Abstract
Background
Immunocompromised individuals are at high risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and subsequent severe or fatal coronavirus disease 2019 (COVID-19), yet they have suboptimal responses to mRNA and inactivated COVID-19 vaccines. The efficacy of tixagevimab–cilgavimab in reducing symptomatic SARS-CoV-2 infection was demonstrated in phase III clinical trials. Nevertheless, real-world data on the effectiveness and safety of tixagevimab–cilgavimab remain limited.
Objective
The aim was to evaluate the effectiveness and safety of tixagevimab–cilgavimab among immunocompromised individuals.
Methods
Adults who were immunocompromised or receiving immunosuppressive therapies were included in this target trial emulation using territory-wide electronic health records in Hong Kong. A sequential trial emulation approach was adopted to compare effectiveness and safety outcomes between individuals who received tixagevimab–cilgavimab and individuals who did not.
Results
A total of 746 tixagevimab–cilgavimab recipients and 2980 controls were included from 1 May 2022 to 30 November 2022. Tixagevimab–cilgavimab significantly reduced the risk of COVID-19 infection (hazard ratio [HR] 0.708, 95% confidence interval [CI] 0.527–0.951) during a median follow-up of 60 days. No significant difference was observed in the risk of COVID-19-related hospitalisation. Zero versus eight COVID-19 mortality cases and zero versus two severe COVID-19 cases were observed in tixagevimab–cilgavimab recipients and controls, respectively. Notably, significant risk reduction in COVID-19 infection was also observed among immunocompromised individuals who had been previously vaccinated with three or more doses of COVID-19 vaccine, or had no prior COVID-19 infection history.
Conclusions
Tixagevimab–cilgavimab was effective in reducing COVID-19 infection among immunocompromised patients during the Omicron wave. Findings were consistent among individuals who previously received three or more doses of COVID-19 vaccine, or had no previous history of COVID-19 infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with a weakened immune system have a poorer response to coronavirus disease 2019 (COVID-19) vaccines; thus, they are in need of additional protection. |
Tixagevimab–cilgavimab significantly reduced the risk of Omicron infection among patients with a weakened immune system, including patients previously vaccinated with booster doses of COVID-19 vaccines. |
Its safety and effectiveness against severe COVID-19 remains to be evaluated in a larger population. |
1 Introduction
Immunocompromised individuals are particularly vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and associated severe and fatal coronavirus disease 2019 (COVID-19). Patients with immunocompromised conditions or on immunosuppressive therapies are not only more likely to become severely ill from COVID-19 [1], they are also more likely to experience suboptimal vaccine response. The low effectiveness of mRNA and inactivated COVID-19 vaccines was observed in several immunocompromised populations, including those with primary immunodeficiencies, autoimmune diseases, haematological malignancies, and prior organ transplantation, as well as those receiving immunomodulatory treatments and dialysis [2,3,4,5].
Tixagevimab–cilgavimab is a combination product of two neutralising IgG1 monoclonal antibodies that bind to distinct, non-overlapping epitopes within the receptor binding domain of the spike protein of SARS-CoV-2. Unlike vaccines that offer protection against COVID-19 by stimulating humoral and adaptive immunity, monoclonal antibodies in general block attachment of SARS-CoV-2 to the human angiotensin-converting enzyme 2 (ACE2) receptor and viral entry, and thus can be particularly helpful for those with weakened immune responses. The available evidence concerning the effectiveness and safety of tixagevimab–cilgavimab remains limited. Insight into the benefits and risks of tixagevimab–cilgavimab for protection against Omicron infection can inform clinical guidelines for prophylaxis of COVID-19 among the immunocompromised population.
In the Phase III Double-blind, Placebo-controlled Study of AZD7442 for Pre-exposure Prophylaxis of COVID-19 in Adult (PROVENT) trial, tixagevimab–cilgavimab was shown to reduce the incidence of symptomatic SARS-CoV-2 infection by 82.8% at a median of 6 months of follow-up in the context of the Alpha variant wave [6]. Consequently, tixagevimab–cilgavimab has been approved by the Food and Drug Administration (FDA) for emergency use as pre-exposure prophylaxis against COVID-19 among adults and adolescents (12 years of age and older weighing at least 40 kg) who are not currently infected or recently exposed with SARS-CoV-2 and have moderate to severe immune compromise or are not recommended to receive the available COVID-19 vaccines due to a history of severe adverse reaction [7]. The recommended initial dosage is 300 mg of tixagevimab and 300 mg of cilgavimab. These two doses should be administered as separate consecutive intramuscular injections. Due to limited neutralising activity against Omicron variants [8], tixagevimab–cilgavimab is not currently authorised for emergency use in the pre-exposure prophylaxis of COVID-19 in the United States (US). However, tixagevimab–cilgavimab retains reduced neutralising activity against the Omicron BA.2 and BA.5 [9], which continue to dominate in most Asian regions, including mainland China and Hong Kong [10,11,12].
As a new pre-exposure prophylaxis agent, tixagevimab–cilgavimab's postmarketing real-world evidence remains limited. Tixagevimab–cilgavimab was first conditionally approved for emergency use in Hong Kong in May 2022. This study aimed to evaluate the safety and effectiveness of tixagevimab–cilgavimab against SARS-CoV-2 infection, other COVID-19-related outcomes, and adverse events in the real-world setting, using territory-wide electronic healthcare records data.
2 Material and Methods
2.1 Data Source
We obtained clinical data from the electronic health records database of the Hospital Authority (HA), vaccination records from the Department of Health (DH), and COVID-19 confirmed case records from the Center of Health Protection (CHP) of the Government of the Hong Kong Special Administrative Region (HKSAR). The HA is the statutory administrative organisation managing the public healthcare sector, providing all public inpatient services and the majority of outpatient services in Hong Kong. The electronic health records database of the HA contains data on patient demographics, diagnoses, procedures, prescriptions, laboratory tests, inpatient admissions, and outpatient and emergency department attendances, providing real-time information to support routine clinical management across all public clinics and hospitals. The DH maintains a database of COVID-19 vaccination records for all Hong Kong citizens. The CHP maintains a database of all confirmed COVID-19 cases, based on both mandatory and voluntary reporting of positive polymerase chain reaction (PCR) and rapid antigen test (RAT) results. These territory-wide databases were integrated using unique anonymised patient identifiers, and have been frequently applied in prior studies about the risk of adverse effects following COVID-19 vaccinations and the effectiveness of COVID-19 oral antivirals [13, 14].
The Hong Kong government has implemented extensive PCR testing for SARS-CoV-2 in public hospitals and clinics for close contacts with confirmed cases. Territory-wide community testing centres were also in place to screen asymptomatic individuals and provide regular testing to various staff groups with a high risk of exposure, such as those working in nursing homes. Compulsory SARS-CoV-2 testing were conducted among three groups: close contacts of confirmed cases, high-risk individuals, and residents in areas suspected of having local outbreaks or positive sewage test results. Individuals were required to fulfill this mandate by reporting either PCR or RAT test results [15]. Reporting of positive RAT results via an online system set up by the HKSAR government was necessary to obtain official proof of SARS-CoV-2 infection, which was required for purposes such as the fulfilment of a compulsory testing notice, exemption of booster vaccination requirements, and application of sick leave [16]. The DH also conduct random checking on voluntarily reported RAT results, and it is an offence to declare false information. Thus, it is expected that the possibility of false-positives is minimal while the proportion of missed asymptomatic infections remains relatively small compared to other regions relying solely on voluntary testing.
2.2 Study Design and Eligibility Criteria
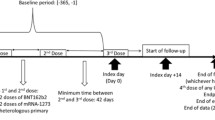
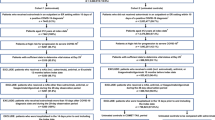
A target trial emulation study was conducted using territory-wide electronic health records databases in Hong Kong. The target trial emulation approach is appropriate to prevent some of the common pitfalls encountered in observational study designs, such as immortal time and selection biases [17]. The setting of this target trial was largely adapted from the PROVENT trial [6], with important differences in the eligibility criteria to reflect real-world clinical practice, which is discussed further below. The specification and emulation of the target trial are presented in Supplementary Table 1 (see the electronic supplementary material). The participant inclusion period was from 1 May 2022 (when tixagevimab–cilgavimab became available in Hong Kong) to 30 November 2022 (to allow at least 60 days of follow-up). Individuals aged ≥ 12 years who had an immunocompromised condition or had received immunosuppressive treatment within 1 year were included (see Supplementary Table 2 for detailed definitions). These eligibility criteria were selected in accordance with real-world clinical practice, since currently the use of tixagevimab–cilgavimab is limited to the immunocompromised population in Hong Kong, despite being approved by the FDA for any individuals aged ≥ 12 years prone to inadequate response to COVID-19 vaccines [7]. The dosage of tixagevimab–cilgavimab was 300 + 300 mg. Patients who had previously received COVID-19 vaccination or had a history of COVID-19 infection (> 90 days before baseline [18]) remained eligible, and their vaccination and infection status were accounted for in the analyses. Patients who had a recent COVID-19 infection within the past 90 days were excluded, to prevent counting re-positivity of a previous SARS-CoV-2 infection as an outcome [19]. Patients who died on or before baseline were also excluded.
2.3 Exposure and Control Matching
A sequential trial emulation approach was adopted to compare the risk of outcomes between individuals who received tixagevimab–cilgavimab and individuals who did not receive tixagevimab–cilgavimab [20, 21]. On each day during the participant inclusion period (index date), all eligible individuals who newly received tixagevimab–cilgavimab (recipients) were matched 1:4 to eligible individuals who had not yet received tixagevimab–cilgavimab on that day (controls), using exact matching (and nearest-neighbour matching with a narrow calliper of 0.2 when exact matching was not feasible). Covariates matched included age, sex, number of COVID-19 vaccine doses received (exact match), months since last COVID-19 vaccine dose, history of COVID-19 infection (exact match), months since previous COVID-19 infection, Charlson Comorbidity Index (CCI) score, type of immunocompromised condition, immunosuppressive therapies received within 1 year, and presence of other comorbidities (chronic kidney disease, respiratory disease, diabetes, cardiovascular disease, dementia). These covariates were selected since they were potential confounders of tixagevimab–cilgavimab treatment and COVID-19 infection and severity. These covariates were time-varying and updated at daily intervals.
The index date for tixagevimab–cilgavimab recipients was defined as the date of first prescription of tixagevimab–cilgavimab, whereas the index date for controls was assigned as per their matched tixagevimab–cilgavimab recipients. Individuals were followed up from the index date until the earliest outcome occurrence, death, 60 days after the index date, or the end of data availability (31 January 2023). Controls who received tixagevimab–cilgavimab after the index date were censored on the date of first tixagevimab–cilgavimab prescription (the corresponding individuals matched with these controls were also censored), and would be re-enrolled as a tixagevimab–cilgavimab recipient with a new set of matched controls.
2.4 Outcomes
The primary outcome was COVID-19 infection, defined as a positive PCR or RAT result. Secondary outcomes included (1) COVID-19-related hospitalisation, defined as hospital admission within 28 days after COVID-19 infection; (2) severe COVID-19, defined as intensive care unit (ICU) admission or use of ventilatory support within 28 days after COVID-19 infection; and (3) COVID-19-related mortality, defined as all-cause mortality within 28 days after COVID-19 infection. Information regarding mortality was extracted from the Hong Kong Deaths Registry, the official government registry documenting all registered deaths in Hong Kong. Use of ventilatory support was identified using International Classification of Diseases-Ninth Revision (ICD-9) procedure codes (39.65, 89.18, 93.90, 93.95, 93.96, 96.7, 96.04).
Safety outcomes for descriptive analyses included a pre-specified list of adverse events of special interest (AESIs) covering various organ systems, identified using ICD-9 codes (Supplementary Table 3; see the electronic supplementary material). These AESIs were adapted from those endorsed by the World Health Organization (WHO) and the European Medicines Agency for the safety surveillance of COVID-19 vaccines, and have been used in previous studies to evaluate the safety of COVID-19 vaccines in Hong Kong [22], and they are also applicable to the safety assessment of pharmaceuticals, particularly biological medicines. The validity of ICD-9 codes in our database has been demonstrated in previous population-based studies, with a high positive predictive value (PPV) for many diagnoses [23, 24].
2.5 Statistical Analysis
Covariate balance in the matched cohort was assessed, and an acceptable threshold for the standardised mean difference (SMD) between tixagevimab–cilgavimab recipients and controls was set at 0.2 or less for all covariates. Survival curves were presented using the Kaplan–Meier estimator, and p values of the log-rank test were reported. Cox proportional hazards regression was used to compare the risk of outcomes between tixagevimab–cilgavimab recipients and controls. Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported. Schoenfeld residuals test was conducted to test the assumption of proportional hazards. For safety outcomes where the number of events was limited, incidence rates were reported with 95% CIs estimated based on Poisson distribution. As the follow-up duration was relatively short, post-baseline time-varying covariates and competing risk of death were not considered in this study.
Pre-specified subgroup analyses stratified by vaccination status (0–2 or ≥ 3 vaccine doses received), age (12–59, ≥ 60 years), sex (male, female), and CCI score (0–4, ≥ 5) were carried out. Interaction effects between treatment and vaccination status, age (continuous variable), sex, and CCI score (continuous variable) were also tested, and the p values for interaction were reported. Sensitivity analyses was conducted where the definition of patients with cancer was restricted to only patients with active cancer (defined as patients with cancer undergoing chemotherapy or radiotherapy or those who had metastasis within 1 year before the index date), rather than all patients with a cancer diagnosis before index.
All statistical tests were two-sided, and p values less than 0.05 were considered statistically significant. Statistical analysis was conducted using R version 4.0.3 (http://www.R-project.org). Two investigators (VY, YY) conducted the statistical analyses independently for quality assurance. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklists were followed to guide transparent reporting of the cohort study.
3 Results
3.1 Patient Characteristics
A total of 229,093 immunocompromised patients were identified during the inclusion period. After exclusion and matching, 746 tixagevimab–cilgavimab recipients and 2980 controls were included (Fig. 1). The mean (SD) age was 61.0 (13.45) and 61.5 (13.2) years respectively, with 54.0% male in both groups (Table 1). The majority in both groups were previously vaccinated, with 67.3% of both groups receiving three or more doses of COVID-19 vaccine (BNT161b2 or CoronaVac), and 88.0% received two or more doses, although on average, around 160 days had passed since their last vaccine dose. A quarter (24.9% in both groups) of individuals had a previous COVID-19 infection, with a mean (SD) duration of 229.32 (86.28) and 237.24 (66.59) days, respectively, since their previous infection. The most prevalent immunocompromised condition was cancer (78.6% in tixagevimab–cilgavimab recipients and 78.4% in controls). More than half of the individuals received chemotherapy (59.9% in both groups) or immunosuppressants (58.7% in both groups) in the past year. All baseline characteristics were well-balanced between the two groups, with an SMD < 0.2 (Table 1).
3.2 Outcomes
After a median (interquartile range, IQR) follow-up of 60 (34) days, 52 COVID-19 infection events were observed in tixagevimab–cilgavimab recipients and 286 in controls. Tixagevimab–cilgavimab was associated with a significantly reduced risk of COVID-19 infection (HR 0.708, 95% CI 0.527–0.951). For COVID-19-related hospitalisation, 21 and 92 COVID-19 infection events were observed in tixagevimab–cilgavimab recipients and controls, respectively. There was no statistically significant difference in the risk of COVID-19-related hospitalisation between tixagevimab–cilgavimab recipients and controls (HR 0.902, 95% CI 0.562–1.449). For COVID-19-related mortality, no events were observed in tixagevimab–cilgavimab recipients, while eight (0.268%) events were observed in controls. Similarly, no events of severe COVID-19 (ICU admission or ventilatory support) were observed in tixagevimab–cilgavimab recipients, while two events were observed in controls (Table 2; Fig. 2). Schoenfeld residuals test for the main outcomes of COVID-19 infection and COVID-19-related hospitalization demonstrated that the proportional hazards assumption was not violated (p = 0.65 and p = 0.42 for the two outcomes, respectively). Findings for COVID-19 infection and COVID-19-related hospitalisation were consistent across subgroups of age, sex, CCI, vaccination status, and COVID-19 infection history. Notably, a significant risk reduction in COVID-19 infection was observed in immunocompromised individuals who had been previously vaccinated with three or more doses of COVID-19 vaccine, or who had no prior COVID-19 infection history (Table 3). Results of sensitivity analyses were consistent with our main analyses (Supplementary Table 4; see the electronic supplementary material).
During the follow-up period, only one case of transient ischaemic attack (also classified as a case of major cardiovascular disease or thromboembolism) and two cases of herpes zoster occurred among tixagevimab–cilgavimab recipients. Nevertheless, the absolute incidence rates for all AESIs were low (< 1 in 10,000 person-days) and no significantly increased risk was observed compared to controls (Table 2). It should, however, be noted that this study has limited statistical power to detect rare adverse events, and further study with a larger sample size is warranted.
4 Discussion
Our findings revealed that tixagevimab–cilgavimab administration was associated with 29.2% (95% CI 4.9–47.3) reduced risk of COVID-19 infection among immunocompromised patients compared with non-users in Hong Kong. Subgroup analysis showed that tixagevimab–cilgavimab remained significantly effective in reducing the risk of COVID-19 infection for individuals who were three-dose vaccinated (36.4%) or without a history of COVID-19 infection (27.2%). Lower incidence rates of COVID-19-related hospitalisation, severe COVID-19, and COVID-19-related mortality were also observed among patients who received tixagevimab–cilgavimab compared with controls. However, due to the limited number of events, our study could not demonstrate statistical significance in severe or fatal COVID-19 and other safety outcomes.
The PROVENT randomised controlled trial (RCT) was conducted during a period when the Alpha variant was dominant. This study reported that the tixagevimab–cilgavimab group had a 76.7% (95% CI 46.0–90.0) relative reduction in the risk of symptomatic COVID-19 infection after 6 months and an 82.8% (95% CI 65.8–91.4) reduction during the extended follow-up period. Three cases of COVID-19-related hospitalisations (0.2%), five cases of severe or critical COVID-19 (0.3%), and two cases of COVID-19-related deaths (0.1%) occurred, all in the placebo group [6]. Another phase III RCT, TACKLE, during the Alpha variant reported that tixagevimab–cilgavimab provided significant protection against progression to severe COVID-19 or death, with a relative risk reduction of 50.5% after 457 days. In this study, there were three COVID-19-related deaths in the tixagevimab–cilgavimab group and six in the placebo group [25]. Recent trials evaluating in vitro neutralising activity of monoclonal antibodies found that tixagevimab lost neutralising activity against most Omicron sub-lineages; hence, cilgavimab was considered the main monoclonal antibody responsible for the drug’s activity against Omicron sub-lineages [26]. Studies on serum neutralisation monoclonal antibodies among patients who received monoclonal antibodies revealed that the neutralising activity of tixagevimab–cilgavimab was reduced against BA.1 (by 344 times) and BA.2 (by 9 times) when compared to the Delta variant [27]. Therefore, the FDA revised its emergency use authorisation for tixagevimab–cilgavimab but may reinstate authorisation if the prevalence of resistant variants decreases to 90% or less on a sustained basis. However, preclinical pseudovirus assay data from the University of Oxford revealed that tixagevimab–cilgavimab still retained neutralisation activity against BA.4 and BA.5 [28]. During our study period, the Omicron BA.2 and BA.4/BA.5 were the dominant variants in Hong Kong from January 2022 until May 2023 [10,11,12]. Therefore, our study showed that tixagevimab–cilgavimab still retained effectiveness against COVID-19 infection among immunocompromised patients in Hong Kong during this Omicron wave, despite reduced effectiveness compared with RCTs [28]. In our study, more than 60% of the participants received three doses of COVID-19 vaccines. This is in contrast to the PROVENT and TACKLE RCTs, which recruited participants with non-COVID-19 vaccination [6, 25], and the ACTIV-3 RCT, in which 26.1% of participants received either full or partial two-dose vaccines [29]. Previous studies reported that vaccine response was significantly reduced in immunocompromised patients [30], such that the risks of COVID-19 infection and severe complications remain high in immunocompromised patients even after they received COVID-19 vaccines. Our results revealed that tixagevimab–cilgavimab pre-exposure prophylaxis was associated with lower breakthrough infection risk in three-dose vaccinated immunocompromised patients during the Omicron wave, which was consistent with a retrospective cohort study among solid-organ transplant recipients [31].
The average days since last dose in our cohort was approximately 160, which suggests that the vaccine response may have already waned [32] and tixagevimab–cilgavimab provided further protection in these immunocompromised patients. Pharmacokinetic studies revealed that the mean half-lives of tixagevimab and cilgavimab were approximately 80 days [33]. Subgroup analysis also revealed that tixagevimab–cilgavimab significantly reduced the risk of COVID-19 infection in immunocompromised patients without previous infection, but not in those with previous infection. The insignificant protective effect among patients with previous infection could be due to the limited sample size of immunocompromised patients with history of COVID-19 infection as well as hybrid immunity produced by previous infection.
In addition, our study found tixagevimab–cilgavimab was safe, with lower absolute incidence rates for AESIs than the control group, including two cases of herpes zoster, one case of major cardiovascular disease, and one case of thromboembolism. One case of transient ischaemic attack was observed among the tixagevimab–cilgavimab recipients, but did not occur in the control group. Anaphylaxis, myocarditis, heart failure, acute liver injury, and acute kidney injury were not observed in this study. There is currently no evidence that tixagevimab–cilgavimab is significantly associated with any serious adverse events. A large population-based propensity-matched study found no increased risk of cardiovascular events up to 90 days after tixagevimab–cilgavimab administration, including in patients with pre-existing cardiovascular disease [34], while the ACTIV-3 RCT reported six cardiac-related deaths in the tixagevimab–cilgavimab group [35]. Another study reported tixagevimab–cilgavimab may increase the risk of venous and arterial thromboembolic events based on the WHO VigiBase database [36]. Our findings did not demonstrate a significantly increased incidence of thromboembolism, though further studies are warranted due to the limited statistical power in this study.
This is the first real-world study on the safety and effectiveness of tixagevimab–cilgavimab among immunocompromised patients in a Chinese population. Currently, there are only a few real-world studies on the effectiveness and safety of tixagevimab–cilgavimab against the Omicron lineages BA.1 and BA.2 [37, 38], and no study has been conducted among the Chinese population. We provided comprehensive real-world analyses of effectiveness and safety-related outcomes among vaccinated and non-vaccinated patients based on an electronic healthcare records database, which can supplement data derived from RCTs. Most Asian regions, including mainland China and the HKSAR, are still BA.2 and BA.5 variant dominated, although the XBB variants are surging fast. Our study found that tixagevimab–cilgavimab may still offer benefit to immunocompromised patients in these regions, where the next generation of monoclonal antibodies for pre-exposure prophylaxis of COVID-19 that are currently unavailable [39].
Our study has some limitations. First, although this study utilised a target trial emulation approach and exact (or nearest) matching of essential confounders to minimize selection biases and biases due to measured confounders, the possibility of residual confounding due to unmeasured factors such as differences in health behaviours could not be ruled out. Second, the sample size of this study was small, which limited the statistical power to evaluate the safety of tixagevimab–cilgavimab. Nevertheless, we observed that the absolute incidence rates for AESIs were very low, and current observational studies on tixagevimab–cilgavimab conducted in Western populations have small sample sizes because of the restrictions on tixagevimab–cilgavimab administration. While our study showed benefit of tixagevimab–cilgavimab against COVID-19 infection, its effectiveness against severe COVID-19 outcomes was not demonstrated due to limited statistical power, and this warrants further research. Third, in common with all other COVID studies, some asymptomatic COVID-19 infection cases could be missed (undiagnosed or not reported). Hence, the rate of COVID-19 infection may be underestimated. Nevertheless, immunocompromised individuals were at higher baseline risk of illness from SARS-CoV-2 infection and thus likely to seek medical care and receive SARS-CoV-2 testing when ill. Fourth, COVID-19-related hospitalisation, severe COVID-19, and COVID-19-related mortality were defined outcomes within 28 days after COVID-19 infection. We could not distinguish whether the patient was hospitalised or died from complications due to COVID-19 or other causes. Finally, the follow-up period of this study was limited, so future studies on long-term safety and effectiveness as well as analyses of different immunocompromised disease subgroups will further our understanding of tixagevimab–cilgavimab use in immunocompromised individuals.
5 Conclusions
Tixagevimab–cilgavimab was effective in reducing COVID-19 infection among immunocompromised patients, including those who were previously vaccinated with three or more doses of COVID-19 vaccine, or had no prior COVID-19 infection history during the Omicron wave. There remains a protective role among those immunocompromised who may have poor antibody response after four or even five booster doses, especially in regions where BA.2 and BA.5 Omicron variants are still the predominantly circulating strains. Further studies are warranted to evaluate tixagevimab–cilgavimab’s safety and effectiveness against severe COVID-19 in a larger population.
References
Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. https://doi.org/10.1038/s41586-020-2521-4.
Monin L, Laing AG, Munoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. https://doi.org/10.1016/S1470-2045(21)00213-8.
Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105(11):e265–6. https://doi.org/10.1097/TP.0000000000003907.
Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161(3):827–36. https://doi.org/10.1053/j.gastro.2021.05.044.
Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transpl. 2021;36(9):1709–16. https://doi.org/10.1093/ndt/gfab179.
Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386(23):2188–200. https://doi.org/10.1056/NEJMoa2116620.
Food and Drug Administration (FDA). Fact sheet for healthcare providers: emergency use authorization for EvusheldTM (Tixagevimab Co-Packaged with Cilgavimab). Available at: https://www.fda.gov/media/154701/download. Accessed May 11 2023.
Focosi D, Casadevall A. A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (Evusheld) for COVID-19 prophylaxis and treatment. Viruses. 2022. https://doi.org/10.3390/v14091999.
Takashita E, Yamayoshi S, Simon V, et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387(5):468–70. https://doi.org/10.1056/NEJMc2207519.
Centre for Health Protection. Archives of latest situation of cases of COVID-19. Available at: https://www.chp.gov.hk/en/features/102997.html. Accessed May 11.
Surveillance Division of the Communicable Disease Branch of the Centre for Health Protection. COVID-19 & flu express. Available at: https://www.chp.gov.hk/en/resources/29/100148.html. Accessed Nov 9 2023.
Mefsin YM, Chen D, Bond HS, et al. Epidemiology of infections with SARS-CoV-2 Omicron BA.2 variant, Hong Kong, January–March 2022. Emerg Infect Dis. 2022;28(9):1856–8. https://doi.org/10.3201/eid2809.220613.
Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021;21:00451–5. https://doi.org/10.1016/S1473-3099(21)00451-5.
Lai FTT, Li X, Peng K, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case–control study. Ann Intern Med. 2022;175(3):362–70. https://doi.org/10.7326/M21-3700.
Home Affairs Department. Distribution of rapid antigen test kits to people in areas with positive sewage test results. Available at: https://www.had.gov.hk/en/whats_new/press_18_2.htm. Accessed Jan 2 2024.
HKSAR Government Press Release. Truthful test declarations urged. Available at: https://www.news.gov.hk/eng/2022/03/20220324/20220324_182818_404.html. Accessed Mar 24 2022.
Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–64. https://doi.org/10.1093/aje/kwv254.
Centers for Disease Control and Prevention. What is COVID-19 reinfection. Available at: https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html. Accessed May 12 2023.
Ukwishaka J, Ndayishimiye Y, Destine E, Danwang C, Kirakoya-Samadoulougou F. Global prevalence of coronavirus disease 2019 reinfection: a systematic review and meta-analysis. BMC Public Health. 2023;23(1):778. https://doi.org/10.1186/s12889-023-15626-7.
Danaei G, Rodríguez LA, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22(1):70–96. https://doi.org/10.1177/0962280211403603.
Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765.
Wong CKH, Lau KTK, Xiong X, et al. Adverse events of special interest and mortality following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines in Hong Kong: A retrospective study. PLoS Med. 2022;19(6): e1004018. https://doi.org/10.1371/journal.pmed.1004018.
Chan EW, Lau WC, Leung WK, et al. Prevention of Dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149(3):586-95 e3. https://doi.org/10.1053/j.gastro.2015.05.002.
Kwok WC, Tam TCC, Sing CW, Chan EWY, Cheung CL. Validation of diagnostic coding for asthma in an electronic health record system in Hong Kong. J Asthma Allergy. 2023;16:315–21. https://doi.org/10.2147/JAA.S405297.
Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022;10(10):985–96. https://doi.org/10.1016/S2213-2600(22)00180-1.
Touret F, Baronti C, Pastorino B, et al. In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5. Sci Rep-Uk. 2022. https://doi.org/10.1038/s41598-022-16964-z.
Bruel T, Hadjadj J, Maes P, et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022;28(6):1297–302. https://doi.org/10.1038/s41591-022-01792-5.
Tuekprakhon A, Huo J, Nutalai R, et al. Further antibody escape by Omicron BA.4 and BA.5 from vaccine and BA.1 serum. bioRxiv. 2022. https://doi.org/10.1101/2022.05.21.492554.
ACTIV-3-Therapeutics for Inpatients with COVID-19 Study Group. Tixagevimab–cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2022;10(10):972–84. https://doi.org/10.1016/S2213-2600(22)00215-6.
Lee A, Wong SY, Chai LYA, et al. Efficacy of Covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376: e068632. https://doi.org/10.1136/bmj-2021-068632.
Al Jurdi A, Morena L, Cote M, Bethea E, Azzi J, Riella LV. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant. 2022;22(12):3130–6. https://doi.org/10.1111/ajt.17128.
Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24): e84. https://doi.org/10.1056/NEJMoa2114583.
Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327(4): 384–5. https://doi.org/10.1001/jama.2021.24931.
Birabaharan M, Hill E, Begur M, Kaelber DC, Martin TCS, Mehta SR. Cardiovascular outcomes after tixagevimab and cilgavimab use for pre-exposure prophylaxis against coronavirus disease 2019: a population-based propensity-matched cohort study. Clin Infect Dis. 2023;76(8):1500–3. https://doi.org/10.1093/cid/ciac894.
Piszczek J, Murthy S, Afra K. Cardiac and vascular serious adverse events following tixagevimab–cilgavimab. Lancet Respir Med. 2023;11(1):e5–6. https://doi.org/10.1016/S2213-2600(22)00452-0.
Montastruc F, Lafaurie M, Flumian C, de Canecaude C. Increased reporting of venous and arterial thromboembolic events reported with tixagevimab–cilgavimab for coronavirus disease 2019. Clin Microbiol Infect. 2023;29(4):543 e1-e3. https://doi.org/10.1016/j.cmi.2022.11.026.
Nguyen Y, Flahault A, Chavarot N, et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect. 2022;28(12):1654 e1-e4. https://doi.org/10.1016/j.cmi.2022.07.015.
Najjar-Debbiny R, Gronich N, Weber G, Stein N, Saliba W. Effectiveness of Evusheld in immunocompromised patients: propensity score-matched analysis. Clin Infect Dis. 2023;76(6):1067–73. https://doi.org/10.1093/cid/ciac855.
ClinicalTrials.gov. Study Understanding Pre-Exposure pRophylaxis of NOVel Antibodies (SUPERNOVA) sub-study. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT05648110. Accessed Sept 11 2023.
Acknowledgements
We gratefully acknowledge the Centre for Health Protection, the Department of Health, and the Hospital Authority for facilitating data access.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by HMRF Research on COVID-19, the Hong Kong Special Administrative Region (HKSAR) Government (Principal Investigator (WP2): EWC; ref. no. COVID1903011). ICKW and FTTL are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by the by AIR@InnoHK administered by the Innovation and Technology Commission. The study funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest
EYFW has received research grants from the Health Bureau of the Government of the HKSAR, and the Hong Kong Research Grants Council, outside the submitted work. FTTL has been supported by a Research Grants Council Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Health Bureau of the Government of the HKSAR, outside the submitted work. CSLC has received grants from the Health Bureau of the Hong Kong Government, the Hong Kong Research Grants Council, the Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; and personal fees from PrimeVigilance; outside the submitted work. XL has received research grants from the Health Bureau of the Government of the HKSAR; research and educational grants from Janssen and Pfizer; internal funding from the University of Hong Kong; and consultancy fees from Merck Sharp & Dohme; unrelated to this work. CKHW has received research grants from the Health Bureau of the Hong Kong Government, the Hong Kong Research Grants Council, and the EuroQol Research Foundation, unrelated to this work. IFNH received speaker fees from MSD. ICKW reports research funding from Amgen, Bristol Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Hong Kong Health and Medical Research Fund, the National Institute for Health Research in England, the European Commission, and the National Health and Medical Research Council in Australia, outside the submitted work; and is a non-executive director of Jacobson Medical in Hong Kong and a consultant to IQVIA and the World Health Organization. EWC reports grants from the Research Grants Council (Hong Kong), the Research Fund Secretariat of the Health Bureau, the National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and the Narcotics Division of the Security Bureau of the HKSAR; and honorarium from the Hospital Authority; outside the submitted work. All other authors declare no competing interests.
Ethics approval
This study was approved by the Central Institutional Review Board of the Hospital Authority of Hong Kong (CIRB-2021-005-4) and the DH Ethics Committee (LM171/2021).
Consent to participate
Informed consent has been waived by the ethics committee, as this study used anonymised data and does not involve patient contact or intervention.
Consent for publication
Consent for publication was not required for this study, which used anonymised data without any patient identifiable information
Availability of data and materials
Data are not available as the data custodians (the Hospital Authority and the Department of Health of HKSAR) have not given permission for sharing due to patient confidentiality and privacy concerns. Local academic institutions, government departments, or non-governmental organisations may apply for access to the data through the Hospital Authority’s data-sharing portal (https://www3.ha.org.hk/data).
Code availability
Software codes used in this study are available upon request from the corresponding author.
Author contributions
VKCY, YY, EYFW, ICKW, and EWC contributed to the study conception and design. VKCY, YY, EYFW, FTTL, CSLC, XL, CKHW, ICKW, and EWC acquired and interpreted the data. VKCY and YY wrote the first draft of the manuscript and performed statistical analysis of the data. ICKW and EWC administered the project and provided technical and material support. EYFW, FTTL, CSLC, XL, CKHW, IFNH, CSL, ICKW, and EWC revised the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yan, V.K.C., Yang, Y., Wan, E.Y.F. et al. Real-World Effectiveness and Safety of Tixagevimab–Cilgavimab: A Target Trial Emulation Study. Drug Saf (2024). https://doi.org/10.1007/s40264-024-01450-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40264-024-01450-4