Abstract
Background and Objective
During the COVID-19 vaccination campaign, over 34,000 reports of heavy menstrual bleeding following the administration of COVID-19 vaccines originating in the Economic European Area were submitted to EudraVigilance, the European Union database of suspected adverse drug reactions. More than 90% of these reports were sent by consumers while the remaining by healthcare professionals. Public concerns regarding menstruation disorders in COVID-19 vaccinees were also covered by the media. We investigated the impact of media attention on the reporting trends of heavy menstrual bleeding to EudraVigilance.
Methods
We used media outlets published in the Economic European Area on menstrual disorders and COVID-19 vaccines from the beginning of the vaccination campaign in the Economic European Area (1 January, 2021) until December 2022 (i.e., after the regulatory request to add the adverse event to the product information) and spontaneous reports from EudraVigilance.
Results
We found that the publication of safety updates from regulatory authorities and subsequent coverage in media outlets preceded increased reporting to EudraVigilance. Furthermore, the heavy menstrual bleeding reported in the cases occurred several weeks or months earlier and were not submitted to the respective date. The analysis suggests that the spikes in reporting of heavy menstrual bleeding were to some extent influenced by media coverage in some countries.
Conclusions
Consumer reporting to the European Union spontaneous data collection system, EudraVigilance, was of high value for regulatory safety reviews, albeit the reporting behaviours were not free of the influence of the media. These sources of information can be investigated to understand the context of safety concerns of public health interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
To support regulatory decision of the European Union Pharmacovigilance Risk Assessment Committee during the continuous safety review of COVID-19 vaccines, the European Medicines Agency performed an analysis of spontaneous reports of heavy menstrual bleeding submitted by consumers to EudraVigilance, and a review of media reports of heavy menstrual bleeding following COVID-19 vaccination in the European Union. |
The analysis did not and could not investigate the causality with COVID-19 vaccines but focused on the reporting trends to EudraVigilance. |
Two other papers (Banovac, 2017 and Candore, 2022) published previously in Drug Safety analysed the features of patients’ reporting to EudraVigilance database. |
The present article adds information on the median reporting rate when high media interest is involved. |
In some European Union countries with high reporting rates (e.g. the Netherlands, France), the consumer reporting was influenced by communications from media and/or health authorities, with high volume of reports shortly after publications. |
1 Introduction
Within the first months of the COVID-19 vaccination campaign in 2021, temporary changes to both menstruation and the menstrual cycle after COVID-19 vaccination were reported to the EudraVigilance database (EV), the system for collecting, managing and analysing suspected adverse reactions to medicines authorised in the European Economic Area (EEA), and discussed in social media forums. Several media outlets cited women enquiring whether their experience of a menstrual cycle disturbance had any link with recent COVID-19 vaccination. Some media sources considered that these menstrual disorders were attributed to stress induced by the pandemic, also noting that menstrual irregularities occur routinely, and therefore dismissed a potential association with COVID-19 vaccination [1,2,3,4].
The European Union (EU) regulatory network reviewed the spontaneous reports of menstrual disorders with COVID-19 vaccines received in EV. Reports of amenorrhoea and heavy menstrual bleeding were reviewed as part of the post-authorisation surveillance of COVID-19 vaccines; regular updates on these assessments were communicated by the European Medicines Agency (EMA) to the public via regular vaccine safety updates and press briefings [5, 6].
Most of the spontaneous reports were reported directly by vaccinees and primarily concerned non-serious [7] case reports of irregularities of the menstrual cycle [8]. Because of the low proportion of medically confirmed [9] cases (10%), limited diagnostic data and information to exclude alternative aetiologies in the case reports particularly given the wide range of medical conditions that can lead to menstrual disorders, the initial evidence was not considered sufficient to warrant regulatory action. However, this safety issue was continuously monitored by EEA regulators in 2021. An increased number of spontaneous reports alone cannot determine whether there is an actual increased rate of an event. In view of the media attention, it seemed nevertheless relevant to further review this safety aspect.
In February 2022, prompted by an observational study from the Norwegian Institute of Public Health [10], a signal procedure dedicated to assessing all data available on heavy menstrual bleeding after COVID-19 vaccination with messenger (mRNA)-based vaccines was initiated by the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC). Previous studies have shown that media coverage can contribute to public concerns about vaccines or medicines and consequently influence the reporting of adverse events by consumers [11,12,13,14,15]. As menstrual disorders were reported mainly by vaccinees, in June 2022, PRAC discussed the possibility of media influence on reporting trends; thus, an analysis of spontaneous reporting to EV in relation to media outlets was considered complementary to the review of all other data sources to support the committee’s decision-making process for the signal procedure. Following the finalisation of the signal procedure on 27 October, 2022, PRAC concluded that there is a reasonable possibility that heavy menstrual bleeding is causally associated with mRNA-based COVID-19 vaccines and therefore recommended that it should be included as a listed side effect for both Comirnaty and Spikevax. Given that its incidence could not be determined based on the available data, it was listed with a frequency category of unknown [16].
In this paper, we describe the analysis of spontaneous reports of heavy menstrual bleeding in relation to media attention. We used media outlets published in the EEA on menstrual disorders and COVID-19 vaccines from the beginning of the vaccination campaign in the EEA (1 January, 2021) until December 2022 (i.e. after the regulatory request to add the adverse event to the product information) and spontaneous reports from EV. This analysis aimed to (1) describe the reporting trend of cases of heavy menstrual bleeding in the EEA following administration of COVID-19 vaccines and (2) investigate a potential association between EV case reporting and media attention. Importantly, the analysis did not investigate the causality between COVID-19 vaccination and episodes of heavy menstrual bleeding.
2 Material and Methods
2.1 EudraVigilance Search Criteria
Cases of heavy menstrual bleeding with an EU-authorised COVID-19 vaccine originating in the EEA were extracted from EV. The study period covered 1 January, 2021 to 13 December, 2022. The MedDRA Preferred Term (PT) “Heavy menstrual bleeding” was used to identify the adverse reaction; vaccines in Table 1 were used to identify the reported COVID-19 vaccine. In addition to cases with mRNA-based COVID-19 vaccines, cases of vaccines based on other platforms (i.e. Vaxzevria, Jcovden and Nuvaxovid) and heavy menstrual bleeding were also extracted to allow for a comparison across all COVID-19 vaccines authorised within the EU at the time of the study.
For each case, the following dates were extracted: the date of vaccination (‘drug start date’), the date of the start of the adverse reaction (‘reaction start date’), and the date when the vaccinee or healthcare professional reported the adverse reaction (‘receive date’).
To investigate to what extent the reports submitted over the course of the vaccination campaign may be influenced by media attention, the median reporting time was calculated. This was defined as the number of days between the ‘reaction start date’ and the ‘receive date’. Under the assumption that media attention has not influenced the reporting behaviour, a constant median reporting time would be expected over time. If a longer median reporting time is observed after a particular media event it could suggest a stimulated report of an earlier occurrence of the adverse reaction.
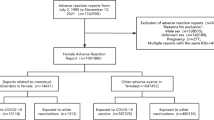
Cases were excluded if (i) any of the receive dates, drug start dates or reaction start dates were missing, (ii) the reaction start date occurred before the drug start date or (iii) the cases were male and cases where the sex of the vaccinee was not specified. The case selection strategy is illustrated in Fig. 1 of the Electronic Supplementary Material (ESM).
2.2 Dynamic Reporting Odds Ratio
The EudraVigilance Data Analysis System (EVDAS) is the main EU pharmacovigilance safety monitoring tool used for signal detection and data analysis, including disproportionality analyses in EV. For a drug-event combination, a signal of disproportionate reporting (SDR) is considered in the presence of at least 3 cases and the lower bound of the 95% confidence interval (LCI) of the reporting odds ratio (ROR) [17] is greater than or equal to 1. To investigate the trend of disproportionate reporting of heavy menstrual bleeding associated with COVID-19 vaccines and to identify the timepoint when the ROR LCI reached the LCI threshold for an SDR, the ROR was calculated in EVDAS monthly from January 2021 to December 2022 using cumulative cases of COVID-19 vaccines originated in the EEA. The ROR was calculated using as the numerator the number of cases in which a COVID-19 vaccine was received and heavy menstrual bleeding reported divided by the number of cases in which a COVID-19 vaccine was received and heavy menstrual bleeding was not reported; and as the denominator the number of cases that used or received other medicinal products including vaccine products and which reported heavy menstrual bleeding divided by the number of cases that used or received other medicinal products and did not report heavy menstrual bleeding (see ESM, Supplementary Note 1). The results were further stratified by COVID-19 vaccine platform (mRNA based vs other) and reporter type (i.e. healthcare professional or patient).
2.3 Mining Media Outlets Across EEA
To perform the search and extract the information, we used the Meltwater media monitoring platform [18], which is used by the EMA for daily monitoring of media articles relevant to its work. The platform is also used to evaluate media coverage of communication campaigns and to gauge media interest in relation to any possible issues with medicinal products.
An automatic search including news sources and print publications, and excluding TV channels and social media, published in the EEA was performed on 23 May, 2023, covering the period from 1 January, 2021 to 13 December, 2022. The search included terms concerning COVID-19 vaccination in connection to terms concerning menstrual bleeding and their variations (ESM, Supplementary Note 2). The terms were translated into the official languages of the EU and EEA Member States.
After manual review, some search terms were excluded (after the term NOT) as they involved news stories not directly related to menstrual bleeding in several outlets, in the respective period. These media outlets were retrieved by the original search strategy because, at the time of the search, a link or short description of an article mentioning menstrual bleeding or related terms was embedded in the webpage.
The following information was extracted for each of the identified articles: date, headline, URL, source, country, sub-region, state, city, language. All figures were produced using the ‘ggplot2’ package in R version 4.2.1.
3 Results
3.1 Demographics of Cases Reported to EV
As of 13 December, 2022, 34,374 EEA cases of the PT ‘heavy menstrual bleeding’ following the administration of any COVID-19 vaccine had been reported to EV. After excluding cases with missing or incomplete information on vaccine administration date or reaction date and selecting cases where sex was specified as female, 23,884 cases remained. Ninety-one percent of the cases were reported following administration of an mRNA-based COVID-19 vaccine: 76% with Comirnaty Original and 15% with Spikevax Original. Table 2 describes the demographics of the EEA cases for both vaccines. The first case of heavy menstrual bleeding in the study period was reported to EV on 6 January, 2021.
In terms of primary source country, over a third of the total cases for Comirnaty Original were reported in the Netherlands, while one third of the cases for Spikevax Original were reported in Germany (Table 2). In fact, for Comirnaty Original, the Netherlands was the primary country (28–39%) in terms of the number of reports of the PT ‘heavy menstrual bleeding’ for all the cases concerning consumers aged under 55 years (Table 2). Furthermore, cases originating from the Netherlands accounted for 29% of the total cases for Comirnaty Original, while the proportion of cases originating from the Netherlands accounted for 8.7% of all the other medicinal products and adverse events (581,044 of 6,703,290 EEA cases of 13 December, 2022). Details on EV cases reported by vaccine (original versions) and country can be found in Table 1 of the ESM.
3.2 Dynamic ROR
We analysed the trend of the LCI of the ROR for heavy menstrual bleeding and COVID-19 vaccines from January 2021 to December 2022 to identify the point in time when the ROR LCI reached the LCI threshold. The analysis was stratified by vaccine type (mRNA-based compared to other COVID-19 vaccines). We found that for mRNA-based COVID-19 vaccines, the LCI of the ROR reached the LCI threshold for an SDR in August 2021, and increased exponentially until March 2022 (Fig. 1A). Over the same period, the increase in the LCI was notably higher when restricting the case reports to those notified directly by consumers. In contrast, for COVID-19 vaccines not based on mRNA platforms, the LCI of the ROR using only spontaneous reports notified directly by consumers did not reach the LCI threshold during the study period (Fig. 1B). For COVID-19 vaccines not based on mRNA platforms, spontaneous reports notified by healthcare professionals reached the LCI threshold for an SDR in June 2021. However, the magnitude of the disproportionality was small compared with mRNA-based COVID-19 vaccines, and the ROR decreased from August 2021.
Reporting odds ratio for messenger RNA (mRNA)-based COVID-19 vaccines (European Economic Area [EEA] cases). Dynamic reporting odds ratio for EEA cases for mRNA based (A) and other (B) COVID-19 vaccines and heavy menstrual bleeding. All the COVID-19 vaccines were used for a pooled reporting odds ratio calculation. The suspected adverse drug reaction was defined using the MedDRA Preferred Term “Heavy menstrual bleeding”. The mRNA-based COVID-19 vaccines included Comirnaty Original, Spikevax Original (previously COVID-19 Vaccine Moderna), Comirnaty Original/Omicron BA.1, Spikevax Original/Omicron BA.1, Comirnaty Original/Omicron BA.4-5 and Spikevax Original/Omicron BA.4-5. Other COVID-19 vaccines included Vaxzevria (previously COVID-19 Vaccine AstraZeneca), Jcovden (previously COVID-19 Vaccine Janssen) and Nuvaxovid. LCI lower confidence interval
3.3 Weekly Reporting Trends and Median Reporting Time
To investigate changes in the reporting of heavy menstrual bleeding, the date when the adverse drug reaction occurred (reaction start date) and the date when the report was first received from the primary source (receive date) were extracted for all the cases and used to visualise the distribution of cases over time. The number of cases per COVID-19 vaccine occurring weekly, based on the reaction start date is shown in Fig. 2A, while the number of cases received per week, based on the receive date is shown in Fig. 2B. A more even distribution is observed when the cases are distributed based on the reaction start date (Fig. 2A) compared to the distribution based on the receive date (Fig. 2B), which could be explained by a delayed reporting of the reaction by the primary source and/or the delay in the processing of reports (weeks 20–35 covering from 1 June to 31 August, 2021; and week 51 covering from 20 December to 26 December, 2021).
Figure 2A shows two peaks in 2021, one that reaches the maximum of 1000 cases on week 31 of 2021 (week starting 2 August) and another that reaches 250 cases in week 51 of 2021 (starting 20 December). Figure 2B shows the same peak in week 31 of 2021 with the highest number of cases (approximately 1500), a second peak in week 39 of 2021 (starting 27 September) of just above 500 cases which is not observed in Fig. 2A, a third peak in week 51 of 2021 with approximately 800 cases and a fourth peak of just above 500 cases in week 29 of 2022. Discrepancies between these figures could be explained by reports submitted days or months after the reaction.
To investigate to what extent media attention could influence the reporting of heavy menstrual bleeding submitted over the course of the vaccination campaign, the median reporting time was calculated as detailed in Methods. From week 24 (starting 14 June) to week 35 (starting 30 August) of 2021, the median reporting time ranged from 10 to 25 days for Comirnaty Original (Fig. 3). From week 36 (starting 6 September), the median reporting time increased and ranged from 30 days to over 3 months. At the beginning of 2022, the median reporting time dropped again to approximately 30 days following an increase in the case reports; however, it soon increased linearly. Similar trends of smaller magnitude were observed for Spikevax Original (Fig. S2 of the ESM). Of note, for this vaccine, the last EEA case of heavy menstrual bleeding was reported to EV in February 2022.
Median reporting time (days) and number of cases received per week for Comirnaty Original. The median reporting time was calculated by week (blue line) using the median value of the difference in days between the receive date and reaction start date per case. The weekly number of cases based on the receive date is indicated by the right axis and shown by the grey area
An alternative approach to investigate the changes in reporting over time would be to explore the median reporting time in relation to the number of weekly cases by reaction start date (Figs. S3–S4 of the ESM). Cases occurring in the early phases of the vaccination campaign had an increased reporting time, while over longer time, the trend is decreasing.
When stratifying the cases by country, the peaks in reporting corresponding to weeks 31, 39 and 51 of 2021, respectively, were explained by cases originating from the Netherlands, and the peak of week 29 of 2022 because of cases received in France (Fig. 4).
3.4 Media Attention in the EEA
We identified a total of 3944 articles for 27 out of the 30 EEA countries. The three countries for which media outlets were not identified were Cyprus, Luxembourg and Lichtenstein; media outlets in these countries may have been covered by the Greek, German or French media outlets, respectively.
Over 100 articles were published across the EEA during 6 weeks (Fig. 5). The coverage was further stratified by country to investigate how each country contributed to the total EEA coverage, and to facilitate the identification of events triggering the spikes in media coverage (Fig. 6).
Media coverage by country and week for articles mentioning COVID-19 vaccines and menstrual disorders. The dashed vertical lines indicate key dates when over 100 articles were published across the European Economic Area. The scale of the y-axis is not fixed to facilitate visualising trends within countries
During week 31 of 2021 (starting 2 August), 114 articles were published across 14 EEA countries, of which 85 (75%) were published in France and the Netherlands. On 30 July, 2021, the French Medicines Agency (ANSM) included menstrual disorders in its latest pharmacovigilance update [19]. The Netherlands Pharmacovigilance Centre (Lareb) also published in their biweekly safety updates that over a thousand menstrual disorder reports had been received following COVID-19 vaccination [20].
The second peak observed during the study period corresponded to week 38 of 2021 (starting 20 September), when 119 articles were published in ten different countries. However, up to 74% of the articles were published in Germany and mentioned a safety update issued on 19 August, 2021 by the Paul-Ehrlich-Institut, the authority responsible for monitoring the safety of vaccines and biomedicines in Germany, on COVID-19 vaccines and suspected adverse events following vaccination, including irregularities of the menstrual cycle [21].
During week 51 of 2021 (starting 20 December), 154 articles were published in 12 different EEA countries, of which 103 (70%) were originated in Norway and the Netherlands. The Norwegian Institute of Public Health published an article on the increased incidence of menstrual changes among young women after coronavirus vaccination [22] on 21 December, 2021, while Lareb published a report on menstrual disorders and postmenopausal bleeding after administration of COVID-19 vaccines 1 day later [23].
The spike observed throughout week 4 of 2022 (starting 24 January) was preceded by articles published in Germany (154 out of 202 articles in the EEA), which were related to a prospective population-based study from the USA with evidence indicating that COVID-19 vaccination was associated with a change of less than 1 day in menstrual cycle length but no change in menses length [24]. On 27 October, 2022 (week 43), PRAC recommended that “heavy menstrual bleeding” should be included as a listed side effect for both Comirnaty and Spikevax, which was reflected in the EMA highlights from the respective PRAC meeting [16]. Within 2 weeks of the press release, over 216 articles were published in 20 countries.
3.5 Overlap of Media Events and Case Reporting
To investigate the relationship between media events identified in the previous section and trends in case reporting, the number of publications were compared to the counts of cases per week in the respective countries (Fig. 7). This analysis showed that in general, media outlet publications were either coincidental or preceded the EV spikes in reporting. For example, there is a temporal correlation between increases in media events and case reporting in the Netherlands and the Lareb safety updates discussing menstruation changes (Fig. 8) after vaccination with COVID-19 vaccines. Similarly, a spike in reporting was observed in France in week 29 of 2022, shortly after a safety communication from the French National Agency for Drug Safety (ANSM) in which the regulators encouraged those who have observed changes in their menstrual cycle to report them [25]. Other countries also showed an overlap of media events and case reporting; however, the reporting to EV was of lower magnitude (Fig. 8).
Nonetheless, reports of heavy menstrual bleeding for Comirnaty Original were received since the beginning of the mass vaccination campaign as shown in Fig. 7. Notably, increased reporting was not observed following the publication of the PRAC recommendation to add heavy menstrual bleeding to the product information as a side effect of unknown frequency of the mRNA-based COVID-19 vaccines Comirnaty and Spikevax.
4 Discussion
In this study, we described the reporting pattern of cases of heavy menstrual bleeding following the administration of COVID-19 vaccines to EV, with a focus on the mRNA-based vaccines, in relation to relevant media coverage. As of 13 December, 2022, EV received 34,374 cases of heavy menstrual bleeding with COVID-19 vaccines originating from EEA countries, of which 23,884 cases were included in the analysis. Seventy-seven percent of these (18,280) were reported for Comirnaty Original, 29% of which occurred in the Netherlands. Such a contribution of Dutch cases to the overall count was unexpected as vaccination in the Netherlands with Comirnaty Original only accounted for 3.8% of the total Comirnaty vaccines administered in the EEA as of 22 July, 2022 (24,603,241 out of 650,605,721, European Centre for Disease Prevention and Control [26]).
We observed that the publication of safety updates from regulatory authorities were often followed by media releases, which in turn led to increased reporting of the respective safety concern in some countries. The increase in reporting included cases that have occurred several weeks or months in the past and which had not been already notified, as shown by the increased median reporting time after the publication date of the aforementioned articles (Fig. 3). We found that the median reporting time for Comirnaty Original before the first spike of media outlets ranged between 10 and 25 days, in line with a previous study showing that the median time to report an adverse drug reaction in the EU is approximately 30 days for spontaneous reports, regardless of whether it was reported by consumers or healthcare professionals [27]. We also observed that after the spike in media outlets, the median reporting time increased and ranged from 30 days to over 3 months.
Specific active safety surveillance tools in national reporting systems put in place during the pandemic may have also contributed to the high volume of reports. In addition, enhanced media coverage could stimulate reporting of events that have happened in the past for which the individuals were unsure or did not associate with vaccine administration (known as reporting bias [28]). Conversely, it may increase awareness of the potential for the occurrence of adverse events in vaccinees (known as notoriety bias [29]). It is noteworthy that cases of heavy menstrual bleeding from the EEA have been received in EV since the beginning of the vaccination campaign, from January 2021, even before the initiation of any public discussion on a possible association. Over time, the number of reports increased reaching a peak during week 31 of 2021 (starting 2 August), also in line with the progression of the vaccination campaign. As shown in Fig. 1, while the LCI of the ROR for the mRNA-based COVID-19 vaccines indicated a consistent significant signal of disproportionate reporting throughout the whole study period, for the three other vaccines, it was only slightly elevated. It should be noted that following the first reports of unusual blood clots after the use of Vaxzevria (previously COVID-19 Vaccine AstraZeneca), several EU countries prioritised the use of mRNA-based COVID-19 vaccines, hence the exposure to other vaccines became significantly lower in the EEA [26].
Drug-related or therapeutic-related changes to menstruation are not routinely evaluated in clinical trials. Spontaneous reporting systems such as EV allow for rapid monitoring and provide a resource for post-marketing surveillance analysis, particularly for rare events or events with a long latency, events not routinely collected during clinical trials. Since November 2012, national competent authorities and marketing authorisation holders are required to report non-serious cases of suspected adverse reactions, while previously they were required to report only serious cases. One of the key provisions of the 2010 EU pharmacovigilance legislation [30] was the new requirement for MAHs and NCAs to report to EV all non-serious cases originating from the EEA. In addition, the new legislation brought an obligation on these stakeholders to record and report cases received from consumers, who cannot report directly to EV. Following technical adjustments in EV, from November 2017, the submission of EEA non-serious reports to EV became mandatory [31]. This encouraged the reporting from European patients and consumers, which amounted to 45% of the total case reports submitted to the EV in 2021 [32].
Notably, our analysis found that for heavy menstrual bleeding and COVID-19 vaccines, over 90% of the case reports were submitted by consumers. Heavy menstrual bleeding was not the only adverse event for which increased reporting had been observed following media attention. For example, a study conducted in New Zealand found that media coverage of the side effects of COVID-19 vaccines was associated with an increased reporting rate of the specific symptoms mentioned in the news items, including myocarditis, pericarditis and anxiety [12]. Previous studies have also investigated the temporal association between media coverage and adverse event reporting for other vaccines, such as the human papillomavirus vaccine, Gardasil. In 2014, a study in the USA showed that media coverage and Internet search activity was associated with increased adverse event reporting following vaccination with Gardasil [15]. Three years later, a study conducted in New Zealand provided further evidence for a correlation between media attention and adverse event reporting for Gardasil by showing that news coverage and Google search volumes were significant predictors of adverse event reporting following Gardasil vaccination [14]. Other medicinal products have also been studied in the context of stimulated media reporting of adverse events. For example, a formulation change of Eltroxin, which was covered by television media in France, influenced the volume and type of symptoms reported by consumers [11]. However, a study performed in the Netherlands found a peak in the reporting of adverse events associated with statins following the broadcast of a television programme; yet such increase was not higher for the adverse events mentioned in the programme [13].
Some limitations of the study are noteworthy. First, spontaneous systems are not primarily useful to detect increased rates of non-serious events that occur commonly in the general population, but rather to detect rare and serious adverse reactions, such as myocarditis with the mRNA-based COVID-19 vaccines or thrombosis with thrombocytopenia syndrome with the COVID-19 vaccine (ChAdOx1-S [recombinant], Vaxzevria) [33]. Analyses that utilise data from spontaneous reports are subject to under-reporting or stimulated reporting, which are difficult to consider when calculating the number of observed cases or when evaluating time trends. As heavy menstrual bleeding can be affected differently by these scenarios (e.g. may be more susceptible to stimulated reporting because of the media coverage, and to under-reporting as a non-serious adverse reaction), caution is needed when interpreting results.
Second, this analysis may be subject to recall bias as some individuals may have been unable to adequately remember when the event occurred, or the nature of the change in their menstrual or bleeding experiences. Third, while the analysis accounted for demographic factors and vaccine product name, it did not include other variables as these were inconsistently available in spontaneous data, for example, reproductive history, hormonal contraception use, systemic vaccine response and COVID-19 infection status, which could have had an influence on the event. Last, reporters to EV could have different coding practices for spontaneous case reports, which could lead to a decreased number of cases subject to this analysis. For example, cases of heavy menstrual bleeding could have been coded under the MedDRA PT “Menometrorrhagia”.
Regarding the media coverage, we searched online outlets located in EEA countries identified as news sources using keywords for COVID-19 vaccination, menstruation, and bleeding and their combination in EEA official languages. While we tried to fine tune the search terms to obtain the most accurate results, some of the articles may be outside the scope; however, we assume that the publication of those articles was randomly distributed during the study period and would not impact the conclusions of the study. In addition, some media outlets may be registered in one country but in fact, they may report in other countries sharing the same language. For example, articles published in Cypriot or Austrian media may indeed appear as articles from Greece or Germany, respectively, simply because the publishing house is located there. Similarly, some outlets publish content in several languages and while the readership of the article may be in one country because of the language, the database may track the source to where the publishing house is located. We also acknowledge that limiting the search to online media outlets (and excluding social media and television), is likely to have underestimated media interest.
5 Conclusions
The publication of safety updates from regulatory authorities related to outcomes of assessment of menstrual disorders after COVID-19 vaccination was in some countries followed by media releases. That in turn led to increased consumer reporting of the adverse event to EV. The high volume of reports was crucial to prompt safety reviews of heavy menstrual bleeding.
While this study did not investigate the causal association between COVID-19 vaccination and episodes of heavy menstrual bleeding, it provided some insights into the influence of media on reporting behaviours. In the case of heavy menstrual bleeding following COVID-19 vaccination, media attention led to increased reporting to EV. While future studies may explore the effect of media attention on reporting behaviours to spontaneous reporting systems, we showed that these sources of information can be investigated to understand the context of safety concerns of public health interest.
References
BBC. Covid vaccine: period changes could be a short-term side effect. BBC News 2021.
Campbell H. Experts cool claims about impact of vaccines on menstrual cycles. Euronews 2021.
HuffPost UK. What to do if your period is bad after your COVID-19 vaccine. HuffPost UK 2021.
Haaretz. Some vaccinated Israeli women report irregular menstrual cycles, bleeding. Israel News, Haaretz.com 2021.
European Medicines Agency. Safety of COVID-19 vaccines. European Medicines Agency. Amsterdam; 2021.
European Medicines Agency. COVID-19: latest updates (archive). 2020. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-public-health-emergency-international-concern-2020-23/covid-19-latest-updates-archive. Accessed 14 Aug 2023.
European Medicines Agency. Note for guidance on clinical safety data management: definitions and standards for expedited reporting. European Medicines Agency. London; 1995.
European Medicines Agency. COVID-19 vaccine safety update. European Medicines Agency. Amsterdam; 2021.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP). Module VI: collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2). European Medicines Agency. London; 2017.
Trogstad, Lill, Increased Occurrence of Menstrual Disturbances in 18- to 30-Year-Old Women after COVID-19 Vaccination (January 1, 2022). Available at SSRN: https://ssrn.com/abstract=3998180
Faasse K, Gamble G, Cundy T, Petrie KJ. Impact of television coverage on the number and type of symptoms reported during a health scare: a retrospective pre-post observational study. BMJ Open. 2012;2: e001607. https://doi.org/10.1136/bmjopen-2012-001607.
MacKrill K. Impact of media coverage on side effect reports from the COVID-19 vaccine. J Psychosom Res. 2023;164: 111093. https://doi.org/10.1016/j.jpsychores.2022.111093.
van Hunsel F, van Puijenbroek E, de Jong-van den Berg L, van Grootheest K. Media attention and the influence on the reporting odds ratio in disproportionality analysis: an example of patient reporting of statins. Pharmacoepidemiol Drug Saf. 2010;19:26–32. https://doi.org/10.1002/pds.1865.
Faasse K, Porsius JT, Faasse J, Martin LR. Bad news: the influence of news coverage and Google searches on Gardasil adverse event reporting. Vaccine. 2017;35:6872–8. https://doi.org/10.1016/j.vaccine.2017.10.004.
Eberth JM, Kline KN, Moskowitz DA, Montealegre JR, Scheurer ME. The role of media and the Internet on vaccine adverse event reporting: a case study of human papillomavirus vaccination. J Adolesc Health. 2014;54:289–95. https://doi.org/10.1016/j.jadohealth.2013.09.005.
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 24-27 October 2022. 2022. Available from: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-24-27-october-2022. Accessed 28 Mar 2023.
European Medicines Agency. Screening for adverse reactions in EudraVigilance. European Medicines Agency. London; 2016.
Meltwater. Media monitoring & analysis. Available from: https://www.meltwater.com/en/products/media-monitoring . Accessed 26 Jun 2023.
ANSM. Actualité: point de situation sur la surveillance des vaccins contre la COVID-19. Période du 16/07/2021 au 22/07/2021. Available from: https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-16-07-2021-au-22-07-2021. Accessed 23 May 2023.
NOS. Vaccinatie en menstruatie: “Iedereen heeft wel eens een verstoorde cyclus”. 2021. https://nos.nl/artikel/2392394-vaccinatie-en-menstruatie-iedereen-heeft-wel-eens-een-verstoorde-cyclus.
Paul-Ehrlich-Institut. Verdachtsfälle von Nebenwirkungen und Impfkomplikationen nach Impfung zum Schutz vor COVID-19 seit Beginn der Impfkampagne am 27.12.2020 bis zum 31.03.2023. https://www.pei.de/DE/newsroom/dossier/coronavirus/sicherheitsbericht-covid-19-impfstoffe-aktuell.html
Norwegian Institute of Public Health. Increased incidence of menstrual changes among young women after coronavirus vaccination. 2022. https://www.fhi.no/en/news/2022/increased-incidence-of-menstrual-disturbances-after-coronavirus-vaccination/.
Netherlands Pharmacovigilance Centre Lareb. Menstrual disorders and postmenopausal bleeding after administration of COVID-19 vaccines. 2021. https://www.lareb.nl/media/uoneih5z/signals_2021_menstrual_disorders-and-postmenopausal_bleeding-and-covid-19-vaccines.pdf.
Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a U.S. cohort. Obstetr Gynecol. 2022;139:481. https://doi.org/10.1097/AOG.0000000000004695.
Agence nationale de sécurité du médicament et des produits de santé. Troubles menstruels et vaccination Covid-19 guide d’aide à la déclaration de pharmacovigilance. 2022 https://ansm.sante.fr/uploads/2022/08/01/20220801-covid-19-guide-troubles-menstruels-patientes-et-ps-26-07-2022.pdf.
European Centre for Disease Prevention and Control. COVID-19 vaccine tracker. Available from: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#distribution-tab. Accessed 25 May 2023.
Banovac M, Candore G, Slattery J, Houÿez F, Haerry D, Genov G, et al. Patient reporting in the EU: analysis of EudraVigilance data. Drug Saf. 2017;40:629–45. https://doi.org/10.1007/s40264-017-0534-1.
Pozsgai K, Szűcs G, Kőnig-Péter A, Balázs O, Vajda P, Botz L, et al. Analysis of pharmacovigilance databases for spontaneous reports of adverse drug reactions related to substandard and falsified medical products: a descriptive study. Front Pharmacol. 2022;13: 964399.
Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E. Existence of notoriety bias in FDA Adverse Event Reporting System database and its impact on signal strength. Hosp Pharm. 2021;56:152–8. https://doi.org/10.1177/0018578719882323.
European Parliament and the Council of the European Union. Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use 2010.
Candore G, Monzon S, Slattery J, Piccolo L, Postigo R, Xurz X, et al. The impact of mandatory reporting of non-serious safety reports to EudraVigilance on the detection of adverse reactions. Drug Saf. 2022;45:83–95. https://doi.org/10.1007/s40264-021-01137-0.
European Medicines Agency. 2021 Annual Report on EudraVigilance for the European Parliament, the Council and the Commission 2022. Available from: https://www.ema.europa.eu/en/documents/report/2021-annual-report-eudravigilance-european-parliament-council-commission_en.pdf. Accessed 14 Aug 2023.
Durand J, Dogné J-M, Cohet C, Browne K, Gordillo-Marañón M, Piccolo L, et al. Safety monitoring of COVID-19 vaccines: perspective from the European Medicines Agency. Clin Pharmacol Ther. 2023;113:1223–34. https://doi.org/10.1002/cpt.2828.
Acknowledgements
The authors are grateful to Kate Browne and Robert Flynn for their review and constructive comments on the manuscript prior to submission. The authors are also thankful to all members of the Pharmacovigilance Office, the Data Analytics and Methods Task Force and the European Pharmacovigilance Risk Assessment Committee, who have supported and continue to support the safety monitoring of the COVID-19 vaccines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to assist with the preparation of this article.
Conflict of Interest
María Gordillo-Marañón, Agnieszka Szmigiel, Vladimíra Yalmanová, Irina Caplanusi, Georgy Genov, David Benee Olsen and Sabine Straus have no conflicts of interest that are directly relevant to the content of this article. María Gordillo-Marañón, Agnieszka Szmigiel, Vladimíra Yalmanova, Irina Caplanusi and Georgy Genov are employees of the European Medicines Agency. Sabine Straus is the Chair of European Medicines Agency’s Pharmacovigilance Risk Assessment Committee and employee of the Medicines Evaluation Board, the Netherlands. David Benee Olsen is a member of the European Medicines Agency Pharmacovigilance Risk Assessment Committee and employee of the Norwegian Medical Products Agency, Norway.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contribution
MGo-M and AS have shared first authorship. All authors contributed towards the writing of the text and have read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gordillo-Marañón, M., Szmigiel, A., Yalmanová, V. et al. COVID-19 Vaccines and Heavy Menstrual Bleeding: The Impact of Media Attention on Reporting to EudraVigilance. Drug Saf (2024). https://doi.org/10.1007/s40264-024-01426-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40264-024-01426-4