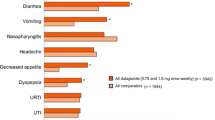
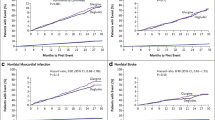
Abstract
Subcutaneous dulaglutide (Trulicity®) is a once-weekly glucagon-like peptide-1 receptor agonist that is approved in numerous countries as an adjunct to diet and exercise for the treatment of adults with type 2 diabetes (T2D). In the clinical trial and real-world settings, once-weekly subcutaneous dulaglutide, as monotherapy or add-on therapy to other antihyperglycaemic agents (including oral antihyperglycaemic drugs and insulin), was an effective and generally well tolerated treatment in adults with inadequately controlled T2D, including in high-risk patients [e.g. obese and elderly patients, those with stage 3 or 4 chronic kidney disease (CKD) and/or cardiovascular (CV) disease]. In the REWIND CV outcomes trial in patients with T2D with or without CV disease, dulaglutide was associated with a significant reduction in the risk of a major adverse cardiac event (MACE; primary composite outcome comprising CV death, nonfatal myocardial infarction or nonfatal stroke) at a median of 5.4 years’ follow-up. Given its durable glycaemic efficacy, beneficial effects on bodyweight and MACE outcomes, low inherent risk of hypoglycaemia and convenient once-weekly regimen, dulaglutide remains an important option in the management of T2D.
Similar content being viewed by others
References
Shin J-I. Second-line glucose-lowering therapy for type 2 diabetes mellitus. Curr Diab Rep. 2019;19(8):54.
International Diabetes Federation. IDF clinical practice recommendations for managing type 2 diabetes in primary care. 2017. https://www.idf.org. Accessed 11 Nov 2019.
American Diabetes Association. 10. Cardiovascular disease and risk management: standards of diabetes care. Diabetes Care. 2019;4(Suppl 1):103–23.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63:221–8.
European Medicines Agency. Trulicity (Dulaglutide): EU summary of product characteristics. 2019. https://www.ema.europa.eu/. Accessed 10 Dec 2019.
Eli Lilly and Company. Trulicity (Dulaglutide): US prescribing information. 2019. https://pi.lilly.com/us/trulicity-uspi.pdf. Accessed 21 Oct 2019.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30.
Burness CB, Scott LJ. Dulaglutide: a review in type 2 diabetes. BioDrugs. 2015;29(6):407–18.
Ferdinand KC, White WB, Calhoun DA, et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64(4):731–7.
Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrin. 2018;6(8):605–17.
Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrin. 2018;6(5):370–81.
Pozzilli P, Norwood P, Jodar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab. 2017;19(7):1024–31.
Dungan KM, Weitgasser R, Perez Manghi F, et al. A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8). Diabetes Obes Metab. 2016;18(5):475–82.
Umpierrez G, Povedano ST, Manghi FP. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168–78.
Nauck M, Weinstock RS, Umpierrez GE, et al. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58.
Dungan K, Povedano ST, Forst T. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–57.
Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added on to pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–67.
Giorgino F, Benroubi M, Sun JH. Efficacy and safety of once weekly dulaglutide vs insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241–9.
Blonde L, Jendle J, Gross J. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–66.
Araki E, Inagaki N, Tanizawa Y, et al. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab. 2015;17(10):994–1002.
Miyagawa J, Odawara M, Takamura T, et al. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab. 2015;17(10):974–83.
Chen YH, Huang CN, Cho YM, et al. Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East-Asian patients with type 2 diabetes in a multicentre, double-blind, randomized, parallel-arm, active comparator, phase III trial. Diabetes Obes Metab. 2018;20(9):2121–30.
Wang W, Nevarez L, Filippova E, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52-week open-label, randomized phase III trial. Diabetes Obes Metab. 2019;21(2):234–43.
Gentilella R, Romera I, Nicolay C, et al. Change in HbA1c across the baseline HbA1c range in type 2 diabetes patients receiving once-weekly dulaglutide versus other incretin agents. Diabetes Ther. 2019;10(3):1113–25.
Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409–18.
Boustani MA, Pittman IT, Yu M, et al. Similar efficacy and safety of once-weekly dulaglutide in patients with type 2 diabetes aged ≥65 and %3c65 years. Diabetes Obes Metab. 2016;18(8):820–8.
Gentilella R, Sesti G, Vazquez L, et al. Dulaglutide is an effective treatment for lowering HbA1c in patients with type 2 diabetes regardless of body mass index. Diabetes Obes Metab. 2019;12:2660–6.
Davidson JA, Manghi FP, Yu M, et al. Efficacy and safety of dulaglutide in Hispanic/Latino patients with type 2 diabetes in the AWARD clinical program. Endocr Pract. 2016;22(12):1406–14.
Mathieu C, Del Prato S, Botros FT, et al. Effect of once weekly dulaglutide by baseline beta-cell function in people with type 2 diabetes in the AWARD programme. Diabetes Obes Metab. 2018;20(8):2023–8.
Weinstock RS, Guerci B, Umpierrez G, et al. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17(9):849–58.
Giorgino F, Yu M, Haupt A, et al. Effect of once-weekly dulaglutide versus insulin glargine in people with type 2 diabetes and different baseline glycaemic patterns: a post hoc analysis of the AWARD-2 clinical trial. Diabetes Obes Metab. 2019;21(11):2570–5.
Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrin. 2018;6(4):275–86.
Dungan KM, Raz I, Skrivanek Z, et al. Achieving the composite endpoint of glycated haemoglobin %3c 7.0%, no weight gain and no hypoglycaemia in the once-weekly dulaglutide AWARD programme. Diabetes Obes Metab. 2016;18(1):49–55.
Yu M, Van Brunt K, Varnado OJ, et al. Patient-reported outcome results in patients with type 2 diabetes treated with once-weekly dulaglutide: data from the AWARD phase III clinical trial programme. Diabetes Obes Metab. 2016;18(4):419–24.
Reaney M, Yu M, Lakshmanan M, et al. Treatment satisfaction in people with type 2 diabetes mellitus treated with once-weekly dulaglutide: data from the AWARD-1 and AWARD-3 clinical trials. Diabetes Obes Metab. 2015;17(9):896–903.
Yu M, Brunt KV, Milicevic Z, et al. Patient-reported outcomes in patients with type 2 diabetes treated with dulaglutide added to titrated insulin glargine (AWARD-9). Clin Ther. 2017;39(11):2284–95.
Fuechtenbusch M, Aberle J, Heitmann E, et al. Weight loss in patients with type 2 diabetes receiving once-weekly dulaglutide plus insulin lispro or insulin glargine plus insulin lispro: a post-hoc analysis of the AWARD-4 study across baseline body mass index subgroups. Diabetes Obes Metab. 2019;21(6):1340–8.
Pantalone KM, Patel H, Yu M, et al. Dulaglutide 1.5 mg as an add-on option for patients uncontrolled on insulin: subgroup analysis by age, duration of diabetes and baseline glycated haemoglobin concentration. Diabetes Obes Metab. 2018;20(6):1461–9.
Tuttle KR, Lakshmanan MC, Rayner B, et al. Body weight and eGFR during dulaglutide treatment in type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7). Diabetes Obes Metab. 2019;21(6):1493–7.
Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States. Diabetes Obes Metab. 2018;21(4):920–9.
Mody R, Huang Q, Yu M, et al. Clinical and economic outcomes among injection-naive patients with type 2 diabetes initiating dulaglutide compared with basal insulin in a US real-world setting: the DISPEL study. BMJ Open Access Res Care. 2019;7(1):e000884.
Mody R, Huang Q, Yu M, et al. Adherence and persistence for dulaglutide (DU) vs basal insulin (BI) in injection-naive patients with type 2 diabetes (T2D): the DISPEL™ study [abstract no 1259-P]. Diabetes. 2019;68(Suppl 1).
Divino V, Boye KS, Lebrec J, et al. GLP-1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Ther. 2019;10(3):1067–88.
Otto T, Myland M, Jung H, et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Germany: a retrospective cohort study. Curr Med Res Opin. 2019;35(5):893–901.
Alatorre C, Fernandez Lando L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953–61.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20(1):42–9.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–8.
Ferdinand KC, Botros FT, Atisso CM, et al. Cardiovascular safety for once-weekly dulaglutide in type 2 diabetes: a pre-specified meta-analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol. 2016;15:38.
Nauck MA, Frossard JL, Barkin JS, et al. Assessment of pancreas safety in the development program of once-weekly GLP-1 receptor agonist dulaglutide. Diabetes Care. 2017;40(5):647–54.
Tuttle KR, McKinney TD, Davidson JA, et al. Effects of once-weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials. Diabetes Obes Metabol. 2017;19(3):436–41.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. 2019. https://www.nice.org.uk/guidance/ng28. Accessed 11 Nov 2019.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl 1):90–102.
American Diabetes Association. Glycemic targets: standards of medical care—2019. Diabetes Care. 2019;42(Suppl 1):61–70.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2019 executive summary. Endocrine Pract. 2019;25(1):69–100.
Wiviott SD, Raz I, Bonca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347–57.
Gentilella R, Pechtner V, Corcos A, et al. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35(1):e3070.
Matza LS, Boye KS, Stewart KD, et al. Crossover clinical trial assessing patient PREFERence between the dulaglutide pen and semaglutide pen (PREFER). Diabetes Obes Met. 2019. https://doi.org/10.1111/dom.13902.
Matza LS, Boye KS, Currie BM, et al. Patient perceptions of injection devices used with dulaglutide and liraglutide for treatment of type 2 diabetes. Curr Med Res Opin. 2018;34(8):1457–64.
Acknowledgements
During the peer review process, the manufacturer of dulaglutide was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Funding
The preparation of this review was not supported by any external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Lesley Scott is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.11356868.
The manuscript was reviewed by:S.C. Bain, Swansea University Medical School, Swansea, UK; D.S.H. Bell, Southside Endocrinology, Birmingham, AL, USA; A.M. Borzì, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Y. Hamamoto, Centre for Diabetes, Endocrinology and Metabolism, Kansai Electric Power Hospital, Osaka, Japan.
Rights and permissions
About this article
Cite this article
Scott, L.J. Dulaglutide: A Review in Type 2 Diabetes. Drugs 80, 197–208 (2020). https://doi.org/10.1007/s40265-020-01260-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01260-9