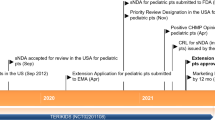
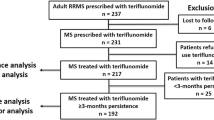
Abstract
Teriflunomide (Aubagio®) is a disease-modifying immunomodulatory drug with anti-inflammatory properties that selectively and reversibly inhibits the mitochondrial enzyme dihydro-orotate dehydrogenase, with consequent inhibition of de novo pyrimidine synthesis and reduced lymphocyte proliferation. Based on extensive evidence from randomized controlled trials (RCTs) and the real-world setting, oral teriflunomide is an effective and generally well tolerated treatment in patients with relapsing multiple sclerosis (MS), with these benefits maintained during long-term treatment (≥ 10 years) and no new safety signals identified. In pivotal RCTs in this patient population, teriflunomide provided significantly better efficacy than placebo (TEMSO and TOWER) and was as effective as interferon β-1a (TENERE) in terms of improvements in clinical outcomes (such as reduced annualized relapse rates, prevention of disability progression) and/or MRI-assessed disease activity measures. Albeit head-to-head trials would definitively establish the relative efficacy of oral disease-modifying therapies, given its convenient oral regimen and beneficial effects in reducing relapses and disease activity, teriflunomide remains an effective option for the management of relapsing–remitting MS (RRMS).
Similar content being viewed by others
References
Faissner S, Gold R. Efficacy and safety of the newer multiple sclerosis drugs approved since 2010. CNS Drugs. 2018;32(3):269–87.
Tintore M, Vidal-Jordana A, Sastre-Garriga J. Treatment of multiple sclerosis: success from bench to bedside. Nat Rev Neurol. 2019;15(1):53–8.
Garnock-Jones KP. Teriflunomide: a review of its use in relapsing multiple sclerosis. CNS Drugs. 2013;27(12):1103–23.
European Medicines Agency. AUBAGIO (teriflunomide) 14 mg film-coated tablets: summary of product characteristics. 2018. http://www.ema.europa.eu/. Accessed 31 Jan 2019.
Aly L, Hemmer B, Korn T. From leflunomide to teriflunomide: drug development and immunosuppressive oral drugs in the treatment of multiple sclerosis. Curr Neuropharmacol. 2017;15(6):874–91.
Bar-Or A, Pachner A, Menguy-Vacheron F, et al. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74(6):659–74.
O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–303.
Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(3):247–56.
Vermersch P, Czlonkowska A, Grimaldi LME, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler J. 2014;20(6):705–16.
Genzyme Corp. AUBAGIO® (teriflunomide): US prescribing information. 2016. http://products.sanofi.us/. Accessed 29 Jan 2019.
Miller AE, O’Connor P, Wolinsky JS, et al. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult Scler J. 2012;18(11):1625–32.
Freedman MS, Wolinsky JS, Comi G, et al. The efficacy of teriflunomide in patients who received prior disease-modifying treatments: subgroup analyses of the teriflunomide phase 3 TEMSO and TOWER studies. Mult Scler J. 2018;24(4):535–9.
Kappos L, Miller A, Poole E, et al. Effect of teriflunomide on substantial disability worsening in patients with relapsing forms of MS in a pooled analysis of the phase-3 TEMSO and TOWER studies [abstract no. EPR2104]. Eur J Neurol. 2018;25(Suppl. 2):433.
O’Connor PW, Lublin FD, Wolinsky JS, et al. Teriflunomide reduces relapse-related neurological sequelae, hospitalizations and steroid use. J Neurol. 2013;260(10):2472–80.
Miller AE, Macdonell R, Comi G, et al. Teriflunomide reduces relapses with sequelae and relapses leading to hospitalizations: results from the TOWER study. J Neurol. 2014;261(9):1781–8.
Sormani MP, Truffinet P, Thangavelu K, et al. Predicting long-term disability outcomes in patients with MS treated with teriflunomide in TEMSO. Neurol Neuroimmunol Neuroinflam. 2017;4(5):1–6.
O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology. 2016;86(10):920–30.
Maurer M, Miller A, Comi G, et al. Impact of long-term teriflunomide treatment on severe relapses: analysis of TEMSO and TOWER extensions [abstract no. P6.361]. Neurology. 2017;88(16 Suppl).
Confavreux C, Li DK, Freedman MS, et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler J. 2012;18(9):1278–89.
Freedman MS, Bar-Or A, Benamor M, et al. Long-term disability outcomes in patients treated with teriflunomide for up to 14 years: group-and patient-level data from the phase 2 extension study [abstract no. P1203]. Mult Scler J. 2017;23(Suppl 3):637–8.
Sprenger T, Lechner-Scott J, Sormani MP, et al. Evaluation of the long-term treatment effect of teriflunomide on cognitive outcomes and association with brain volume change: data from TEMSO and its extension study [abstract no. 068]. J Neurol. 2018;89(6):e28.
Lublin F, Miller A, Truffinet P, et al. Long-term disability outcomes in teriflunomide-treated patients in TEMSO and TOWER: an EDSS and FSS categorical analysis [abstract no. EP1715]. Mult Scler J. 2017;23(Suppl 3):903.
Vermersch P, Truffinet P, Poole EM, et al. Baseline characteristics and long-term disability outcomes: subgroup analysis of the TEMSO and TOWER core and extension studies [abstract no. P1191]. Mult Scler J. 2017;23(Suppl 3):629–30.
Oh J, Freedman MS, Miller AE, et al. Long-term efficacy of teriflunomide in patients recently diagnosed with relapsing forms of MS [abstract no. P6.339]. Neurology. 2017;88(16 Suppl).
Wolinsky JS, Narayana PA, Nelson F, et al. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler J. 2013;19(10):1310–9.
Radue EW, Sprenger T, Gaetano L, et al. Teriflunomide slows BVL in relapsing MS: a reanalysis of the TEMSO MRI data set using SIENA. Neurol Neuroimmunol Neuroinflam. 2017;4(e390):1–7.
Miller AE, Rovira A, Lebrun-Frenay C, et al. Assessing the effect of teriflunomide on unique active lesions in patients with relapsing remitting multiple sclerosis [abstract no. P946]. Mult Scler J. 2018;24(Suppl 2):515–6.
Sprenger T, Gaetano L, Mueller-Lenke N, et al. Correlation between brain volume loss and long-term disability worsening in patients with MS: SIENA analysis of TEMSO MRI data [abstract no. P-22]. Mult Scler J. 2018;24(3):388–9.
Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(10):977–86.
Zivadinov R, Dwyer MG, Carl E, et al. Slowing of cortical grey matter atrophy with teriflunomide is associated with delayed conversion to clinically definite MS [abstract no. P671]. Mult Scler J. 2017;23(Suppl 3):321–2.
Zivadinov R, Dwyer MG, Carl E, et al. Evaluating the effect of teriflunomide on whole brain atrophy in the phase 3 TOPIC study [abstract no. P870]. Mult Scler J. 2018;24(Suppl 2):463.
Miller AE, Freedman MS, Oh J, et al. Long-term outcomes in patients with early multiple sclerosis treated with teriflunomide: TOPIC extension study [abstract no. PR2085]. Eur J Neurol. 2017;24(Suppl 1):576.
Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in patients with relapsing forms of MS switching to teriflunomide from other disease-modifying therapies: results from the global phase 4 Teri-PRO study in routine clinical practice. Mult Scler Relat Disord. 2018;26:211–8.
Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in relapsing forms of MS: real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord. 2017;17:107–15.
Coyle PK, Kharti B, Edwards KR, et al. Teriflunomide real-world evidence: global differences in the phase 4 Teri-PRO study. Mult Scler Relat Disord. 2019;31:157–64.
Kalincik T, Spelman T, Jokubaitis V, et al. Effectiveness of fingolimod, dimethyl fumarate and teriflunomide in relapsing-remitting multiple sclerosis: a comparative longitudinal study [abstract no. P677]. Mult Scler J. 2017;23(Suppl 3):325–7.
Braune S, Grimm S, van Hovell P, et al. Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry. J Neurol. 2018;265(12):2980–92.
Rosenkranz T, Engelmann U, Kullmann JS. Teriflunomide for relapsing-remitting multiple sclerosis: a multicentre, non- interventional, prospective study in Germany (TAURUS-MS I) [abstract no. P918]. Mult Scler J. 2018;24(Suppl 2):496–7.
Laplaud DA, Barbin L, Casey R, et al. Comparative efficacy of teriflunomide versus dimethyl-fumarate on clinical and MRI outcomes: a two years French multicenter observational study [abstract no. 226]. Mult Scler J. 2018;24(Suppl 2):84–5.
Buron M, Magyari M, Chalmer TA, et al. Comparative effectiveness of teriflunomide and dimethyl fumarate in relapsing remitting multiple sclerosis. A Danish nationwide cohort study [abstract no. 227]. Mult Scler J. 2018;24(Suppl 2):85–6.
Magyari M, Buron M, Illes Z. The Danish experience of teriflunomide treatment in relapsing remitting multiple sclerosis [abstract no. P883]. Mult Scler J. 2018;24(Suppl 2):472.
D’Amico E, Zanghi A, Callari G, et al. Comparable efficacy and safety of dimethyl fumarate and teriflunomide treatment in relapsing-remitting multiple sclerosis: an Italian real-word multicenter experience. Ther Adv Neurol Disord. 2018;11:1–14.
Da Silva MCV, Conway D, Cox G, et al. Time to treatment failure following initiation of fingolimod versus teriflunomide for the treatment of multiple sclerosis: a retrospective U.S. claims study [abstract no. P1.372]. Neurology. 2018;90(15 Suppl).
Bowen J, Kozma CM, Grosso M, et al. Real-world assessment of relapse in patients with multiple sclerosis newly initiating scIFNbeta1a compared with oral disease-modifying drugs [abstract no. P1245]. Mult Scler J. 2017;23(Suppl 3):665.
Ontaneda D, Nicholas J, Carraro M, et al. Comparative effectiveness of dimethyl fumarate versus fingolimod and teriflunomide among MS patients switching from first-generation platform therapies in the US. Mult Scler Relat Disord. 2019;27:101–11.
Zivadinov R, Kresa-Reahl K, Weinstock-Guttman B, et al. Comparative effectiveness of teriflunomide and dimethyl fumarate in patients with relapsing forms of MS in the retrospective real-world Teri-RADAR study. J Comp Eff Res. 2019;85(5):305–16.
Vermersch P, Saiz A, Grigoriadis N, et al. Assessing teriflunomide treatment satisfaction in clinical trial and real- world settings: TENERE and TAURUS-MS I [abstract no. P885]. Mult Scler J. 2018;24(Suppl 2):473–4.
Duquette P, Yeung M, Mouallif S, et al. A retrospective claims analysis: compliance and discontinuation rates among Canadian patients with multiple sclerosis treated with disease-modifying therapies. PLoS One. 2019;14(1):e0210417.
Johnson KM, Zhou H, Lin F, et al. Real-world adherence and persistence to oral disease-modifying therapies in multiple sclerosis patients over 1 year. J Manag Care Spec Pharm. 2017;23(8):844–52.
D’Amico E, Zanghi A, Sciandra M, et al. Discontinuation of teriflunomide and dimethyl fumarate in a large Italian multicentre population: a 24-month real-world experience. J Neurol. 2019;266(2):411–6.
Comi G, Freedman MS, Kappos L, et al. Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions. Mult Scler Relat Disord. 2016;5:97–104.
Coyle P, Miller A, Gold R, et al. Lymphocyte counts in patients treated with teriflunomide: observations from phase 3 clinical trials and the real-world Teri-PRO study [abstract no. P5.376]. Neurology. 2018;90(15 Suppl).
Comi G, Miller AE, Benamor M, et al. No association of infection with reduced lymphocyte counts: results from up to 6 years of teriflunomide treatment in patients with relapsing forms of MS (RMS) in the TOWER core and extension studies [abstract no. PR1113]. Eur J Neurol. 2017;24(Suppl 1):510.
Comi G, Miller A, Benamor M, et al. Limited impact of long-term teriflunomide treatment on lymphocyte counts and infection rates in the pooled TEMSO and TOWER core and extension trials [abstract no. P041]. Mult Scler J. 2018;24(Suppl 1):29–30.
Vukusic S, Coyle PK, Jurgensen S, et al. Pregnancy outcomes in patients with multiple sclerosis treated with teriflunomide: clinical study data and 5 years of post-marketing experience. Mult Scler J. 2019. https://doi.org/10.1177/1352458519843055.
Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther. 2014;3(2):133–8.
Vukusic S, Coyle PK, Jurgensen S, et al. Pregnancy outcomes in patients with MS treated with teriflunomide: clinical study and postmarketing data [abstract no. P4.361]. Neurology. 2018;90(15 Suppl).
Andersen JB, Moberg JY, Spelman T. Pregnancy outcomes in teriflunomide exposed men and women: a nationwide Danish registry-based study [abstract no. P354]. Mult Scler J. 2018;24(Suppl 2):137–8.
Leray E, Reilhac A, Kerbrat S. Incidence of pregnancy in women with multiple sclerosis treated with teriflunomide in France [abstract no. P1253]. Mult Scler J. 2018;24(Suppl 2):715.
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25(2):215–37.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):777–88.
National Institute for Health and Care Excellence. Teriflunomide for treating relapsing-remitting multiple sclerosis. 2014. http://www.nice.org.uk/guidance/ta303. Accessed 13 Mar 2019.
Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):789–800.
Lorefice L, Fenu G, Gerevini S, et al. PML in a person with multiple sclerosis: is teriflunomide the felon? Neurology. 2018;90(2):83–5.
Acknowledgements
During the peer review process, the manufacturer of teriflunomide was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Lesley Scott is a salaried employee of Adis International Ltd./Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by:E. Bernitsas, Department of Neurology, Wayne State University, Detroit, MI, USA; S. de Biase, Neurology Unit, Department of Experimental and Clinical Medical Sciences, University of Udine Medical School, Udine, Italy; F. Ladeira, Hospital de Egas Moniz, Centro Hospitalar de Lisboa Ocidental, Lisbon, Portugal.
Rights and permissions
About this article
Cite this article
Scott, L.J. Teriflunomide: A Review in Relapsing–Remitting Multiple Sclerosis. Drugs 79, 875–886 (2019). https://doi.org/10.1007/s40265-019-01135-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01135-8