevention has most commonly relied on maintenance immunosuppression with the IV anti-CD20 monoclonal antibody rituximab, the oral purine analog anti-metabolites mycophenolate mofetil and azathioprine, and, to a lesser extent, oral corticosteroids [1, 8, 17]. However, none of these agents are specifically approved for the treatment of NMOSD and their off-label use is almost entirely supported by the results of uncontrolled observational studies [1, 8, 17]. Moreover, between a third and three-quarters of patients receiving these agents in such studies have not remained relapse-free [1], highlighting an urgent need for additional preventive treatments with proven effectiveness [18].
The identification of the central role of the complement cascade in the pathogenesis of AQP4-IgG-seropositive NMOSD provided a rationale for evaluating an inhibitor of this system in the preventive treatment of the disease [17]. Eculizumab (Soliris®), a humanized monoclonal antibody (mAb), targets human complement component 5, to which it binds with high affinity, thereby inhibiting activation of the terminal complement pathway [19]. In the EU, eculizumab is approved for the treatment of NMOSD in adult patients who are AQP4-IgG-seropositive with a relapsing course of disease [20]. Similarly, in the USA [21], Canada [22] and Japan [23], eculizumab is approved for the treatment of NMOSD in adult patients who are AQP4-IgG-seropositive. This article reviews clinical efficacy and tolerability data relevant to the use of eculizumab in the treatment of AQP4-IgG-seropositive NMOSD, with the key pharmacological properties of the drug summarized in Table 1. Eculizumab is also approved for the treatment of paroxysmal nocturnal haemoglobinuria and atypical haemolytic uremic syndrome (in adults and children [20]), as well as for the treatment of refractory generalized myasthenia gravis in adults [21] who are anti-acetylcholine receptor antibody-positive [20, 21]. However, the use of the drug in these additional indications, which has been discussed in detail elsewhere [24,25,26], is beyond the scope of this article.
2 Therapeutic Efficacy of Eculizumab
The efficacy of eculizumab in the treatment of AQP4-IgG-seropositive NMOSD has been evaluated in the phase III PREVENT study (Prevention of Relapses in Neuromyelitis Optica) [27]. This pivotal, randomized, double-blind, placebo-controlled, multinational, time-to-event trial in 143 patients with AQP4-IgG-seropositive disease was conducted on the basis of encouraging findings from an earlier pilot study in 14 AQP4-IgG–seropositive patients [28]. The PREVENT study enrolled individuals aged ≥ 18 years with NMOSD according to 2007 diagnostic criteria [29] (or neuromyelitis optica according to 2006 diagnostic criteria [30]) and confirmed AQP4-IgG seropositivity. They were also required to have a history of ≥ 2 relapses during the previous 12 months or 3 relapses during the previous 24 months (with ≥ 1 relapse in the previous 12 months) and an Expanded Disability Status Scale (EDSS) score of ≤ 7. Patients receiving immunosuppressive therapies (ISTs) for relapse prevention were eligible for inclusion, provided they were on stable-dose regimens [27]. Key exclusion criteria included treatment with mitoxantrone or rituximab ≤ 3 months prior to screening, the use of IV immunoglobulin (IVIg) ≤ 3 weeks prior to screening, the receipt of prednisone doses > 20 mg/day (or equivalent) at screening, and the presence of a systemic bacterial or other infection that was considered to be clinically significant or inappropriately treated. Unresolved meningococcal disease was another exclusion criterion. Patients were vaccinated against Neisseria meningitidis in accordance with the then prevailing Advisory Committee on Immunization Practices (ACIP) recommendations prior to receiving a study medication, unless a previous vaccination provided adequate coverage; a 14-day course of antibiotics was administered if there were < 14 days between vaccination and starting trial treatment [27].
Participants were stratified by EDSS score and use of concomitant ISTs before being randomized to receive eculizumab (900 mg weekly for the first four doses then 1200 mg every 2 weeks from the following week; n = 96) or matching placebo (n = 47) administered via IV infusion over ≈ 35 (range 25–45) minutes. IVIg and PLEX were permitted as acute rescue therapies in the event of relapse [27].
The primary endpoint was (time to) the first adjudicated on-trial relapse. Briefly, treating physicians identified relapses on the basis of the following criteria: a new onset of neurological symptoms or worsening of existing neurological symptoms, with an objective change on neurological examination that persisted for > 24 h, signs and symptoms attributable to NMOSD rather than other causes, and onset preceded by ≥ 30 days of clinical stability. All physician-identified relapses were then adjudicated retrospectively using the same criteria by a blinded independent committee. Patients continued with their blinded treatment for 6 weeks following a relapse [27]. The primary endpoint and six pre-specified secondary efficacy endpoints were tested in a hierarchical order. Regarding the latter, the adjudicated annualized relapse rate (ARR) was followed by two assessments of neurological disability [EDSS and Modified Rankin Scale (mRS) score], one of functional disability [Hauser Ambulation Index (HAI) score] and two of generic health-related quality of life [European Quality of Life 5-Dimension 3-Level (EQ-5D-3L) visual analogue scale and EQ-5D-3L summary index score] [27].
Patients who completed the core study could enter an open-label extension (OLE) of PREVENT (NCT02003144), where, following a 4-week, blinded induction phase, they received maintenance therapy with eculizumab 1200 mg every 2 weeks; their concomitant ISTs could be changed at the discretion of the treating physician [27]. Some results of these trials are available from abstracts/posters [31,32,33,34,35,36,37].
At baseline in PREVENT, patients had a mean age of 44.3 years, a mean ARR during the previous 24 months of 1.99, and median scores on the EDSS, mRS and HAI that indicated moderate-to-severe disability. Overall, the vast majority (91%) were women, and three-quarters (76%) were receiving concomitant ISTs (glucocorticoids and/or another drug). Approximately one-third (32%) had received rituximab, albeit ≥ 3 months prior to screening. The two treatment groups were generally well balanced in terms of demographic and clinical characteristics [27].
Eculizumab significantly reduced the risk of the primary endpoint of adjudicated relapse relative to placebo in PREVENT. The trial was terminated after 23 of 24 pre-specified adjudicated relapses (there was uncertainty in estimating when the final event would occur); these relapses occurred in 3 (3%) of the 96 patients in the eculizumab group and 20 (43%) of the 47 patients in the placebo group [hazard ratio (HR) 0.06; 95% CI 0.02–0.20; p < 0.001] (Table 2) [27]. The proportions of eculizumab- and placebo-treated patients who remained relapse-free were, respectively, 97.9% and 63.2% at week 48, 96.4% and 51.9% at week 96, and 96.4% and 45.4% at week 144 (Kaplan–Meier estimates) [27].
In post hoc analyses, the treatment effect of eculizumab on adjudicated relapse risk reduction was robust across all pre-specified subgroups analysed [31]. These subgroups included those based on age (< 45 years; ≥ 45 years), sex, geographical region (Americas; Asia–Pacific; Europe) and ethnicity (Asian; Black or African American; White) [31]. Among Asian patients, an estimated 97.3% of those treated with eculizumab (n = 37) versus 72.2% of those treated with placebo (n = 15) were free from relapse at week 48 (HR 0.054; 95% CI 0.007–0.453; p = 0.0002) [36]. In terms of baseline demographics and concomitant IST use, the Asian subgroup was similar to the overall study population [36].
Likewise, the treatment effect of eculizumab on adjudicated relapse risk reduction was robust across pre-specified subgroups based on prior rituximab use (yes; no) and baseline IST use [corticosteroids alone; azathioprine (with or without corticosteroids); mycophenolate mofetil (with or without corticosteroids); other] [31]. Among patients not receiving concomitant ISTs at baseline, adjudicated relapses occurred in none (0%) of 21 eculizumab recipients versus 7 (54%) of 13 placebo recipients (p < 0.0001) [31]. Similar results were seen in a post hoc analysis of all patients receiving concomitant ISTs at baseline (albeit not a pre-defined subgroup), with adjudicated relapses occurring in 3 (4%) of 75 eculizumab recipients versus 13 (38%) of 34 placebo recipients (between-group statistical comparison not reported) [27].
Regarding the six pre-specified secondary endpoints, the adjudicated ARR significantly favoured eculizumab over placebo (rate ratio 0.04; 95% CI 0.01–0.15; p < 0.001) (Table 2) [27]. However, the mean change from baseline in EDSS score, the next pre-specified secondary endpoint to be tested hierarchically, did not differ significantly between the two groups; hence, no statistical inferences based on p-values could be made for the remaining pre-specified assessments of disability and quality of life in the sequence (Table 2) [27]. The observed lack of improvement in the EDSS score with eculizumab could have reflected, in part, the short (6-week) period of post-relapse follow-up prior to entering the OLE, as per the trial design.
The results of the hierarchical testing strategy notwithstanding, improvements in disability and quality of life endpoints assessed as secondary or tertiary outcomes consistently favoured eculizumab over placebo (Table 2) [27, 32, 33]. In post hoc analyses of disability worsening, significantly fewer eculizumab than placebo recipients experienced a protocol-defined increase in EDSS score (11.5 vs. 23.4%; p < 0.05) or HAI score (8.3 vs. 23.4%; p < 0.01) between baseline and study end [32]. Also in post hoc analyses, changes from baseline to study end in the proportions of patients reporting problems significantly (p < 0.05) favoured eculizumab over placebo for 3 of the 5 EQ-5D-3L dimension scores, namely self-care, pain/discomfort and usual activities [33]. Generic health-related quality of life was further assessed using the 36-item Short-Form Health Survey (SF-36); improvements between baseline and study end were significantly (p < 0.05) greater with eculizumab than placebo for the physical component score (PCS) and 2 of the 8 scales, namely physical functioning and role limitations due to physical health [33]. Based on a categorical analysis of clinically meaningful changes in SF-36 score, eculizumab, compared with placebo, significantly (p < 0.05) decreased the probability of PCS deterioration and increased the probability of PCS improvement [33].
Interestingly, in terms of the impact on healthcare resource utilization, eculizumab relative to placebo significantly reduced the annualized rate of adjudicated relapse-related hospitalization (0.012 vs. 0.267; p < 0.0001), as well as the annualized rates of use of IV methylprednisolone (0.012 vs. 0.286; p < 0.0001), high-dose oral corticosteroids (0.012 vs. 0.114; p = 0.0021) and PLEX (0.012 vs. 0.134; p = 0.0006) in relation to the acute treatment of such relapses [34].
The observed durable efficacy of eculizumab in the core study has been supported by findings from the ongoing OLE. According to the most recent interim analysis (database cut-off date of 31 October 2018), 119 patients had been enrolled and treated in the OLE (78 and 41 who had previously received eculizumab and placebo, respectively, in PREVENT); the median duration on treatment during the study was 20 (range 0.1–198) weeks. Relative to the historical ARR (i.e. based on the 24 months prior to screening in PREVENT; mean value: 2.013), the median reduction in the primary efficacy endpoint of the on-trial, physician-determined ARR [− 1.829 (minimum, maximum: − 6.38, 1.63)] represented a statistically significant (p < 0.0001) and clinically meaningful effect of eculizumab [18]. The majority (95.8%) of patients in the OLE did not experience an on-trial, adjudicated relapse, with an estimated 94.6%, 91.1% and 91.1% of patients being free from adjudicated relapses at weeks 48, 96 and 144, respectively [18].
Combined data from patients receiving at least one dose of eculizumab in PREVENT or the OLE (based on the same database cut-off date of 31 October 2018) are also available [35, 37]. According to this pooled analysis, only 8 (5.8%) out of a total of 137 patients who were treated with eculizumab and followed for a median of 107.8 (range 5.1–237.9) weeks experienced an on-trial, adjudicated relapse [three in PREVENT and five in the OLE (one patient had two relapses)]. An estimated 96.8%, 93.9%, 93.9% and 93.9% of patients were relapse-free at weeks 48, 96, 144 and 192, respectively, and the adjudicated ARR was 0.032 (95% CI 0.017–0.062) [35]. On-trial, adjudicated relapse findings for the Asian subgroup (n = 50) were consistent with those for the overall study population, with an estimated 95.2% of Asian patients being free from relapse at week 192 [37].
3 Tolerability of Eculizumab
Eculizumab has been generally well tolerated in the short- and longer-term in patients with AQP4-IgG-seropositive NMOSD who received the drug in the PREVENT study and/or its OLE, which is ongoing [18, 27, 35] (Sect. 2). In PREVENT, similar proportions of eculizumab and placebo recipients (n = 96 and 47) reported treatment-emergent adverse events (TEAEs; 92% and 96%). However, overall exposure to treatment with eculizumab [in patient-years (PY)] was ≈ 3.3 times that to placebo [18]; the overall exposure-adjusted rates of TEAEs were 749 per 100 PY with eculizumab versus 1161 per 100 PY with placebo [745 vs. 1127 per 100 PY, when NMOSD relapses that met the definition of a serious AE (SAE) were excluded] [27]. The most common TEAEs (incidence ≥ 15% in either group; excluding NMOSD relapses) were upper respiratory tract infection [URTI; 29% (31 events per 100 PY) with eculizumab vs. 13% (19 events per 100 PY) with placebo], headache [23% (55) vs 23% (38)], nasopharyngitis [21% (29) vs. 19% (28)], nausea [17% (17) vs. 26% (36)], diarrhoea [16% (13) vs. 15% (36)], dizziness [15% (11) vs. 13% (11)], back pain [15% (10) vs. 13% (17)], urinary tract infection [UTI; 14% (26) vs. 21% (24)], limb pain [11% (8) vs. 21% (21)], cough [11% (8) vs. 15% (17)] and vomiting [10% (6) vs. 17% (19)]. Most TEAEs were mild to moderate in severity (97.5 and 96.4% with eculizumab and placebo, respectively) and considered to be unrelated to the study medication, as determined by the investigator (71.7% and 85.9%, respectively) [27].
Treatment-emergent SAEs (TESAEs) were reported by 31% and 55% of eculizumab- and placebo-treated patients (rates of 31 and 88 per 100 PY, respectively). The most common TESAE was NMOSD relapse meeting the definition of an SAE; this occurred in seven (7.3%) and 16 (34.0%) of eculizumab and placebo recipients (rates of 4 and 34 events per 100 PY, respectively). The only other TESAEs reported in more than one patient in the eculizumab group were pneumonia (n = 3; also reported in one patient in the placebo group), cellulitis (n = 2), sepsis (n = 2) and UTI (n = 2) [27].
The adverse event profile of eculizumab was consistent across all patient subgroups evaluated, regardless of previous use of rituximab or use of various different concomitant ISTs at baseline [31]. Similarly, the adverse event profile of eculizumab in the subgroup of Asian patients was generally consistent with that seen in the overall study population [36].
Due to its mechanism of action (Sect. 1), eculizumab increases the risk of meningococcal and encapsulated bacterial infection [20, 21]. In this regard, all PREVENT participants received meningococcal vaccination, and no cases of meningococcal infection were reported. Additionally, there were no cases of Aspergillosis [18]. Other serious infections occurred in 11 (11.5%) and 6 (12.8%) of eculizumab and placebo recipients (rates of 8 and 15 events per 100 PY, respectively) [18, 27].
No eculizumab recipient discontinued the PREVENT study due to TEAEs (including infusion-related reactions), although one (an active smoker with an extensive history of pulmonary disease who was receiving concomitant azathioprine) died from pulmonary empyema after 108 weeks in the trial [20, 21, 27]. This event was considered by the investigator as probably related to the trial agent [27]; however, the micro-organisms that were implicated (Streptococcus intermedius and Peptostreptococcus micros [27]) are not associated with complement deficiency. In comparison, two placebo recipients discontinued the study because of TEAEs (pneumonia in one patient and prerenal failure and pancytopenia in the other) [27]. As with all proteins, there is a potential for immunogenicity with eculizumab. However, both of the eculizumab recipients in PREVENT who showed an antidrug antibody response post-baseline were negative for neutralising antibodies; no correlation of antibody development to adverse events was observed [20] (Sect. 1). The long-term tolerability profile of eculizumab in the ongoing OLE (database cut-off date of 31 October 2018) was consistent with that observed in the core study [18]. In the pooled analysis of patients who received at least one dose of eculizumab in the PREVENT study and/or the OLE (n = 137; overall follow-up of 282.3 PY) [35], the rates of TEAEs and TESAEs were 763.1 and 37.6 per 100 PY (758.5 and 32.9 per 100 PY, excluding NMOSD relapses); findings for Asian patients were consistent with those for the overall study population [37]. The most common TEAEs (incidence ≥ 15%) included headache (27.0%), URTI (25.5%), nasopharyngitis (22.6%), UTI (16.8%), nausea (16.1%), back pain (15.3%) and diarrhoea (15.3%); the most common SAEs other than NMOSD relapses were pneumonia (2.9%), UTI (2.9%) and optic neuritis (2.2%). Of note, the incidence of TEAEs (including infections) did not increase with increasing duration of exposure to eculizumab [35].
There were no deaths or cases of meningococcal or aspergillus infection in the OLE [18]. One patient developed Neisseria gonorrhoeae, which was considered to be related to eculizumab, and made a full recovery [18, 35].
4 Dosage and Administration of Eculizumab
The recommended regimen of eculizumab in adults with AQP4-IgG-seropositive NMOSD is 900 mg weekly for the first four doses, then 1200 mg every 2 weeks (± 2 days [20]) from the following week [20, 21]. Eculizumab should be administered as a 25–45 min (in the EU [20]) or 35 min (in the USA [21]) IV infusion, and patients should be monitored for 1 h following each infusion. If an adverse event occurs during administration, the infusion may be slowed or stopped at the discretion of the physician, although the total infusion time may not exceed 2 h [20, 21]. Supplemental dosing of eculizumab is required in patients receiving concomitant PLEX or infusion (see local prescribing information for further details).
The use of eculizumab is associated with an increased susceptibility to opportunistic meningococcal infection (N. meningitidis) [20, 21]; the US prescribing information carries a black box warning regarding the risk of serious (life-threatening or fatal) meningococcal infections [21]. To reduce the risk of meningococcal infection, all patients must be vaccinated ≥ 2 weeks prior to the first dose of eculizumab; the drug is contraindicated in patients who are not currently vaccinated against N. meningitidis, unless they receive prophylactic treatment with appropriate antibiotics until 2 weeks after vaccination. Patients should be vaccinated in accordance with current national guidelines [20, 21]. Eculizumab is also contraindicated in patients with unresolved N. meningitidis infection [20, 21].
Local prescribing information should be consulted for further information, including dosage and administration details, contradindications, warnings and precautions.
5 Current Status of Eculizumab in the Management of NMOSD
The terminal complement protein (C5) inhibitor eculizumab is the first drug to be approved in the EU [20], USA [21], Canada [22] and Japan [23] specifically for use in adults with NMOSD who are AQP4-IgG-seropositive [20,21,22,23] (and have a relapsing course of disease [20]). The EU approval was based on the final results of the phase III, randomized, double-blind, placebo-controlled, PREVENT study and interim data from its ongoing OLE (expected completion date mid-2020). In the core study, eculizumab reduced the risk of adjudicated relapse relative to placebo in patients with AQP4-IgG-seropositive NMOSD (Sect. 2). This benefit of eculizumab, which was seen across all patient subgroups analysed, including those based on ethnicity, geographical region, historic use of rituximab and concomitant use of ISTs, was associated with reductions in relapse-related hospitalizations and medication use, and was accompanied by improvements in neurological and functional disability assessments, as well as in generic quality of life measures (Sect. 2). Preventing relapse-related accrual of disability is the primary goal of maintenance therapy for NMOSD (Sect. 1) and in this respect, eculizumab demonstrated durable efficacy, with, for example, the vast majority (≈94%) of patients who received at least one dose of the drug in the core study or OLE remaining free from adjudicated relapse through 4 years of treatment (Sect. 2).
Eculizumab was generally well tolerated in PREVENT and/or its OLE (Sect. 3). Overall, the safety profile of eculizumab in patients with AQP4-IgG-seropositive NMOSD, including in the longer-term, was consistent with that seen for the drug in other approved indications [18, 35]; there were no unexpected findings, with the most common TEAEs being URTI, headache, nasopharyngitis and nausea (Sect. 3). The main safety concern with eculizumab is the increase in patient susceptibility to infections, especially with Neisseria species and encapsulated bacteria, which is related to its mechanism of action (Sect. 3). All patients receiving eculizumab should be vaccinated against meningococcal infection and, if necessary, administered appropriate prophylactic antibiotics (Sect. 4). All PREVENT participants received meningococcal vaccination/appropriate prophylactic antibiotics and no cases of meningococcal infection have been reported to date, including in the ongoing OLE (Sect. 3). It should be noted, however, that 16 cases of meningococcal disease have been identified in eculizumab-treated patients over an 8-year period (2008–2016) in the USA [38]. All patients had meningococcemia; six also had evidence of meningitis. Fourteen patients had received ≥ 1 dose of meningococcal vaccine; 11 cases were due to non-groupable strains of N. meningitides, which are not impacted by vaccination [38].
Additional studies evaluating the efficacy of eculizumab relative to that of other ISTs commonly used (albeit off-label) as preventative treatments for NMOSD would be of benefit. Comparisons with rituximab, in particular, are of interest, since this humanized anti-CD20 mAb is widely used and, increasingly, as a first-line therapy [8]. The availability of real-world data demonstrating the effectiveness of eculizumab in the treatment of AQP4-IgG-seropositive NMOSD is also awaited with interest. Moreover, given that concern has been expressed over the cost of eculizumab in clinical practice, the conduct of robust pharmacoeconomic studies designed to determine the cost-effectiveness of the drug relative to other commonly used ISTs is desirable.
In conclusion, eculizumab is an effective and generally well tolerated therapy for AQP4-IgG-seropositive NMOSD; it is the first agent to be specifically approved for the treatment of this rare, debilitating and potentially life-threatening condition.
Data Selection Eculizumab: 105 records identified
Duplicates removed | 17 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 17 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 33 |
Cited efficacy/tolerability articles | 9 |
Cited articles not efficacy/tolerability | 29 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were eculizumab, Soliris, NMOSD, neuromyelitis optica spectrum disease, Devic’s disease, aquaporin-4-positive. Records were limited to those in English language. Searches last updated 18 March 2020 | |
Change history
25 June 2022
video update
21 April 2020
A Correction to this paper has been published: https://doi.org/10.1007/s40265-020-01315-x
22 April 2020
A Correction to this paper has been published: https://doi.org/10.1007/s40265-020-01316-w
References
Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc. 2017;92(4):663–79.
Oh J, Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int. 2012;2012:460825.
Borisow N, Mori M, Kuwabara S, et al. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol. 2018;9:888.
Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–89.
Mealy MA, Kessler RA, Rimler Z, et al. Mortality in neuromyelitis optica is strongly associated with African ancestry. Neurol Neuroimmunol Neuroinflamm. 2018;5:e468.
Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014;176:149–64.
Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain. 2012;135:1834–49.
Collongues N, Ayme-Dietrich E, Monassier L, et al. Pharmacotherapy for neuromyelitis optica spectrum disorders: current management and future options. Drugs. 2019;79(2):125–42.
Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017;264(10):2088–94.
Lana-Peixoto MA, Talim N. Neuromyelitis optica spectrum disorder and anti-MOG syndromes. Biomedicines. 2019;7(2):42.
Pittock SJ, Lucchinetti CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann N Y Acad Sci. 2016;1366(1):20–39.
Verkman AS, Phuan PW, Asavapanumas N, et al. Biology of AQP4 and anti-AQP4 antibody: therapeutic implications. Brain Pathol. 2013;23(6):684–95.
Ratelade J, Verkman AS. Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol. 2012;44(9):1519–30.
Jasiak-Zatonska M, Kalinowska-Lyszczarz A, Michalak S, et al. The immunology of neuromyelitis optica: current knowledge, clinical implications, controversies and future perspectives. Int J Mol Sci. 2016;17:273.
Tradtrantip L, Yao X, Su T, et al. Bystander mechanism for complement initiated early oligodendrocyte injury in neuromyelitis optica. Acta Neuropathol. 2017;134:35–44.
Duan T, Smith AJ, Verkman AS. Complement-dependent bystander injury to neurons in AQP4-IgG seropositive neuromyelitis optica. J Neuroinflamm. 2018;15(1):294.
Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
European Medicines Agency. Soliris: European public assessment report (EPAR). 2019. https://www.ema.europa.eu/. Accessed 14 Oct 2019.
Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25(11):1256–64.
European Medicines Agency. Soliris® (Eculizumab): summary of product characteristics. 2019. https://ec.europa.eu/. Accessed 30 Aug 2019.
Alexion Pharmaceuticals Inc. Soliris® (Eculizumab) US Prescribing Information. 2007 [Revised version 06/2019]. https://alexion.com/. Accessed 2019.
Alexion Pharma GmbH. SOLIRIS® (eculizumab for injection): Canadian product monograph. 2019. https://www.alexion.com. Accessed 3 Feb 2020.
Alexion Pharma LLC. Soliris for intravenous infusion 300 mg: Japanese prescribing information. 2019. https://www.pmda.go.jp. Accessed 8 Dec 2019.
Dhillon S. Eculizumab: a review in generalized Myasthenia Gravis. Drugs. 2018;78(3):367–76.
Keating GM. Eculizumab: a review of its use in atypical haemolytic uraemic syndrome. Drugs. 2013;73(18):2053–66.
McKeage K. Eculizumab. Drugs. 2011;71(17):2327–45.
Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–25.
Pittock SJ, Lennon VA, McKeon A, et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 2013;12(6):554–62.
Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–15.
Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–9.
Pittock SJ, Berthele A, Fujihara K, et al. Subgroup analyses from the phase 3 PREVENT study in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder [abstract no. P605 plus poster]. In: 35th ECTRIMS congress; 2019.
Palace J, Pittock SJ, Berthele A, et al. Impact of eculizumab on disability measures in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder: phase 3 PREVENT study [abstract no. P1343 plus poster]. In: 35th ECTRIMS congress; 2019.
Pittock, SJ, Berthele A, Fujihara K et al. Impact of eculizumab on reported quality of life in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder: findings from the PREVENT study [abstract no. P612 plus poster]. In: 35th ECTRIMS congress; 2019.
Kim HJ, Pittock SJ, Berthele A, et al. Impact of eculizumab on hospitalization rates and relapse treatment in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder: findings from the phase 3 PREVENT study [abstract no. P604 plus poster]. In: 35th ECTRIMS congress; 2019.
Wingerchuk DM, Pittock SJ, Berthele A, et al. Long-term safety and effectiveness of eculizumab in neuromyelitis optica spectrum disorder [abstract no. 142 plus oral presentation]. In: 35th ECTRIMS congress; 2019.
Kim HJ, Berthele A, FujiharaK, et al. Efficacy and safety of eculizumab in Asian patients with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder: subgroup analysis from the phase 3 PREVENT study [poster no. P.P.107]. In: 12th PACTRIMS congress; 2019.
Fujihara K, Berthele A, Kim HJ, et al. Long-term safety and efficacy of eculizumab in Asian patients with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder [poster no. P.P106]. In: 12th PACTRIMS congress; 2019.
McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR. 2017;66(27):734–7.
Acknowledgements
During the peer review process, the manufacturer of eculizumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
James Frampton is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The original version of this article was revised due to a retrospective Open Access request.
The original version of this article was revised: Due to Table 2 abbreviation update.
Enhanced material
for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.11955258.
The manuscript was reviewed by: M.A. Lana-Peixoto, CIEM MS Research Center, Federal University of Minas Gerais Medical School, Belo Horizonte, Brazil; M. Ralli, Department of Sense Organs, Sapienza University of Rome, Rome, Italy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Frampton, J.E. Eculizumab: A Review in Neuromyelitis Optica Spectrum Disorder. Drugs 80, 719–727 (2020). https://doi.org/10.1007/s40265-020-01297-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01297-w