Abstract
Oral dapagliflozin (Edistride®, Forxiga®) is approved in the EU at a dosage of 5 mg/day as an adjunct to insulin in adults with type 1 diabetes (T1D) and a body mass index (BMI) of ≥ 27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy. As a highly selective SGLT2 inhibitor, dapagliflozin decreases plasma glucose levels independently of insulin action and enables glycaemic control improvement without increasing the risks associated with intensive insulin therapy. In the phase III DEPICT-1 and -2 trials, dapagliflozin 5 mg/day as an adjunct to insulin improved glycaemic control and reduced total daily insulin dose and bodyweight relative to placebo in adults with inadequately controlled T1D, including in patients with a BMI of ≥ 27 kg/m2, over 24 weeks of treatment. In extensions of these trials, these improvements were maintained up to 52 weeks. Dapagliflozin was generally well tolerated with a manageable safety profile and a hypoglycaemia profile generally similar to placebo. The incidence of diabetic ketoacidosis with dapagliflozin in patients with a BMI ≥ 27 kg/m2 was less than half that of the overall population who received dapagliflozin. Dapagliflozin is the first SGLT2 inhibitor to be approved for use in T1D and, while further clinical experience in T1D is required to more definitively establish its efficacy and safety profile, it provides a promising adjunctive treatment option for adults with T1D and a BMI of ≥ 27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First oral treatment indicated for T1D in the EU |
Approved as an adjunct to insulin in adults with T1D and a BMI ≥ 27 kg/m2 in whom insulin alone does not provide adequate glycaemic control |
Reduces plasma glucose independently of insulin |
Improves glycaemic control and reduces total daily insulin dose and bodyweight without increasing the risk of hypoglycaemia events |
Generally well tolerated; manageable safety profile |
1 Introduction
Insulin replacement therapy is the mainstay of treatment for patients with type 1 diabetes (T1D) [1, 2]. Despite the improvements over the years in insulin delivery and glucose monitoring systems, glycaemic control in individuals with T1D is often suboptimal, with less than a third of this population achieving optimal glycaemic control [i.e. glycated haemoglobin (HbA1c) < 7%] [3]. Although intensive insulin treatment may be used to improve poor glycaemic control, its therapeutic potential is limited by the increased risk of hypoglycaemia and weight gain, which are associated with a greater risk of adverse cardiovascular (CV) outcomes [4]. Severe hypoglycaemic episodes may also lead to events such as seizures, coma or death [5]. Furthermore, glycaemic variability (the fluctuations in blood glucose levels throughout the day) is an independent risk factor for hypoglycaemia in T1D [6]. Obesity and insulin resistance are also associated with intensive insulin therapy and have become more prevalent in T1D [7]. Therefore, improving glycaemic control without increasing the risk of hypoglycaemia and other related comorbidities is an important objective in the management of T1D.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a class of antidiabetic drugs used in the treatment of type 2 diabetes (T2D). By inhibiting reabsorption of filtered glucose in the proximal tubule to increase urinary glucose excretion, SGLT2 inhibitors lower blood glucose levels independently of insulin [4]. Therefore, when used alongside insulin, SGLT2 inhibitors offer a means of improving glycaemic control without increasing the risk of insulin-related adverse effects [8]. As SGLT2 inhibitors may improve CV outcomes and bodyweight [9, 10], they may be of particular benefit to patients with high body mass indices (BMI).
Dapagliflozin (Edistride®, Forxiga®), an SGLT2 inhibitor, is the first oral treatment approved in T1D in the EU where it is indicated as an adjunct to insulin in adults with T1D and a BMI of ≥ 27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy [11]. This review discusses therapeutic efficacy and tolerability data relevant to the use of dapagliflozin in this setting, focusing on the approved dosage of 5 mg/day. The pharmacological properties of dapagliflozin have been reviewed in detail previously [12, 13] and are summarized in Table 1.
2 Therapeutic Efficacy of Dapagliflozin
The therapeutic efficacy of dapagliflozin as an adjunct to insulin in adult patients with insufficiently controlled T1D was assessed in two 24-week, randomized, double-blind, multinational, phase III studies, DEPICT-1 [14] and DEPICT-2 [15]. In these studies, patients received oral dapagliflozin 5 mg (n = 259 [14] and 271 [15]), dapagliflozin 10 mg (n = 259 [14] and 270 [15]) or placebo (n = 260 [14] and 272 [15]) once daily with insulin, the dose of which was adjusted as per glucose readings, local guidance and individual circumstances [14, 15]. After the first dose of the study drug, the daily insulin dose was recommended to be reduced by up to 20% to reduce the risk of hypoglycaemia [but no greater, due to the risk of diabetic ketoacidosis (DKA) from excessive insulin dose reduction]. The subsequent doses were titrated back as far as possible to baseline levels [14, 15].
Eligible patients (aged 18–75 years) in both studies had HbA1c levels of 7.7–11.0% at screening and 7.5–10.5% at randomization, were prescribed daily insulin for ≥ 12 months before the study, had C-peptide levels of < 0.7 ng/mL and a BMI of ≥ 18.5 kg/m2 [14, 15]. Exclusion criteria included (but were not limited to) a history of T2D, previous pancreatic surgery or any pancreatic disorder resulting in reduced β-cell capacity, diabetes insipidus, DKA requiring medical intervention ≤ 1 month before screening, severe hypoglycaemia, symptoms of poorly controlled diabetes, and previous treatment with any SGLT2 inhibitor. At baseline, the mean age of patients was ≈ 43 years, the mean BMI was 28 kg/m2 and the mean disease duration was ≈ 20 years [14, 15].
In each study, patients underwent an 8-week run-in period to optimize insulin before being randomized to study treatment [14, 15]. Randomization was stratified according to the use of continuous glucose monitoring (CGM), method of insulin administration [i.e. multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII)] and HbA1c range (7.5 to < 9.0% or 9.0–10.5%) [14, 15].
The primary efficacy endpoint in both studies was the change in HbA1c from baseline at 24 weeks [14, 15]. Statistical analyses for secondary endpoints were only performed if significance was found in the primary endpoint for dapagliflozin 5 mg versus placebo and dapagliflozin 10 mg versus placebo [14, 15]. A pooled post hoc subgroup analysis of patients with a BMI of ≥ 27 kg/m2 (i.e. the approved population; Sect. 4) was also performed using 24-week data from both trials (abstract) [16]. In both trials, only small differences in efficacy were evident between the dapagliflozin 5 mg/day and 10 mg/day treatment arms in terms of HbA1c reductions [14, 15]. Consequently, dapagliflozin 5 mg/day was selected as the approved dosage in the EU [17] and is the focus of the discussion.
Following the 24-week main study phase, DEPICT-1 and DEPICT-2 had 28-week extensions in which most patients participated (90% [18] and 88% [19]); most of these patients continued treatment until the end of the extension (i.e. week 52; ≈ 85% [18] and 83% [19]). In the extensions, efficacy endpoints were exploratory (Sect. 2.2) and CGM-related endpoints were not evaluated [17].
2.1 Outcomes at Week 24
2.1.1 Glycaemic Control
Dapagliflozin 5 mg/day or 10 mg/day (as an adjunct to insulin) significantly improved glycaemic control relative to placebo in adult patients with T1D in the DEPICT-1 [14] and DEPICT-2 [15] studies. The improvement in HbA1c from baseline at week 24 was significantly greater with dapagliflozin 5 mg/day than placebo (Table 2), with the reduction in HbA1c with dapagliflozin 5 mg/day seen from week 4 of treatment and maintained to week 24 [14, 15].
Twice as many dapagliflozin 5 mg/day recipients as placebo recipients had a reduction from baseline in HbA1c of ≥ 0.5% at week 24 without experiencing severe hypoglycaemia, with odds ratios significantly favouring dapagliflozin (Table 2) [14, 15]. In a pooled analysis of the DEPICT studies, 39% and 11% of dapagliflozin 5 mg/day and placebo recipients achieved an HbA1c reduction of ≥ 0.5% without weight gain at week 24, and 44% and 22% of patients achieved this reduction without hypoglycaemia or DKA [abstract data] [20].
In a pooled subgroup analysis, dapagliflozin 5 mg/day recipients with a BMI ≥ 27 kg/m2 experienced similar improvements in glycaemic parameters at week 24 to those in the overall study population (Table 2) [16]. The odds of achieving an HbA1c reduction of ≥ 0.5% without severe hypoglycaemia were also higher in the dapagliflozin than placebo group (Table 2) [16].
CGM-assessed glycaemic parameters were also significantly improved (nominal p-values) with dapagliflozin 5 mg/day relative to placebo in both DEPICT studies, including daily glucose levels, glycaemic stability (as per MAGE) and the percentage of glucose readings in the target range (Table 2) [14, 15]. Pooled data from both studies showed that blood glucose levels with dapagliflozin 5 mg/day were the lowest between 4 a.m. and 8 a.m. [abstract] [21].
Pooled analyses of DEPICT-1 and -2 support the utility of dapagliflozin 5 mg/day as an adjunct to insulin in providing glycaemic stability [22, 23]. In one analysis, adjusted mean changes from baseline in CGM-assessed parameters with dapagliflozin 5 mg/day versus placebo at week 24 were consistent with those seen in DEPICT-1 and -2 and not accompanied by increases in the time spent in hypoglycaemic blood glucose ranges (abstract) [22]. In another pooled analysis, the mean standard deviations of 24-h CGM readings were reduced with dapagliflozin 5 mg/day from 3.7 mmol/L at baseline to 3.3 mmol/L at 12 weeks and 3.4 mmol/L at 24 weeks (placebo group changed from 3.7 mmol/L to 3.6 mmol/L and 3.7 mmol/L) [abstract] [23].
2.1.2 Other Outcomes
In DEPICT-1 [14] and DEPICT-2 [15], patients receiving dapagliflozin 5 mg/day had significant reduction in total daily insulin dose at 24 weeks relative to placebo recipients (Table 2). The reduction from baseline in total daily insulin dose was seen from weeks 2–24 [14, 15], with exploratory data indicating that this was maintained until week 52 (Sect. 2.2.2). Reductions in basal and bolus insulin doses with dapagliflozin 5 mg/day were proportionally similar to that of the total daily insulin dose (adjusted mean changes from baseline with dapagliflozin 5 mg/day vs placebo: basal insulin − 11.6 vs − 0.6% [14] and − 11.2 vs + 1.5% [15]; bolus insulin − 14.3 vs − 4.6% [14] and − 11.6 vs − 2.6% [15]). A pooled analysis showed that, in patients who discontinued dapagliflozin 5 mg/day at any point in DEPICT-1 and DEPICT-2, the difference in mean total daily insulin dose at 2 weeks post-discontinuation was + 4.0 IU versus 2 weeks prior to discontinuation [abstract] [24].
Dapagliflozin 5 mg/day was associated with a significant reduction in bodyweight relative to placebo at 24 weeks (Table 2), with dapagliflozin recipients experiencing a continuous decrease in bodyweight throughout the 24-week period (more marked initially) versus minimal changes in the placebo group [14, 15].
At week 24, changes in total daily insulin and bodyweight from baseline in dapagliflozin 5 mg/day recipients with a BMI ≥ 27 kg/m2 were consistent with those seen in the overall population (Table 2) [16].
2.2 Outcomes at Week 52
2.2.1 Glycaemic Control
Exploratory analyses of the DEPICT-1 and DEPICT-2 extensions indicated that the improvements in glycaemic control with dapagliflozin 5 mg/day were maintained at week 52 [17, 18]. At week 52, the adjusted mean HbA1c changes from baseline in dapagliflozin 5 mg/day and placebo recipients were − 0.27% and + 0.06% (change vs placebo − 0.33%) in DEPICT-1 [18] and − 0.11% and + 0.09% (change vs placebo − 0.20%) in DEPICT-2 [17]. Adjusted mean changes from baseline in fasting plasma glucose levels were − 1.1 and + 0.4 mmol/L in DEPICT-1; 7.0% and 2.7% of patients in the respective groups achieved an HbA1c of < 7% in the DEPICT-1 extension [18].
In the dapagliflozin 5 mg/day and placebo groups, 40% and 24% of patients in DEPICT-1 [18] and 33% and 21% of patients in DEPICT-2 [17] achieved an HbA1c reduction of ≥ 0.5% without severe hypoglycaemia. Moreover, in a pooled analysis of these extensions, patients had significantly (p < 0.0001) greater odds of achieving this endpoint with dapagliflozin than placebo at weeks 24 (OR 2.90, 95% CI 2.19–3.83) and 52 (OR 2.04, 95% CI 1.55–2.70) [abstract] [25].
2.2.2 Other Outcomes
In both DEPICT studies, the total daily insulin dose with dapagliflozin 5 mg/day at week 52 was similar to that at week 24 (data not reported) [17]; the dose remained low and below that of placebo throughout weeks 2–52 [18, 26].
Reductions in bodyweight with dapagliflozin 5 mg/day were maintained at week 52; the adjusted mean change from baseline with dapagliflozin 5 mg/day compared with placebo was − 2.95% (95% CI − 3.83, − 2.06) [adjusted mean changes − 2.80 vs + 0.15%] in DEPICT-1 [18] and − 4.42% (95% CI − 5.19, − 3.64) [mean changes in each treatment group not reported] in DEPICT-2 [17]. In the DEPICT-1 extension, bodyweight continued to decrease until week 32 in dapagliflozin 5 mg/day recipients, from which point it was generally maintained to week 52 [18].
In a pooled DEPICT analysis of patients with hypertension at baseline [systolic/diastolic blood pressure (BP) ≥ 140/90 mmHg], dapagliflozin 5 mg/day recipients (n = 75) had an adjusted mean change from baseline in systolic BP versus placebo (n = 85) of − 2.86 mmHg at 24 weeks and − 3.72 mmHg at 52 weeks [25].
The effect of dapagliflozin on renal protection was assessed in T1D patients with albuminuria at baseline in another pooled analysis of these trials [27]. At week 52, the adjusted mean percentage difference in urine albumin-to-creatinine ratio (UACR) with dapagliflozin 5 mg/day (n = 80) versus placebo (n = 87) was − 13.3% (95% CI − 37.2, + 19.8) and the adjusted mean difference in estimated glomerular filtration rate (eGFR) was + 3.3 (95% CI − 0.9, + 7.5) [27].
3 Tolerability of Dapagliflozin
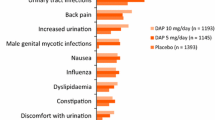
Dapagliflozin 5 mg/day as an adjunct to insulin was generally well tolerated with a manageable safety profile in adult patients with inadequately controlled T1D in the DEPICT-1 [14, 18] and DEPICT-2 [15] studies. Across both studies, patients receiving dapagliflozin 5 mg/day (n = 548) or placebo (n = 532) had a mean of ≈ 160 exposure days, with most of these patients (89.6% and 88.9%) having 121–180 exposure days [17]. In this pooled analysis, at least one adverse event (AE) during study treatment occurred in 70.1% and 62.4% of dapagliflozin 5 mg/day and placebo recipients after 24 weeks [17] and in 79.9 vs 74.1% of patients after 52 weeks [25]. Most AEs were of mild severity [17]. In the first 24 weeks of study, 28.6% and 11.8% of patients in the dapagliflozin 5 mg/day and placebo groups experienced a treatment-related AE (TRAE), with this difference between the groups being driven predominantly by AEs associated with increased urinary frequency and genital infections [17]. The most common (> 5%) AEs with a numerically greater incidence among dapagliflozin 5 mg/day than placebo recipients in the first 24 weeks of study were upper respiratory tract infection (5.7 vs 4.3%), pollakiuria (5.7 vs 2.6%) and urinary tract infection (UTI; 5.1 vs 4.1) [17].
Across the two studies, 6.8% and 3.8% of dapagliflozin 5 mg/day and placebo recipients experienced at least one serious AE (SAE) during 24 weeks of treatment, with the most common SAEs involving metabolism and nutrition disorders (3.1 vs 0.9% of patients) and infections and infestations (1.1 vs 0.6%); 2.7% and 1.1% of patients in the respective groups experienced an SAE leading to discontinuation [17]. After 52 weeks, 12.6% and 8.6% of dapagliflozin 5 mg/day and placebo recipients had experienced at least one SAE, with 4.0% and 1.7% of patients experiencing an SAE leading to treatment discontinuation [25]. In the DEPICT-1 extension, 2.9% of dapagliflozin 5 mg/day recipients (vs 0.8% of placebo recipients) experienced an SAE that was considered to be related to treatment (AEs not specified) [18]. Death occurred in one dapagliflozin 5 mg/day recipient in DEPICT-2 (motor vehicle accident under the influence of narcotics and alcohol [26]) and one placebo recipient in DEPICT-1 (cause not specified [18]).
3.1 Adverse Events of Special Interest
The hypoglycaemia profile of dapagliflozin 5 mg/day was generally similar to that of placebo in DEPICT-1 and DEPICT-2 [25]. Over 52 weeks, 83.6% of dapagliflozin 5 mg/day and 84.4% of placebo recipients experienced a hypoglycaemia event, with incidence rates (IR) per 100 patient-years’ (PYs) exposure of 3040 and 3332. Most hypoglycaemia events were documented symptomatic events [based on American Diabetes Association (ADA) categorization, i.e. those with typical symptoms and plasma glucose ≤ 3.9 mmol/L], occurring in 80.3% of dapagliflozin 5 mg/day and 80.3% of placebo recipients (IR 2388 and 2593 events/100 PYs’ exposure). Severe hypoglycaemic events (requiring assistance from another person) occurred in 9.7 and 10.0% of patients (IR 28.7 and 31.8 events/100 PYs’ exposure) [25]. In the DEPICT-1 extension, one dapagliflozin 5 mg/day recipient and one placebo recipient discontinued treatment due to a hypoglycaemia-related SAE [18].
DKA is a potential concern in patients with T1D receiving adjunctive SGLT inhibitors [28]. After 52 weeks of treatment, 4.0% of dapagliflozin 5 mg/day and 1.1% of placebo recipients in the overall pooled population experienced DKA events adjudicated to be definite [25]. In patients with a BMI of ≥ 27 kg/m2, 1.7% of dapagliflozin 5 mg/day recipients (5/286) and 1.0 of placebo recipients (3/289) experienced a definite DKA event; among patients with a BMI of < 27 kg/m2, definite DKA events occurred in 17 (6.5% of 262 patients) and three (1.2% of 243 patients) patients [25]. DKA incidence rates were 1.9 and 1.2 per 100 PYs in dapagliflozin 5 mg/day and placebo recipients in the BMI ≥ 27 kg/m2 subgroup, and 4.6 and 1.3 per 100 PYs in the overall population [29]. The most common causal factor for DKA events was inadequate insulin doses, through missed doses or pump failure (52% of 23 dapagliflozin 5 mg/day recipients; placebo data not reported) [25]. In DEPICT-1, 37.0, 37.0 and 25.9% of definite DKA events were adjudicated to be mild, moderate and severe, respectively [18]. Most of the definite DKA events in DEPICT-1 (70%) [18] and DEPICT-2 (77%) [15] were treated with standard therapy, intravenous fluids and additional insulin.
In terms of other AEs of special interest, dapagliflozin 5 mg/day was associated with a low incidence of adjudicated CV events (0.4 vs 0.8% of placebo recipients), renal function events (1.3 vs 0.8%) and fractures (2.2 vs 2.3%) over 52 weeks in the pooled analysis [25]. By contrast, UTIs an genital infections were common with dapagliflozin 5 mg/day overall (10.4 vs 7.3% of placebo recipients had UTIs; 13.3 vs 3.4% had genital infections), but occurred predominantly among female participants (17.0 vs 12.5% had UTIs; 19.3 vs 6.4% had genital infections) [incidence among males was 1.7 vs 1.6% for UTIs and 5.5 vs 0% for genital infections] [25]. In DEPICT-1, hypersensitivity reactions occurred in 5.4% and 2.3% of dapagliflozin 5 mg/day and placebo recipients; most reactions were mild and skin-related (e.g. rash, dermatitis, eczema) [18].
4 Dosage and Administration
Dapagliflozin is approved in the EU as an adjunct to insulin in adult patients with T1D and a BMI of ≥ 27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy [11]. In T1D, dapagliflozin must only be administered as an adjunct to insulin. The recommended dose of dapagliflozin is 5 mg once daily administered orally, with or without food. In patients with a known risk of frequent and/or severe hypoglycaemia, the starting insulin dose may need to be reduced to decrease the risk of hypoglycaemia. If required, insulin dose reduction should be done cautiously to avoid DKA and ketosis. Dapagliflozin should not be initiated in patients with a GFR of < 60 mL/min and should be discontinued if the GFR is persistently < 45 mL/min. No dose adjustment is necessary based on renal function or in patients with mild or moderate hepatic impairment [11].
Before initiating dapagliflozin, patients should be assessed for risk factors for DKA and ketone levels obtained [11]. Ketone levels should be monitored during treatment and dapagliflozin should not be administered if ketone levels are elevated. Patients should be assessed for ketoacidosis, regardless of glucose level, if they have non-specific symptoms of DKA (e.g. nausea, vomiting, anorexia, sleepiness). If DKA is suspected or diagnosed, dapagliflozin should be discontinued immediately. Patients using an insulin infusion pump are at a greater risk of DKA and should check their ketone levels with any suspected insulin interruption regardless of blood glucose levels [11]. Local prescribing information should be consulted for detailed information, including contraindications, precautions, drug interactions, use in special patient populations, and blood glucose and ketone monitoring recommendations.
5 Current Status of Dapagliflozin in the Management of T1D
Insulin therapy is vital for the survival of T1D patients, in whom insulin deficiency may be idiopathic or occur due to autoimmune β-cell destruction [30]. The 2016 National Institute for Health and Care Excellence (NICE) [1] and 2019 ADA guidelines [2] recommend treating adult T1D patients with MDIs of prandial and basal insulin; while CSII is recommended in both guidelines, NICE guidelines only recommend CSII if patients have experienced debilitating hypoglycaemia, or if HbA1c levels remain high, with MDI therapy. Insulin therapy should be tailored to the individual (with respect to e.g. dose, dose timing, injection methods) [1, 2]. However, insulin therapy alone is often not enough for T1D patients to achieve glycaemic control, with adverse effects such as hypoglycaemia becoming more likely with intensive insulin therapy, and obesity and insulin resistance becoming more prevalent in T1D (Sect. 1). To overcome these limitations, research into the adjunctive use of various agents with different mechanisms of action is currently underway in T1D [31].
SGLT2 inhibitors act independently of insulin to facilitate the improvement of glycaemic control without exacerbating insulin-related risks, such as hypoglycaemia and weight gain (Sect. 1). Recent research suggests that SGLT2 inhibitors may also have renal and CV protective effects [10, 32], which would be of particular benefit in the context of suboptimal insulin control and in obesity. DKA is a known risk with SGLT2 inhibitor therapy, potentially more so with higher doses and with certain patient lifestyles (e.g. excessive drinking and ketogenic diets) [28]. Certain strategies may mitigate the risk of DKA in T1D patients, such as initiating SGLT2 inhibitor therapy at the lowest possible dose, and tailoring insulin dose reductions to the blood glucose and ketone levels of the patient and the SGLT2 inhibitor used [28, 33]. Initially approved for use in T2D, dapagliflozin is the first SGLT2 inhibitor to be approved in the EU as an adjunct to insulin in T1D patients with a BMI of ≥ 27 kg/m2 (Sect. 4). Recently, sotagliflozin, a SGLT1/2 dual inhibitor, was also approved in the EU for the same indication [34]. Nevertheless, the US FDA has issued complete response letters for both dapagliflozin [35] and sotagliflozin [36] in T1D.
In the placebo-controlled DEPICT-1 and DEPICT-2 studies, dapagliflozin 5 mg/day was effective as an adjunct to insulin therapy in adults with T1D (Sect. 2). After 24 weeks of treatment with the drug, patients with a BMI of ≥ 27 kg/m2 experienced similar improvements in glycaemic control to the overall study population, as measured by HbA1c. Improvements in CGM-assessed glycaemic control parameters (although of nominal statistical significance) were also seen with dapagliflozin (Sect. 2.1.1). These parameters included glycaemic stability, an important unmet need in T1D [37], and time in the glycaemic target range, which may be a useful metric in treatment optimization as it was reported to be valuable in assessing treatment efficacy [38] and the risk for complications [39]. Dapagliflozin was also associated with reductions in total daily insulin dose and bodyweight, which may be of particular benefit to patients with a high BMI (Sect. 2.1.2). While extension study data were exploratory, efficacy findings at 52 weeks were consistent with those seen after 24 weeks (Sect. 2.2). At week 52, improvements in BP were also observed in patients with hypertension (i.e. BP ≥ 140/90 mmHg) and UACR data suggested that dapagliflozin 5 mg/day may have a renoprotective effect in patients with albuminuria (Sect. 2.2.2).
Dapagliflozin 5 mg/day was generally well tolerated with a manageable safety profile over 52 weeks of treatment in these trials (Sect. 3). The hypoglycaemic profile of dapagliflozin was similar to that of placebo after 52 weeks (Sect. 3.1). Although uncommon overall, definite DKA events were over three times as frequent in dapagliflozin 5 mg/day recipients than placebo recipients in the overall study population (Sect. 3.1). However, among dapagliflozin 5 mg/day recipients, the incidence rate of DKA events was 2.4-fold lower in those with a BMI ≥ 27 kg/m2 compared with the overall population (Sect. 3.1). Nevertheless, careful monitoring and management of any risks of DKA are crucial with dapagliflozin treatment [28]. Further long-term clinical experience with dapagliflozin in T1D is needed to more definitively establish the efficacy and safety profile of the drug for this chronic disease, especially in those with a high BMI (given the increasing prevalence of obesity in the T1D population [7]) and its relative position to that of other adjunctive therapies.
According to the draft final guidance from NICE, analyses using 52-week DEPICT data predicted dapagliflozin, as an adjunct to insulin, to be a cost-effective use of National Health Service resources for the treatment of T1D in patients with a BMI of ≥ 27 kg/m2 when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy [40]. Another UK-based cost-utility analysis using 24-week DEPICT-1 data predicted dapagliflozin as an adjunct to insulin to be cost effective relative to placebo, with a corresponding incremental cost-effectiveness ratio of £13,449 per quality-adjusted life years [41]. Further robust pharmacoeconomic data concerning the cost effectiveness of dapagliflozin 5 mg/day in T1D would be of interest.
In conclusion, dapagliflozin 5 mg/day as an adjunct to insulin improves glycaemic control and reduces total daily insulin dose and bodyweight without increasing the risk of hypoglycaemia events in adults with T1D. While further clinical experience is required, dapagliflozin 5 mg/day as an adjunct to insulin provides a promising treatment option in adult patients with T1D and a BMI of ≥ 27 kg/m2 when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy.
Data Selection Dapagliflozin: 247 records
Duplicates removed | 64 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 85 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 55 |
Cited efficacy/tolerability articles | 16 |
Cited articles not efficacy/tolerability | 27 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were dapagliflozin, Farxiga, Forxiga, Type 1 diabetes mellitus. Records were limited to those in English language. Searches last updated 27 Sep 2019 | |
Change history
26 November 2019
The article Dapagliflozin: A Review in Type 1 Diabetes, written by Julia Paik and Hannah A. Blair, was originally published Online First without Open Access.
References
National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management. 2016. https://www.nice.org.uk/guidance/ng17. Accessed 27 Sep 2019.
American Diabetes Association. Standards of Medical Care in Diabetes: 2019 abridged for primary care providers. Clin Diabetes. 2019;37(1):11–34.
Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8.
Bode BW, Garg SK. The emerging role of adjunctive noninsulin antihyperglycemic therapy in the management of type 1 diabetes. Endocr Pract. 2016;22(2):220–30.
International Hypoglycaemia Study Group. Minimizing hypoglycaemia in diabetes. Diabetes Care. 2015;38(8):1583–91.
Kilpatrick ES, Rigby AS, Goode K, et al. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50(12):2553–61.
Priya G, Kalra S. A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin? Diabetes Ther. 2018;9(1):349–61.
Ahmed-Sarwar N, Nagel AK, Leistman S, et al. SGLT-2 inhibitors: is there a role in type 1 diabetes mellitus management? Ann Pharmacother. 2017;51(9):791–6.
Chen J, Fan F, Wang JY, et al. The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta-analysis. Sci Rep. 2017;7:44128.
Patel DK, Strong J. The pleiotropic effects of sodium-glucose cotransporter-2 inhibitors: beyond the glycemic benefit. Diabetes Ther. 2019;10(5):1771–92.
European Medicines Agency. Dapagliflozin (Edistride): summary of product characteristics. 2019. http://ec.europa.eu/. Accessed 27 Sep 2019.
Plosker GL. Dapagliflozin: a review of its use in patients with type 2 diabetes. Drugs. 2014;74(18):2191–209.
Dhillon S. Dapagliflozin: a review in type 2 diabetes. Drugs. 2019;79(10):1135–46.
Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):864–76.
Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care. 2018;41(9):1938–46.
Mathieu C, Dandona P, Philip M, et al. Analysis of benefit/risk in the subgroup of patients with BMI of ≥ 27 kg/m2 in the dapagliflozin DEPICT-1 and -2 trials in type 1 diabetes [abstract no. SAT-LB025]. In: Endocrine Society Annual Meeting. 2019.
European Medicines Agency. Dapagliflozin (Forxiga/Edistride): public assessment report. 2019. https://www.ema.europa.eu/. Accessed 27 Sep 2019.
Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care. 2018;41(12):2552–9.
Rudofsky G, Mathieu C, Dandona P, et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type I diabetes: the DEPICT-2 study [abstract]. In: International Congress of Endocrinology. 2018.
Dandona P, Thoren F, Langkilde AM, et al. Pooled data analysis of composite endpoints from the DEPICT-1 and DEPICT-2 studies using dapagliflozin compared to placebo added to adjustable insulin in type 1 diabetes [abstract no. 612]. Diabetologia. 2018;61 (Suppl 1):S296.
Mathieu C, Dandona P, Phillip M, et al. Glucose variables in T1D studies with dapagliflozin: pooled analysis of continuous glucose monitoring data from DEPICT-1 and 2 [abstract no. 125-LB plus poster]. Diabetes. 2018;67(Suppl 1):LB35.
Mathieu C, Dandona P, Philip M, et al. Glucose variables in type 1 diabetes studies with dapagliflozin: pooled analysis of continuous glucose monitoring data from DEPICT-1 and -2. Diabetes Care. 2019;42(6):1081–7.
Handelsman Y, Dandona P, Mathieu C, et al. Dapagliflozin treatment reduces glycemic variability as assessed by standard deviation of interstitial glucose [abstract no. 32 plus poster]. In: AACE 28th Annual Congress. 2019.
Gordon J, Danne T, Beresford-Hulme LM, et al. Discontinuation of dapagliflozin in DEPICT-1 and DEPICT-2 led to clinically meaningful increases in HbA1c and body weight [abstract no. 2355-PUB]. In: ADA 79th Scientific Sessions. 2019.
Dandona P, Mathieu C, Phillip M, et al. Dapagliflozin (DAPA) in type 1 diabetes (T1D): pooled outcomes from DEPICT-1 and -2 [abstract no. 1231-P plus poster]. Diabetes. 2019;68(Suppl 1).
Rudofsky G. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-2 study. Oral presentation (International Congress of Endocrinology). 2018.
Edelman S, Jendle J, Dandona P, et al. Effect of adding dapagliflozin as an adjunct to insulin on urinary albumin-to-creatinine ratio over 52 weeks in adults with type 1 diabetes [abstract no. TH-PO1156 plus poster]. In: ASN Kidney Week. 2018.
Danne T, Garg S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42(6):1147–54.
Mathieu C, Dandona P, Phillip M, et al. Analysis of benefit:risk in the subgroup of patients with a BMI of ≥ 27 kg/m2 in the dapagliflozin DEPICT-1 and -2 trials in type 1 diabetes [abstract no. 7914 plus poster]. In: Endocrine Society Annual Meeting. 2019.
American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes. Diabetes Care. 2019;42(Suppl 1):S13–28.
Mittermayer F, Caveney E, De Oliveira C, et al. Addressing unmet medical needs in type 1 diabetes: a review of drugs under development. Curr Diabetes Rev. 2017;13(3):300–14.
Nagahisa T, Saisho Y. Cardiorenal protection: potential of SGLT2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes. Diabetes Ther. 2019;10(5):1733–52.
McCrimmon RJ, Henry RR. SGLT inhibitor adjunct therapy in type 1 diabetes. Diabetologia. 2018;61(10):2126–33.
Markham A, Keam SJ. Sotagliflozin: first global approval. Drugs. 2019;79(9):1023–9.
AstraZeneca. Update on US regulatory decision for Farxiga in type-1 diabetes [media release]. 15 Jul 2019. https://www.astrazeneca.com/.
Lexicon Pharmaceuticals. FDA issues complete response letter for Zynquista (sotagliflozin) [media release]. 22 Mar 2019. http://www.lexpharma.com.
Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39(4):273–82.
Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–5.
Runge AS, Kennedy L, Brown AS, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes. 2018;36(2):112–9.
National Institute for Health and Care Excellence. Dapagliflozin with insulin for treating type 1 diabetes: final appraisal document. 2019. https://www.nice.org/uk. Accessed 27 Sep 2019.
Bennett H, McEwan P, Evans M, et al. Cost-effectiveness of dapagliflozin in adult patients with inadequately controlled type 1 diabetes: a health economic analysis using 24-week results from the DEPICT-1 randomised controlled trial [abstract no. DB4]. Value Health. 2018;21(Suppl 3):S7.
Tang W, Leil TA, Johnsson E, et al. Comparison of the pharmacokinetics and pharmacodynamics of dapagliflozin in patients with type 1 versus type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18(3):236–40.
Henry RR, Rosenstock J, Edelman S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38(3):412–9.
Acknowledgements
During the peer review process, the manufacturer of dapagliflozin was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Julia Paik and Hannah Blair are salaried employees of Adis International Ltd/Springer Nature, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The original version of this article was revised due to a retrospective Open Access order.
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.9937097.
The manuscript was reviewed by:M. Evans, Diabetes Resource Centre, Llandough Hospital, Cardiff, UK; J. Jendle, Diabetes Endocrinology and Metabolism Research Center, School of Medical Sciences, Örebro University, Örebro, Sweden.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit original author(s) and the source, provide a link to the Creative Commons licence and indicate if changes were made.
About this article
Cite this article
Paik, J., Blair, H.A. Dapagliflozin: A Review in Type 1 Diabetes. Drugs 79, 1877–1884 (2019). https://doi.org/10.1007/s40265-019-01213-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01213-x