Abstract
Introduction
Effectiveness and respiratory adverse events following coronavirus disease-2019 (COVID-19) vaccines have not been well investigated in Chinese patients with chronic obstructive pulmonary disease (COPD) and asthma.
Methods
Using electronic health care records in Hong Kong, we included adults with COPD or asthma or both and hospitalised for severe respiratory exacerbation in a self-controlled case series (SCCS) study between 23/02/2021 and 30/11/2022. Conditional Poisson regression models were used to estimate the incidence of outcomes within exposure periods (28 days after each dose) compared with baseline periods. Cox proportional hazard models evaluated vaccine effectiveness (VE) against COVID-related mortality, hospitalisation, and severe complications, including admission to intensive care units or ventilatory support. The VE assessment was based on vaccine types and the number of doses.
Results
In the SCCS, 343 CoronaVac recipients and 212 BNT162b2 recipients were included. No increased risk of outcomes was observed within the exposure periods. In the cohort study, 108,423 and 83,323 patients received ≥ 2 doses of CoronaVac and BNT162b2, respectively. The VE (95% CI) against COVID-related mortality, hospitalisation, and severe complications after two-dose CoronaVac was 77% (74–80%), 18% (6–23%), and 29% (12–43%), respectively, while for the two-dose regimen of BNT162b2, it was 92% (91–94%), 33% (30–37%), and 57% (45–66%), respectively. Higher VE against COVID-related mortality, hospitalisation, and severe complications was found for the three-dose regimen of CoronaVac (94%, 40%, and 71%) and BNT162b2 (98%, 65%, and 83%). Administering a fourth dose of either vaccine showed additional reductions in COVID-related outcomes.
Conclusions
Among people with COPD and asthma, the COVID-19 vaccines CoronaVac and BNT162b2 did not increase severe exacerbations and achieved moderate-to-high effectiveness against COVID-related outcomes. COVID-19 vaccination remains essential and should be encouraged to protect this vulnerable population in future epidemic waves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ongoing coronavirus disease 2019 (COVID-19) infection, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has put people with chronic obstructive pulmonary disease (COPD) [1] and severe asthma [2,3,4] at serious risk, resulting in high rates of hospitalisation and mortality. However, patients with mild to moderate asthma have similar clinical outcomes as those without asthma [5, 6].
With the national and global vaccination programme rollouts, patients with COPD and asthma were advised to get vaccinated to prevent severe COVID-related outcomes such as death and hospitalisation [7]. However, information regarding the exacerbation following vaccination and the effectiveness of vaccines in this patient group is inconclusive. Since the rollout of the vaccination programmes, emerging case reports [8,9,10,11] have shown that patients with COPD and asthma may experience acute exacerbations or acute respiratory distress syndrome after the second dose of the messenger RNA (mRNA) vaccines (e.g., Moderna, BNT162b2), possibly due to transient systemic inflammation following vaccination [12]. Nevertheless, data on respiratory exacerbations from these vaccines among these patient groups are limited.
COVID-19 vaccine trials and real-world vaccine effectiveness (VE) studies have demonstrated high-level VE in the general population and older people [13, 14]. However, VE in patients with COPD and asthma has yet to be thoroughly explored. Although some studies [2, 7] found that two doses of BNT162b2 reduced COVID-19 hospitalisation in patients with asthma and COPD, these studies mainly focused on effectiveness during the early phase of the pandemic (Delta variant) and excluded those with the Omicron variant. In addition, the follow-up periods were short (28 days after the second dose), and medium-term vaccine effectiveness remains unexplored. Furthermore, the VE of the third and fourth doses has yet to be investigated in these patient groups. Moreover, limited studies have investigated the VE of inactivated vaccines (CoronaVac) in this group. Given that this group of people is prone to severe COVID-related outcomes after infection, understanding the VE of COVID-19 vaccines against COVID-related severe outcomes may provide insights to reduce adverse events after infection.
Since late December 2021, the Omicron variant has affected over 2 million people in Hong Kong (HK). The city has reported the highest COVID-related mortality rate relative to its population size during the Omicron variant infection, which is currently the dominant variant in HK. The vaccination programme in HK commenced in early 2021, utilising two widely administered vaccines globally, the BNT162b2 (Fosun-BioNTech, equivalent to Pfizer-BioNTech outside China) mRNA vaccine and the CoronaVac inactivated vaccine (Sinovac) [15]. Up to the third quarter of 2022, over 5 million people have received the third vaccination, and 45 thousand have received a fourth vaccination.
Accordingly, our study aimed to examine (1) the respiratory exacerbation within 28 days after each dose of CoronaVac and BNT162b2 and (2) the effectiveness of two, three, and four doses of these vaccines in reducing the risks of COVID-related mortality and hospitalisation in people with COPD or asthma or both. We conducted this study using a territory-wide electronic health record database in HK.
2 Methods
2.1 Data Sources
This study utilised routine electronic health records from the clinical management system under the Hospital Authority (HA) of HK and vaccination records from the Department of Health (DH) of the HK Government. These two databases are linked using the unique HK Identity Card Number or other personal identification numbers. The HA is a statutory administrative organisation in HK that manages all public inpatient and outpatient services. The clinical management system, which includes demographics, diagnoses, prescriptions, and laboratory tests, provides real-time data support and monitoring for routine clinical management across all clinics and hospitals in HA. On the other hand, the DH manages and retains the database for all vaccination records in HK. The two population-based databases have been used previously to conduct studies on the risk of adverse effects after COVID-19 vaccinations and other COVID-19 pharmacovigilance studies [16,17,18].
2.2 Study Population
The study population included those aged ≥ 18 years who had either been hospitalised for COPD or asthma before the start of the study (specifics outlined in each study design). The International Classification of Disease Ninth version, Clinical Modification (ICD-9-CM) codes, and British National Formula (BNF) codes are shown in Supplementary Table 1. Our primary analysis included people with COPD or asthma, or both, as some may have both conditions concurrently. Subgroup analysis was conducted to differentiate between admissions related to COPD and asthma.
2.3 A Self-Controlled Case Series (SCCS) for Severe Exacerbation
The SCCS design was applied to investigate the risk of severe respiratory exacerbation following administration of CoronaVac or BNT162b2 between 23 February 2021 and 30 November 2022 (observational period). The SCCS is a within-individual study design that compares the incidence of outcomes during the exposure period to the non-exposure period within the same individual. Therefore, it inherently controls for known and unmeasured time-invariant confounders. Additionally, time-varying confounders such as seasonality effects can be adjusted for within the regression model.
We identified people with COPD and asthma who had exacerbation during the observational period for the SCCS. The flowchart outlining the selection process for the SCCS population is shown in Fig. 1. The primary outcome of interest for the SCCS was severe respiratory exacerbation, defined as the first hospitalisation for asthma or COPD or respiratory failure of any cause. The exacerbations were determined according to published case reports [8,9,10,11]. Patients who received heterologous COVID-19 vaccines or were diagnosed with COVID-19 infection before 30 November 2022 were excluded from the analysis since the COVID-19 infection may increase exacerbations that would bias the results.
Since respiratory exacerbation after vaccination could potentially impact subsequent exposure, we applied a modified SCCS extension developed for event-dependent exposure [19]. The modified SCCS model incorporated unvaccinated patients who experienced outcome events during the observational periods to account for the probability of receiving vaccination after the event. Including unvaccinated patients was a control to provide a baseline comparison for the vaccinated group.
The risk periods were defined as 28 days after administering the first, second, third, and fourth doses. Based on case reports showing that the acute respiratory events occurred within 7 days after vaccination, we further divided the risk periods into 0–7 days and 8–28 days after each dose. The rest of the study period was defined as the baseline period. A schematic presentation of the SCCS design is shown in Fig. 2. Since no trial was conducted to examine the risk of respiratory exacerbation after COVID-19 vaccination, a relative risk of 1.1–3.0 was assumed based on our team’s previous COVID-19 vaccine safety studies [20,21,22]. This corresponded to a required sample size of 70–6430 events to achieve 80% power at a significance level of 0.05 for the SCCS analysis, considering an observation period of 650 days.
2.4 Retrospective Cohort Study for Vaccine Effectiveness
We identified patients with COPD and asthma (study population) before 23 February 2021. Vaccinated patients were those who had received two homogenous doses of COVID-19 vaccines. The index date for vaccinated patients was defined as the date of their second dose. People who had received heterogeneous first two doses before 30 November 2022 were excluded from the analysis. Before 30 November 2022, patients who did not receive COVID-19 vaccines were considered unvaccinated. For each vaccinated patient, a randomly selected unvaccinated patient of the same age and sex was matched, and the second dose vaccination date from the vaccinated patient was assigned as the pseudo-index date for the matched unvaccinated individual. Patients in the control group who died before the pseudo-index date were excluded from the analysis.
People were followed up from the index date (pseudo-index date for unvaccinated individuals) until the end of the study (30 November 2022), the occurrence of the outcome, the date of the next vaccine dose, or the date of death, whichever came first. For the analysis of VE regarding the fourth dose, the cohort was followed up until the end of the study, the occurrence of the outcome or date of death, whichever came first. The schematic presentation of the cohort study is shown in Supplementary Figure 1.
The primary outcomes were defined as COVID-related mortality and hospitalisation. COVID-related mortality was defined as non-injury or poisoning-related mortality within 28 days after a positive polymerase chain reaction (PCR) test for COVID-19. This definition aligns with the guidelines and recommendations provided by the World Health Organisation [23]. COVID-related hospitalisation was defined as all-cause hospitalisation within 28 days after a positive PCR test for COVID-19. The rationale for choosing this co-primary outcome was that it provides important information about disease severity and its impact on people’s health. In addition, this result may provide evidence to guide policy decisions and resource needs in future pandemics. The secondary outcome was COVID-related severe complications, defined as admission to the intensive care unit or use of ventilatory support within 14 days after a positive PCR test for COVID-19 [24].
We identified the baseline characteristics, including sex and age, at the index date. Comorbidities and Charlson score were identified using a 3-year lookback period before 23 February 2021. Prior medications were identified within a 1-year lookback period before 23 February 2021. The comorbidities and previous medications are shown in Table 1.
2.5 Statistical Analysis
The safety study estimated the incidence of outcomes following COVID-19 vaccines, reported as cases per 100,000 doses. The analysis utilised a conditional Poisson regression model to estimate the incidence rate ratio (IRR) and its corresponding 95% confidence interval (CI). This involved comparing the incidence rates of respiratory exacerbation during different risk periods to the baseline period while adjusting for the monthly seasonality effect.
For the effectiveness study, a propensity score-based inverse-probability-of-treatment weighted Cox proportional hazard model was used to estimate the weighted hazard ratio (HR) of COVID-related outcomes. This analysis compared people who received two, three, and four vaccine doses to those who were unvaccinated. The VE was evaluated by (1 − adjusted HR) × 100%. The propensity score was calculated using multinomial logistic regression models, including all covariates as potential predictors for the COVID-19 vaccinated group. Extreme weight values were truncated at the 5th and 95th percentile of the distribution to mitigate the influence of outliers.
2.6 Subgroup Analysis
Several subgroup analyses were conducted to examine the robustness of the primary analysis results. Stratification was carried out based on people with admission for COPD or asthma, sex, and age group (< 65 years and ≥ 65 years) for subgroup analysis in both exacerbations and effectiveness studies. A subgroup analysis was also conducted on patients who had previously experienced respiratory failure (before 23 February 2021), as they may be at a higher risk of severe outcomes following COVID-19 infection. However, subgroup analyses were not performed for people who received the third and fourth vaccine doses due to the limited sample size available for analysis.
All statistical tests were two-sided, and p values < 0.05 were considered significant. Statistical analyses were conducted using R version 4.1.2 and SAS version 9.4 (SAS Institute, Inc, Cary, NC). To ensure quality assurance, two authors (co-first authors) independently performed the statistical analyses. The transparent reporting of this cohort study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklists to enhance the clarity and completeness of the study report.
3 Results
We initially identified 4,830,617 patients with COPD or asthma or both, aged ≥ 18 years before 23 Feb 2021, for SCCS and cohort studies. Following the applications of our exclusion criteria for the SCCS analysis (Fig. 1), we included 3627 CoronaVac recipients, 2108 BNT162b2 recipients, and 2381 patients without vaccination during the observational period. After removing ineligible patients from the cohort study (Fig. 1), 108,423 received two doses of CoronaVac, 83,323 received two doses of BNT162b2, and 24,624 were unvaccinated.
3.1 Patient Characteristics
The proportion of males in the unvaccinated group and among BNT162b2 recipients was similar, at 53%, slightly higher than those in CoronaVac (48%). Among both CoronaVac and BNT162b2 recipients, 81% received a third dose, while 13% of CoronaVac recipients and 9% of BNT162b2 recipients received a fourth dose (Table 1).
3.2 Exacerbations (SCCS)
Compared to the baseline period, no increased risk of respiratory exacerbation was observed in any risk periods for CoronaVac (IRR: first dose: 0.98, 0.81–1.18; second dose: 0.93, 0.76–1.13; third dose: 1.04, 0.84–1.30; fourth dose: 1.53, 0.83–2.83) nor BNT162b2 (IRR: first dose: 1.01, 0.78–1.30; second dose: 1.22, 0.98–1.51; third dose: 0.97, 0.74–1.29; fourth dose: 1.07, 0.48–2.37) (Table 2).
3.3 Vaccine Effectiveness (Cohort Study)
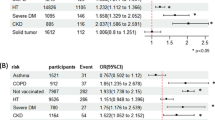
The VE against COVID-related mortality after two doses of CoronaVac and BNT162b2 was estimated to be 77% (74–80%) and 92% (91–94%), respectively. Similarly, the VE against COVID-related hospitalisation following two doses of CoronaVac and BNT162b2 was 18% (6–23%) and 33% (30–37%), respectively. For severe complications, the VE with two-dose CoronaVac was 29% (12–43%), while for two-dose BNT162b2, it was 57% (45–66%). When considering three doses of homogeneous CoronaVac and BNT162b2, both vaccines demonstrated significant effectiveness against COVID-related mortality (CoronaVac: 94%, 91–95%; BNT162b2: 98%, 96–99%), hospitalisation (CoronaVac: 40%, 34–44%; BNT162b2: 65%, 62–69%) and severe complications (CoronaVac: 71%, 57–81%; BNT162b2:81%, 73–88%). Although the number of events was limited for the fourth dose, four homogeneous doses of CoronaVac and BNT162b2 showed additional effectiveness in mitigating COVID-related outcomes compared to two and three doses (Table 3 and Supplementary Table 2).
3.4 Subgroup Analysis
Supplementary Tables 3 and 4 present the subgroup analyses for the exacerbation after receiving CoronaVac and BNT162b2. No increased risk of respiratory exacerbations was observed following each vaccination in people with COPD. This is consistent with those with asthma. Other subgroups showed consistent results with the primary analysis. Additionally, Supplementary Table 5 provides the subgroup analysis for the association between two doses of vaccines and the risks of COVID-related mortality, hospitalisation, and severe complications. Across the subgroups, we observed that the VE against COVID-related outcomes was higher for BNT162b2 compared to CoronaVac. In people with COPD, the VE against COVID-related mortality, COVID-related hospitalisation, and COVID-related complications following two doses of CoronaVac and BNT162b2 was 74% and 90%, 27% and 33%, 30% and 33%, respectively. In people with asthma, the VE against COVID-related mortality, COVID-related hospitalisation, and COVID-related complication following two doses of CoronaVac and BNT162b2 was 81% and 89%, 18% and 29%, and 67% and 79%, respectively. The VE for COVID-related mortality for other subgroups ranged from 85–97% for BNT162b2 to 69–82% for CoronaVac. For COVID-related hospitalisation, the VE for other subgroups ranged from 10–97% for BNT162b2 to 9–44% for CoronaVac. Furthermore, for COVID-related complications, the VE for other subgroups ranged from 47–79% for BNT162b2 to 17–67% for CoronaVac.
4 Discussion
To our knowledge, this study is the first to evaluate respiratory exacerbation and medium-term effectiveness following two, three, and four doses of vaccines in the Chinese population with two prevalent chronic respiratory conditions: COPD and asthma. With a population exceeding 1 million, our study revealed no increased risk of respiratory exacerbation after each vaccine dose. The VE of two doses of CoronaVac and BNT162b2 persisted against COVID-19 outcomes in the medium term. Furthermore, booster doses (three and four) provided additional protection against adverse events of COVID-19, building upon the efficacy of the initial two-dose vaccination. These findings suggest that CoronaVac and BNT162b2 can be safely and effectively administered to people with COPD and asthma. It is recommended that booster vaccinations be implemented for this patient group in future epidemics.
Our study combined both asthma and COPD with several implications. Chronic obstructive pulmonary disease and asthma are distinct pulmonary diseases with different aetiologies, prognoses, and management strategies. Thus, focusing exclusively on one disease may have certain advantages, such as providing more specific insights into the impact of COVID-19 and vaccines on that condition. Nonetheless, asthma and COPD do not infrequently coexist, and asthma is one of several risk factors for developing COPD. Furthermore, asthma and COPD often share similar triggering factors, such as respiratory infections and air pollution. By including both conditions, we aimed to capture the real-world scenario faced by health care professionals and individuals with these respiratory diseases during the COVID-19 pandemic and beyond. Additionally, combining the conditions allowed us to investigate and provide important insights into the effect on exacerbations and effectiveness of COVID-19 vaccines in a broad population of people with the two most common chronic underlying respiratory diseases.
Earlier case reports [8,9,10,11] have highlighted an increase in respiratory adverse events at a population-based level following COVID-19 vaccination. However, our study did not observe such an increase. There are several possible explanations. First, it is known that major inflammatory responses and subsequent hospital admissions resulting from mRNA vaccines are rare [25]. Second, patients who received the mRNA vaccine in this study were younger, with a mean age of 50.25 years, and had fewer comorbidities. It is possible that these factors made them better able to tolerate any adverse events from the vaccine compared to older patients. Notably, the case reports that reported increased respiratory adverse events included older patients (mean age 65 years) with poorer lung function. Therefore, the absence of an increased risk of exacerbations in our study in relation to the use of BNT162b2 is plausible. Further, our study did not observe respiratory exacerbation in CoronaVac recipients, consistent with our published study investigating adverse events of special interest associated with CoronaVac in patients aged ≥ 65 years [26].
Since the roll-out of BNT162b2, some observational studies [2, 7] have demonstrated effectiveness in reducing COVID-related hospitalisation and mortality in people with COPD and asthma. One cohort study [2], including 561,279 patients with asthma aged ≥ 18 years, reported an 85% reduction in the hazard of 28-day COVID-related hospitalisation following two doses of BNT162b2 compared to unvaccinated patients. Another UK-based cohort study [7] included 1 million patients aged ≥ 16 years with clinical risk groups, including asthma and COPD, and reported an 89% reduction in the 28-day composite endpoint of COVID-19 hospitalisation and mortality. Our estimates for two doses of BNT162b2 on COVID-related mortality were in line with the previous literature. However, the VE of two doses of BNT162b2 against COVID-related hospitalisation was lower than reported in the literature. A plausible explanation for the lower VE observed in our study is that our follow-up period was longer than previous studies, which typically focused on a shorter period (e.g., 28 days after vaccination). It is known that vaccine effectiveness can wane over time, and a recent review showed a decrease of approximately 20–30% in COVID-19 vaccine effectiveness six months after the second vaccination [27]. Although the VE against COVID-related hospitalisation was slightly lower than previous studies, our findings indicate that the VE of two doses of BNT162b2 against adverse events remains at 30% in the medium term. The lower risk of severe complications observed after two doses of BNT162b2 is in line with our recently published study [24] in the general population, which indicated that the VE against severe complications also applies to people with chronic respiratory diseases.
CoronaVac is more commonly utilised in developing countries than in developed countries [28, 29]. While CoronaVac has demonstrated effectiveness in reducing infection, hospitalisation, and mortality in the general population [30,31,32], its effectiveness in patients with COPD and asthma has not been extensively investigated, unlike BNT162b2. However, current findings were consistent with previous studies, suggesting that CoronaVac is effective in preventing hospitalisation, severe complications, and mortality, and these results can be extrapolated to people with COPD and asthma.
We observed that three doses offered extra protection beyond the first two doses, a finding aligned with other studies in the general population [30, 31]. Despite the limited number of events, to our knowledge, our study is the first to report on the VE of the fourth dose against COVID-related outcomes. Our results showed that the fourth dose offered additional protection compared to the second and third doses, emphasising the need to administer a second booster (fourth dose) in this population. These findings align with a recently published article on the reduction of all-cause mortality in older people in Sweden [33]. However, it is important to note that our study may have overestimated the effectiveness of the fourth dose due to the short follow-up period. Nonetheless, our results indicate the VE of the fourth dose in people with COPD and asthma. Future studies investigating the waning of protection after the fourth dose in this patient group are warranted. Our findings highlight a continued reduction in people with COPD and asthma, underscoring the importance of the third and fourth doses in mitigating severe outcomes in this population.
4.1 Limitations
This study has several limitations. First, there may be some potential unmeasured confounders in the effectiveness study. In HK, the increasing awareness of vaccines and public health campaigns to encourage vaccination among older people have led to a rise in vaccination rates in recent months. However, some people, particularly those of older age and with multimorbidity, remain unvaccinated [34]. In addition, in this study, the participants who received the CoronaVac vaccine were older and had more comorbidities than those who received the BNT162b2 vaccine. Since patients had the freedom to choose which vaccine they received, it is possible that older individuals or those with more health issues opted for a vaccine that is perceived to have fewer side effects [35]. This study could not determine such preferences directly, but to account for potential differences in characteristics like age and comorbidities between the two vaccination groups, we utilised inverse probability of treatment weighting (IPTW) adjustment. This adjustment helped control for these common indicators and reduce indication bias in the effectiveness study. Second, the outcomes of interest were hospitalisation and mortality related to COVID-19 rather than outcomes caused by COVID-19. Assessing the latter would require a comprehensive causality assessment for each outcome event, which was not feasible within the scope of this study due to limited information in the database. Third, our study may have excluded mild and moderate exacerbations of COPD and asthma, as we only identified cases requiring hospitalisation. Fourth, it is important to note that the population in HK is predominantly ethnic Chinese. Therefore, the generalisability of the analysis to other ethnicities needs to be tested through replication studies. Lastly, while some participants in the present study received a different type of vaccine for their third and fourth doses, the sample size for this subgroup remains small. Further studies are needed to elucidate the risk of COVID-related outcomes in this group.
5 Conclusion
In this population-based study in HK, we observed no elevated risk of respiratory exacerbation following each dose of the COVID-19 vaccine (CoronaVac and BNT162b2) in patients with COPD or asthma or both. Both vaccines were associated with reduced risks of COVID-related mortality, hospitalisations, and severe complications. Booster vaccines (third and fourth doses) exhibited higher VE against COVID-19 outcomes than a two-dose regimen. It is recommended that patients with COPD and asthma receive vaccination to mitigate the risk of adverse outcomes associated with COVID-19, both in the current and future epidemic waves.
References
Singh D, Mathioudakis AG, Higham A. Chronic obstructive pulmonary disease and COVID-19: interrelationships. Curr Opin Pulm Med. 2022;28:76–83. https://doi.org/10.1097/mcp.0000000000000834.
Shi T, Pan J, Vasileiou E, Robertson C, Sheikh A, Collaborators PHS and the EI. Risk of serious COVID-19 outcomes among adults with asthma in Scotland: a national incident cohort study. Lancet Respir Med. 2022;10:347–354. https://doi.org/10.1016/s2213-2600(21)00543-9
Bloom CI, Drake TM, Docherty AB, Lipworth BJ, Johnston SL, Nguyen-Van-Tam JS, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:699–711. https://doi.org/10.1016/s2213-2600(21)00013-8.
Conway FM, Bloom CI, Shah PL. Susceptibility of patients with airway disease to SARS-CoV-2 infection. Am J Resp Crit Care. 2022;206:696–703. https://doi.org/10.1164/rccm.202111-2547pp.
Sunjaya AP, Allida SM, Tanna GLD, Jenkins C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. 2022;59:866–79. https://doi.org/10.1080/02770903.2021.1888116.
Bloom CI, Cullinan P, Wedzicha JA. Asthma phenotypes and COVID-19 risk: a population-based observational study. Am J Resp Crit Care. 2021;205:36–45. https://doi.org/10.1164/rccm.202107-1704oc.
Whitaker HJ, Tsang RSM, Byford R, Andrews NJ, Sherlock J, Pillai PS, et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response amongst individuals in clinical risk groups. J Infect. 2022;84:675–83. https://doi.org/10.1016/j.jinf.2021.12.044.
Mumm T, Elbashir M. A COPD exacerbation that occurred after the MRNA COVID-19 vaccine. Chest. 2021;160:A1764–A1764. https://doi.org/10.1016/j.chest.2021.07.1607.
Colaneri M, Filippo MD, Licari A, Marseglia A, Maiocchi L, Ricciardi A, et al. COVID vaccination and asthma exacerbation: might there be a link? Int J Infect Dis. 2021;112:243–6. https://doi.org/10.1016/j.ijid.2021.09.026.
Durdevic M, Arora A, Durdevic D, Stan AC, Naaraayan A. Acute COPD exacerbation after the second dose of COVID-19 vaccine. Chest. 2021;160:1760. https://doi.org/10.1016/j.chest.2021.07.1603.
Tutak AS, Söylemez F, Konuk HB, Çakmak E, Karakaya B, Doğan A, et al. A patient presenting with ARDS after COVID-19 vaccination: a COVID-19 case report. J Infect Public Heal. 2021;14:1395–7. https://doi.org/10.1016/j.jiph.2021.05.017.
Bekkat-Berkani R, Wilkinson T, Buchy P, Santos GD, Stefanidis D, Devaster J-M, et al. Seasonal influenza vaccination in patients with COPD: a systematic literature review. Bmc Pulm Med. 2017;17:79. https://doi.org/10.1186/s12890-017-0420-8.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl J Med. 2020;383:2603–15. https://doi.org/10.1056/nejmoa2034577.
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–35. https://doi.org/10.1016/s0140-6736(21)00790-x.
Chui CSL, Fan M, Wan EYF, Leung MTY, Cheung E, Yan VKC, et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: a self-controlled case series study. Eclinicalmedicine. 2022;50: 101504. https://doi.org/10.1016/j.eclinm.2022.101504.
Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22:64–72. https://doi.org/10.1016/s1473-3099(21)00451-5.
Chua GT, Kwan MYW, Chui CSL, Smith RD, Cheung EC-L, Ma T, et al. Epidemiology of acute myocarditis/pericarditis in hong kong adolescents following comirnaty vaccination. Clin Infect Dis. 2021;75:ciab989. https://doi.org/10.1093/cid/ciab989.
Li X, Tong X, Yeung WWY, Kuan P, Yum SHH, Chui CSL, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81:564–8. https://doi.org/10.1136/annrheumdis-2021-221571.
Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, Bertrand M, Baricault B, Drouin J, et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med. 2022;41:1735–50. https://doi.org/10.1002/sim.9325.
Ye X, Ma T, Blais JE, Yan VKC, Kang W, Chui CSL, et al. Association between BNT162b2 or CoronaVac COVID-19 vaccines and major adverse cardiovascular events among individuals with cardiovascular disease. Cardiovasc Res. 2022;118:2329–38. https://doi.org/10.1093/cvr/cvac068.
Lai FTT, Yan VKC, Ye X, Ma T, Qin X, Chui CSL, et al. Booster vaccination with inactivated whole-virus or mRNA vaccines and COVID-19–related deaths among people with multimorbidity: a cohort study. CMAJ. 2023;195:E143–52. https://doi.org/10.1503/cmaj.221068.
Cheng FWT, Fan M, Wong CKH, Chui CSL, Lai FTT, Li X, et al. The effectiveness and safety of mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines among individuals with chronic kidney diseases. Kidney Int. 2022;102:922–5. https://doi.org/10.1016/j.kint.2022.07.018.
Msemburi W, Karlinsky A, Knutson V, Aleshin-Guendel S, Chatterji S, Wakefield J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature. 2023;613:130–7. https://doi.org/10.1038/s41586-022-05522-2.
Yan VKC, Wan EYF, Ye X, Mok AHY, Lai FTT, Chui CSL, et al. Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case–control study. Emerg Microb Infec. 2022;11:2304–14. https://doi.org/10.1080/22221751.2022.2114854.
Tahtinen S, Tong A-J, Himmels P, Oh J, Paler-Martinez A, Kim L, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022;23:532–42. https://doi.org/10.1038/s41590-022-01160-y.
Wan EYF, Wang Y, Chui CSL, Mok AHY, Xu W, Yan VKC, et al. Safety of an inactivated, whole-virion COVID-19 vaccine (CoronaVac) in people aged 60 years or older in Hong Kong: a modified self-controlled case series. Lancet Heal Longev. 2022;3:e491–500. https://doi.org/10.1016/s2666-7568(22)00125-8.
Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–44. https://doi.org/10.1016/s0140-6736(22)00152-0.
Premikha M, Chiew CJ, Wei WE, Leo Y-S, Ong B, Lye DC, et al. Comparative effectiveness of mRNA and inactivated whole virus vaccines against COVID-19 infection and severe disease in Singapore. Clin Infect Dis Official Publ Infect Dis Soc Am. 2022. https://doi.org/10.1093/cid/ciac288.
Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, et al. Effectiveness of CoronaVac among health care workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Heal Am. 2021;1: 100025. https://doi.org/10.1016/j.lana.2021.100025.
Cerqueira-Silva T, Andrews JR, Boaventura VS, Ranzani OT, Oliveira V de A, Paixão ES, et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case-control study. Lancet Infect Dis. 2022;22:791–801. https://doi.org/10.1016/s1473-3099(22)00140-2.
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. New Engl J Med. 2021;385:875–84. https://doi.org/10.1056/nejmoa2107715.
Palacios R, Batista AP, Albuquerque CSN, Patiño EG, Santos J do P, Conde MTRP, et al. Efficacy and safety of a COVID-19 inactivated vaccine in health care professionals in Brazil: the PROFISCOV study. Ssrn Electron J. 2021. https://doi.org/10.2139/ssrn.3822780.
Nordström P, Ballin M, Nordström A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: a nationwide, retrospective cohort study in Sweden. Lancet Reg Heal Europe. 2022;21: 100466. https://doi.org/10.1016/j.lanepe.2022.100466.
Yuan J, Lam WWT, Xiao J, Ni MY, Cowling BJ, Liao Q. Why do Chinese older adults in Hong Kong delay or refuse COVID-19 vaccination? A qualitative study based on grounded theory. Ssrn Electron J. 2022. https://doi.org/10.2139/ssrn.4078966.
Elnaem MH, Taufek NHM, Rahman NSA, Nazar NIM, Zin CS, Nuffer W, et al. COVID-19 vaccination attitudes, perceptions, and side effect experiences in malaysia: do age, gender, and vaccine type matter? Vaccines. 2021;9:1156. https://doi.org/10.3390/vaccines9101156.
Acknowledgements
The authors thank the Hospital Authority and the Department of Health for the generous provision of data for this study and Vincent Yan for technical support. A research grant has been received from The Food and Health Bureau, the Government of Hong Kong Special Administrative Region, China. The funders did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. FTTL and ICKW’s post were partly funded by D24H; hence this work was partly supported by AIR@InnoHK, administered by the Innovation and Technology Commission.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was regulatory-initiated pharmacovigilance and was funded by a research grant from the Food and Health Bureau of The Government of the Hong Kong Special Administrative Region (Reference COVID19F01).
Competing interests
SXWQ, FWTC, LWYF, HWCK, and TTM declare that there is no conflict of interest. CB is funded by the National Institute for Health and Care Research (NIHR UK) and Asthma and Lung UK, for work outside this study. CKHW reports the receipt of the General Research Fund, Research Grant Council, Government of Hong Kong SAR; EuroQol Research Foundation, AstraZeneca, and Boehringer Ingelheim all outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, MSD, and Amgen; personal fees from Primevigilance Ltd.; outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, outside the submitted work. XL received research grants from the Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, and the Hong Kong Research Grants Council, outside the submitted work. EWC reports grants from the Research Grants Council (RGC, Hong Kong), grants from the Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, Narcotics Division of the Security Bureau of HKSAR; honorarium from Hospital Authority, outside the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice in the previous 3 years. He is also an independent non-executive director of Jacobson Medical in Hong Kong.
Ethics approval and consent to participant
The ethic of this study has been approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB; ethical number UW21-377). There is no participant consent included in this study.
Consent for publication
Non-applicable.
Code availability
Non-applicable.
Availability of data and materials
The data are provided by the Hospital Authority of HK and the Department of Health HK. The data cannot be circulated out of HK if not permitted.
Authors’ contributions
SXWQ, FWTC, ICKW, and EWYC got the concept of the study. SXWQ and FWTC analysed the data and cross-checked it. SXWQ wrote the main manuscript text and prepared all the results. HWCK, CB, and CM provided clinical interpretation. SXWQ, CSLC, EYFW, ICKW, and EWYC provided a statistical plan. ICKW and EWYC acquired the data from the Hospital Authority. All authors reviewed the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Qin, S.X., Cheng, F.W.T., Kwok, W.C. et al. Effectiveness and Respiratory Adverse Events Following Inactivated and mRNA COVID-19 Vaccines in Patients with COPD and Asthma: A Chinese Population-Based Study. Drug Saf 47, 135–146 (2024). https://doi.org/10.1007/s40264-023-01364-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01364-7