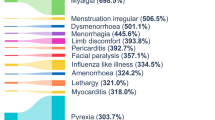
Abstract
Introduction
In 2021, the massive Covid-19 vaccination campaign in France was accompanied by an intensified pharmacovigilance monitoring of their potential adverse drug reactions. The importance of this reporting might have led to an important selective reporting and overloading of Pharmacovigilance Centres, delaying the recording of some reports in the national pharmacovigilance database. In this context, we aimed to evaluate the impact of the Covid-19 vaccination campaign in France and related reports on spontaneous reporting of adverse drug reactions that were not related to the Covid-19 vaccine.
Methods
We performed time-series analyses considering the monthly number of adverse drug reactions reported between January 1, 2018 and April 30, 2022 using the French Pharmacovigilance database. The impact of the Covid-19 vaccination campaign on the monthly reporting not Covid-19 vaccine related was estimated using interrupted time-series. January 2021, marking the start of the campaign, was the intervention date in the models. Analyses were run globally first considering all adverse drug reaction reports, and second according to notifier type and to case seriousness.
Results
We included 170,294 reports registered in the French Pharmacovigilance database between January 1, 2018 and April 30, 2022 that were not Covid-19 vaccine-related. Among these, 77,067 (45.3%) were serious and 146,683 (86.1%) had been reported by health care professionals. The campaign start was associated with a nearly 35.0% decrease in average monthly reporting that was not Covid-19 vaccine-related, with a significant level decrease in the monthly number of reports of −658.0 (p < 10−3) immediately after the vaccination campaign start and a subsequent slope decrease of −50.0 (p < 10−3). This decrease was mainly due to a significant level and slope decrease (level: −739.2 p < 10−3; slope: −39 [p < 10−2]) for health care professional reports. A similar level decrease was found for the monthly number of both serious and non-serious reports (−402.3, p < 10−3; and −311.9, p = 10−2, respectively). According to the ATC 1 level, the decrease in the monthly number of reports showed similar patterns for all drugs. However, a potential increase in the number of serious reports suspecting antineoplastic and immunomodulating drugs (ATC L) or drugs targeting blood was observed (ATC B).
Conclusion
Our study showed a significant impact of the Covid-19 campaign vaccination in the reporting of adverse drug reactions that were not Covid-19 vaccine-related, of roughly 35%. This leads to a loss of information regarding the monitoring of drug safety that could have impacted the system capacity to detect safety signals for drugs other than Covid-19 vaccines.




Similar content being viewed by others
References
Vial T. French pharmacovigilance: Missions, organization and perspectives. Therapies. 2016;71:143–50.
Moore N, Berdaï D, Blin P, Droz C. Pharmacovigilance – The next chapter. Therapies. 2019;74:557–67.
Faillie J-L. Case-non case studies: Principles, methods, bias and interpretation. Therapie. 2018;73:247–55.
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29:385–96.
Costa C, Abeijon P, Rodrigues DA, Figueiras A, Herdeiro MT, Torre C. Factors associated with underreporting of adverse drug reactions by patients: a systematic review. Int J Clin Pharm. 2023;
García-Abeijon P, Costa C, Taracido M, Herdeiro MT, Torre C, Figueiras A. Factors associated with underreporting of adverse drug reactions by health care professionals: A systematic review update. Drug Saf. 2023;46:625–36.
Raschi E, Poluzzi E, Salvo F, Pariente A, De Ponti F, Marchesini G, et al. Pharmacovigilance of sodium-glucose co-transporter-2 inhibitors: What a clinician should know on disproportionality analysis of spontaneous reporting systems. Nutr Metab Cardiovasc Dis NMCD. 2018;28:533–42.
Mouly S, Roustit M, Bagheri H, Perault-Pochat M-C, Molimard M, Bordet R. The French Levothyrox® crisis: We did the best we could but…. Therapie. 2019;74:431–5.
Langlade C, Gouverneur A, Bosco-Lévy P, Gouraud A, Pérault-Pochat M-C, Béné J, et al. Adverse events reported for Mirena levonorgestrel-releasing intrauterine device in France and impact of media coverage. Br J Clin Pharmacol. 2019;85:2126–33.
Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30:891–8.
Arnaud M, Salvo F, Ahmed I, Robinson P, Moore N, Bégaud B, et al. A Method for the Minimization of Competition Bias in Signal Detection from Spontaneous Reporting Databases. Drug Saf. 2016;39:251–60.
Caster O, Juhlin K, Watson S, Norén GN. Improved statistical signal detection in pharmacovigilance by combining multiple strength-of-evidence aspects in vigiRank. Drug Saf. 2014;37:617–28.
Lacroix C, Salvo F, Gras-Champel V, Gautier S, Massy N, Valnet-Rabier M-B, et al. French organization for the pharmacovigilance of COVID-19 vaccines: A major challenge. Therapie. 2021;76:297–303.
Khouri C, Revol B, Lepelley M, Mallaret M, Cracowski J-L. Impact of the “French Levothyrox crisis” on signal detection in the World Health Organization pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2018;27:1427–8.
Viard D, Parassol-Girard N, Romani S, Van Obberghen E, Rocher F, Berriri S, et al. Spontaneous adverse event notifications by patients subsequent to the marketing of a new formulation of Levothyrox® amidst a drug media crisis: atypical profile as compared with other drugs. Fundam Clin Pharmacol. 2019;33:463–70.
MedDRA | [Internet]. [cited 2019 Jan 23]. Available from: https://www.meddra.org/
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309.
Jandoc R, Burden AM, Mamdani M, Lévesque LE, Cadarette SM. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol. 2015;68:950–6.
Micallef B, Dogné J-M, Sultana J, Straus SMJM, Nisticò R, Serracino-Inglott A, et al. An Exploratory Study of the Impact of COVID-19 Vaccine Spontaneous Reporting on Masking Signal Detection in EudraVigilance. Drug Saf. 2023;
Vidlin S. Unmasking data in the COVID-19 vaccine era [Internet]. Upps. Rep. 20230201 [cited 2023 Sep 21]. Available from: https://www.uppsalareports.org/articles/unmasking-data-in-the-covid-19-vaccine-era/
Harpaz R, DuMouchel W, Van Manen R, Nip A, Bright S, Szarfman A, et al. Signaling COVID-19 Vaccine Adverse Events. Drug Saf. 2022;45:765–80.
Adopo D, Daynes P, Benkebil M, Debs A, Jonville-Berra AP, Polard E, Patient involvement in pharmacovigilance: determinants and evolution of reporting from, et al. to 2020 in France. Eur J Clin Pharmacol. 2011;2022:1–8.
Rolfes L, van Hunsel F, van der Linden L, Taxis K, van Puijenbroek E. The Quality of Clinical Information in Adverse Drug Reaction Reports by Patients and Healthcare Professionals: A Retrospective Comparative Analysis. Drug Saf. 2017;40:607–14.
Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin Cancer Inform. 2020;4:1059–71.
Mathieu C, Pambrun E, Bénard-Laribière A, Noize P, Faillie J-L, Bezin J, et al. Impact of the COVID-19 pandemic and its control measures on cardiovascular and antidiabetic drugs use in France in 2020: a nationwide repeated cohort study. Eur J Epidemiol. 2022;37:1049–59.
Mathieu C, Bezin J, Pariente A. Impact of COVID-19 epidemic on antihypertensive drug treatment disruptions: results from a nationwide interrupted time-series analysis. Front Pharmacol. 2023;14:1129244.
Hauben M, Hung E. Effects of the COVID-19 Pandemic on Spontaneous Reporting: Global and National Time-series Analyses. Clin Ther. 2021;43:360-368.e5.
Dörks M, Jobski K, Hoffmann F, Douros A. Global COVID-19 pandemic and reporting behavior - An analysis of the Food and Drug Administration adverse events reporting system. Pharmacoepidemiol Drug Saf. 2021;30:707–15.
Acknowledgements
The authors would like to thank all members of the French Network of Pharmacovigilance Centres and the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) for the availability and the accuracy of the data.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This research received no funding.
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
In accordance with French regulations, formal approval by an investigational review board is not required for this type of study.
Consent to participate
As all data recorded in the French Pharmacovigilance database are anonymous, informed consent is waived.
Consent to publication
Not required.
Availability of data and material
The approval to access the anonymised data maintained by the French Network of Pharmacovigilance requires the data to be treated as confidential with protected and secure access. For this reason, the data cannot be shared publicly.
Code availability
The code will be made available upon reasonable request.
Author contributions
All authors contributed to the study conception and design. Validation of the data and analysis were performed by SG and AS. SG drafted the manuscript which was commented and reviewed by all authors. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Germay, S., Singier, A., Salvo, F. et al. Impact of Covid-19 Vaccination on Spontaneous Pharmacovigilance Reporting in France. Drug Saf 46, 1381–1389 (2023). https://doi.org/10.1007/s40264-023-01359-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01359-4