Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment and care of patients with cancer owing to unique features, including the occurrence of the so-called immune-related adverse events (irAEs). A multidisciplinary team, possibly including a cardio-oncology specialist, is warranted to achieve a favorable patient outcome. Cardiovascular toxicity, especially myocarditis, emerged as a life-threatening irAE in the real-word setting, and the European Society of Cardiology has recently published the first guideline on cardio-oncology to increase awareness and promote a standardized approach to tackle this complex multimodal issue, including diagnostic challenges, assessment, treatment, and surveillance of patients with cancer receiving ICIs. In this article, through a question & answer format made up of case vignettes, we offer a clinically oriented overview on the latest advancements of ICI-related cardiovascular toxicity, focusing on myocarditis and associated irAEs (myositis and myasthenia gravis within the so-called overlap syndrome), with the purpose of assisting clinicians and healthcare professionals in daily clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The spectrum of cardiovascular toxicity with immune checkpoint inhibitors (ICIs) is heterogeneous, from myocarditis to noninflammatory manifestations (e.g., heart failure, arrhythmias, and atherosclerosis-related events). |
The mechanisms subtending ICI-related cardiovascular toxicity remain speculative, although translational approaches have described an auto-antigen-driven mechanism as a potential immunological basis of myocarditis. |
The incidence of cardiovascular toxicity is estimated to reach 10%; myocarditis is rare albeit possibly hyper-acute (within first two cycles) and fatal (mortality 50%). ICI combination regimens represent the most important risk factor for myocarditis (twofold increased risk). |
Myocarditis may frequently (40%) co-occur with myositis/myasthenia gravis (the so-called overlap syndrome), with a fulminant (after first ICI infusion) and fatal (75%) outcome. Neurological symptoms may precede the occurrence of myocarditis. |
The 2022 European Society of Cardiology (ESC) guideline on cardio-oncology provided, for the first time, recommendations on cardiovascular assessment and monitoring before, during, and after anticancer treatments, mostly on the basis ofexpert opinion. |
The actual transferability of ESC recommendations depends on local aspects, including the availability of a dedicated cardio-oncology service. |
Increased awareness is needed across different medical specialties, including general practitioners and emergency practitioners, to timely inform a multidisciplinary workup. |
The ESC guidelines recommended cardiac biomarkers (troponin and natriuretic peptides) and ECG at baseline and before each of the first three cycles of ICI administration. Transthoracic echocardiogram is also recommended at baseline in high-risk patients (e.g., those receiving ICI combination). Conversely, there is no consensus among oncological guidelines (e.g., ASCO, ESMO) on the need for baseline ECG and troponin testing. Of note, the risk of myocarditis cannot be predicted on the basis of baseline parameters. |
Additional serial monitoring of creatin kinase, aldolase, and acetylcholine receptor antibodies could be considered in high-risk patients to early detect myocarditis and overlap syndrome. |
Timely high-dose corticosteroids for 3 days represent the first-line approach, whereas targeted immunomodulating drugs are used in refractory cases. Rechallenge with ICIs deserves a careful case-by-case evaluation. |
1 Introduction
In 2018, it was estimated that up to 43% of patients with cancer may be eligible for immune checkpoint inhibitor (ICI) treatment [1], with an exponential expansion across cancer types, stages and settings, and combination regimens (with other immunotherapies, targeted therapies, traditional cytotoxic drugs, or radiation therapy). ICIs have led to unprecedented results, including sustained durable long-term survival, resulting in a plateau of the survival curves and improved health-related quality of life over conventional therapies as well as the occurrence of unique and distinct patterns of adverse events (AEs) and the so-called immune-related adverse events (irAEs) [2].
By blocking either cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed cell death 1 (PD-1), or its ligand (PD-L1), ICIs hyperactivate the immune system with off-target toxicities, which can virtually affect all organs or systems in the body, with a potential for multisystem severe toxicities [3, 4], thus requiring new skills to make a timely diagnosis, and a multidisciplinary approach for successful patient management, including, among others, cardiologists, endocrinologists, dermatologists, neurologists, internists, and clinical pharmacologists [5, 6]. The potential association between the occurrence of immune-related toxicities and improved outcomes (i.e., irAEs as biomarkers of efficacy) remains controversial [7].
Type, incidence, and onset of irAEs vary depending on pharmacological class, ICI dose, type of cancer, and patient susceptibility. A recent overview of 129 systematic reviews found that the three AEs with the highest median incidence were fatigue (18.3%), diarrhea (15.3%), and rash (14.4%) [8]. In general, irAEs occur in 70–90% of patients (10–15% for grade ≥ 3); all-grade colitis, hypophysitis, and rash are more frequent with CTLA-4 inhibitors, whereas pneumonitis, hypothyroidism, arthralgia, and vitiligo are more common with anti-PD-1/PD-L1 drugs [9]. Patients receiving anti-PD-1/PD-L1 monoclonal antibodies have a lower incidence of any-grade irAEs than those treated with anti–CTLA-4 drugs, whereas subjects treated with ICI combination showed increased incidence, severity, and earlier onset of irAEs. Heterogeneous latencies have been described, ranging from fulminant occurrences (within days, especially hyper-acute myocarditis) [10] to delayed onset up to 26 weeks (e.g., diabetes, arthritis), and chronic unresolved toxicities (e.g., endocrinopathies after > 6 months, even after ICI interruption) [11], with a median onset of 40 days [12].
A recent online survey of 155 resident and faculty physicians in internal medicine, emergency medicine, and family medicine found important knowledge gaps regarding recognition and treatment of irAEs [13], although efforts have been pursued in improving our understanding, diagnosis, and prediction. Since 2017, different guidelines are constantly updated to support timely recognition and management of irAEs by major societies, including the American Society for Clinical Oncology (ASCO) [14], the European Society for Medical Oncology (ESMO) [15], the National Comprehensive Cancer Network (NCCN) [16], and the Society for Immunotherapy of Cancer (SITC) [17]. Notably, the relevant recommendations mainly rely on retrospective cohort or case-control studies, case reports/series, or even expert opinions.
The European Society of Cardiology (ESC) has recently published the first guideline on cardio-oncology [18], including chemotherapeutics, targeted therapies, and immunotherapies, thus strengthening the importance of increasing awareness on this emerging safety issue. To this end, the ESC guidelines supported the creation of dedicated cardio-oncology services. To promote harmonization on diagnosis and management, the ESC guideline endorsed recent international definitions of cancer-therapy-related cardiovascular toxicity [19]. Of note, among 272 recommendations, only 3% were supported by Level of Evidence (LOE) A, whereas 76% by LOE C (expert opinion, small or retrospective studies, or registries).
In this question & answer (Q&A) article, made up of case vignettes, we address the complex multimodal issue of cardiovascular toxicity with ICIs, including diagnostic challenges, assessment, treatment, and surveillance of patients with cancer receiving ICIs, with a focus on myocarditis. The intent is to assist clinicians and healthcare professionals in the management of ICI-related cardiovascular toxicity, especially from the perspective of a cardiologist. For details on literature selection, please see the Electronic Supplementary Material.
2 What Is the Spectrum of ICI-Related Cardiovascular Toxicity?
The spectrum of ICI-related cardiovascular toxicity is variegate, embracing not only myocarditis, but also nonmalignant pericardial disorders, acute coronary syndromes, congestive heart failure, atrioventricular block, supraventricular and ventricular arrhythmias, Takotsubo-like syndrome, vasculitis, and venous thromboembolism [20, 21]. Although initially a retrospective case series of 11 patients found a potential beneficial effect of PD-1 blockade on complicated plaque burden, with documented regression of atheromatous plaque in 3 patients [22], the current body of evidence supports an increased risk of accelerated atherosclerosis and atherosclerotic cardiovascular events mainly driven by hypercholesterolemia, with further interest in exploring the potential synergism of immunotherapy with statin and non-statin drugs [23]. Basically, apart from Torsade de Pointes occurrence due to remarkable QT prolongation (a common safety issue with tyrosine kinase inhibitors), hypertension, and valvular disease, any cardiovascular event is potentially attributable to ICIs (Fig. 1).
Multifaceted spectrum of cardiovascular toxicity and related events with immune checkpoint inhibitors. Albeit rare, myocarditis is the most common and serious cardiovascular presentation, with potential fulminant manifestation. Although myocarditis may occur alone, potential overlaps with other cardiovascular (e.g., heart failure, arrhythmias) or non-cardiovascular (e.g., myositis and myasthenia gravis, the so-called overlap syndrome) immune-related adverse events are possible. Please refer to the full text for details. Created with biorender.com
As compared with other forms of myocarditis (e.g., infective, vaccine-related, antipsychotic-related), the prognosis of ICI-related myocarditis is significantly worse, with mortality rates ranging from 25% to 50%. Co-existence of cardiovascular events, especially coronary artery disease when revascularization is performed [24], and other irAEs, especially those involving skeletal muscle and/or the neuromuscular junction, that is, myositis and/or myasthenia gravis (MG) [4], may portend poorer outcome. Several other predictors of poor prognosis have been identified, including dual ICI combination (Table 1). The ESC guidelines defined high-risk patients as those receiving dual ICI and/or combination ICI-cardiotoxic therapy, with ICI-related non-cardiovascular events, prior cancer therapy-related cardiac dysfunction, or cardiovascular disease.
Emerging data suggest that myocarditis typically presents early after ICI start. Mahmood et al. [25] reported a median time to onset of 34 days (81% of cases presented within 3 months from the first dose); Escudier et al., [26] reported a median time to presentation of 65 days (after a median of three infusions); Moslehi et al. [27] reviewed 101 myocarditis cases from VigiBase, the WHO’s global database of individual case safety reports, and found that 64% (38 of 59 cases with dosing details available) occurred after the first or second ICI dose. Myocarditis has also been described up to several months after starting ICI therapy, although it is uncertain whether it may related to late diagnosis, actual delayed myocarditis, or a cardiomyopathy related to a persistent subtle systemic inflammation [28].
3 What Is the Epidemiology of ICI-Related Cardiovascular Toxicity?
The precise incidence of cardiovascular toxicity remains uncertain, especially due to the (unexpected) underreporting from pivotal clinical trials [29], challenges in diagnosis and definition, and an initial lack of awareness [30]. Most of the current understanding is based on real-world data, including retrospective multi-/single-institutional case series, and nationwide cohort studies.
Initially, the evidence on ICI-related cardiovascular toxicity emerged from case reports and post-marketing surveillance. In 2016, Johnson and colleagues reported two cases of fatal fulminant myocarditis following ICI combination regimen early after the first dose of therapy; 18 myocarditis cases were found in the Bristol-Myers Squibb pharmacovigilance database (0.27% in patients receiving the nivolumab-ipilimumab combination versus 0.06% in those receiving nivolumab alone) [31]. Subsequent pharmacovigilance studies using VigiBase confirmed the life-threatening nature of myocarditis, and the heterogeneous spectrum of cardiovascular toxicity [27, 32].
Different estimates emerged from randomized controlled trials (RCTs). A pooled analyses of 6925 participants enrolled in the National Cancer Institute-Cancer Therapy Evaluation Program found a higher incidence with anti-PD-1/PD-L1 plus targeted therapies compared with anti-PD-1/PD-L1 plus anti-CTLA-4 combinations (2.1% versus 0.9%). Remarkably, a multisystem involvement with non-cardiac irAEs was found in 65% of patients [33]. A systematic review of 63 RCTs found a (higher) wide range of incidence, from 3.2 (myocarditis) to 19.3 (dyslipidemia) per 1000 patients, with a number needed to harm of 462 and 74, respectively [34]. Another meta-analysis of 51 RCTs confirmed a higher rate of cardiovascular irAEs with ICI combination therapies as compared with monotherapies (5.8% versus 3.1%) [35].
With regard to cohort studies, Drobni et al. [36] reported an incidence of cardiovascular events (not including myocarditis) of 4.2% in 2842 patients, with a rate of progression (imaging study) of total aortic plaque volume more than threefold higher with ICIs and partially attenuated by concomitant use of statins or corticosteroids. A Danish nationwide study reported an absolute risk of cardiovascular events (including myocarditis) at 1 year of 9.7% in 743 patients with lung cancer and 6.6% in 145 patients with melanoma [37]. Very recently, Laenens et al. [38] reported an incidence of cardiovascular events (mainly consisting of heart failure) of 10.3% with a median time to event of 5 months, especially in subjects with a history of heart failure and valvular heart disease.
4 What Is the Mechanism(s) Underlying ICI-Related Cardiovascular Toxicity?
The pathophysiology of cardiovascular irAEs with ICIs remains poorly understood, but multiple interrelated mechanisms are plausibly involved [39]. Several preclinical in vivo (genetic knockout) and in vitro models support crucial roles of CTLA-4, PD-1, and PD-L1 signaling in cardiac–immune crosstalk. A post mortem analysis of patients with ICI-related myocarditis and associated myositis treated with nivolumab plus ipilimumab revealed infiltration of CD4+, CD8+ T cells, and macrophages in the myocardium and conduction system, with subsequent cardiomyocytes necrosis, whereas no B cells nor autoantibodies were found. Similar T-cell clones were observed in the infiltrated skeletal muscle and tumors. Moreover, PD-L1 was expressed on the membrane of injured myocytes [31].
Different mechanistic hypotheses have been proposed for ICI-related myocarditis [40], including: (a) shared antigen between the tumor and myocardium (“epitope sharing”), resulting in cross-reactivity; (b) tumor cell death with subsequent release of previously inaccessible intracellular antigens in the cancer microenvironment (“epitope spreading”) [41]; (c) activation of a preexisting T-cell clone reactive to self-antigens; in this regard, preexisting autoantibodies targeting cardiac troponin were detected in preclinical models; (d) triggering antibody-dependent cellular toxicity T-reg depletion, with a loss of peripheral tolerance [42], especially in case of previous cardiovascular injury; (e) T-cell infiltration secondary to overexpression of PD-L1 on cardiovascular cells (known to be protective with regard to myocardial ischemia reperfusion and atherosclerosis); (f) release of proinflammatory cytokines (although cytokine release syndrome is more frequent with CAR T-cell therapy as compared with ICIs); and (g) dysregulation of myocardial metabolism induced by smouldering inflammation.
Very recently, Gergely et al. [43] found increased IL-17 signalling in the thymus of mice with cardiac dysfunction induced by PD-1 blockade, with relevant pharmacological inhibition capable of preventing cardiotoxicity, thus paving the way for potential repurposing of IL-17 blocking drugs. Axelrod et al. [44], using a genetic mouse model with homozygous knockout of Pdcd1 and heterozygous deletion of Ctla4, showed that the myocardial immune infiltrate was primarily composed of CD8+ T cells and identified the cardiac-specific protein α-myosin as a potential autoantigen in ICI-related myocarditis. The full translational potential of these findings needs to be further investigated.
5 What Are Presenting Signs and Symptoms?
ICI-related cardiovascular toxicity may present with non-organ specific symptoms (e.g., unusual fatigue, weakness, muscle pain, syncope), or typical cardiac symptoms (e.g., palpitations, shortness of breath, chest pain, pulmonary or lower extremity edema, irregular heartbeat), including subtle signs and symptoms (potentially masked by non-cardiac irAEs). Therefore, the diagnosis of ICI-related cardiotoxicity is challenging, especially with regard to fatigue, a likely complication of cancer per se.
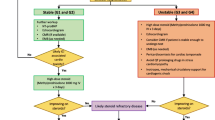
A subclinical rise of cardiac biomarkers in the absence of clear symptoms could be an early sign of cardiotoxicity. Therefore, the ESC guidelines stated that any abnormal finding (presence of symptoms, a new increase in troponin associated with either cardiovascular symptoms or other irAEs, and new ECG abnormalities) should prompt urgent cardiovascular imaging, and other causes of myocardial injury (e.g., acute coronary syndrome, acute infectious myocarditis) should be excluded [18] (Fig. 2).
Proposed flowchart for the diagnosis, assessment, and management of cardiovascular toxicity with immune checkpoint inhibitors. Adapted from [18, 21]. *Class of recommendations according to the 2022 ESC Cardio-Oncology guidelines [18]. AChR acetylcoline receptor antibodies, ACS acute coronary syndrome, CK creatin kinase, CV cardiovascular, CMR cardiac magnetic resonance, ECG electrocardiogram, EMB endomyocardial biopsy, HbA1c glycated hemoglobin, ICI immune checkpoint inhibitors, ICU intensive care unit, irAEs immune-related adverse events, IM immunomodulating, LV left ventricular, MCS mechanical circulatory support, NT-proBNP N-terminal pro-brain-natriuretic-peptide, TSH thyroid-stimulating hormone, TTE transthoracic echocardiography, TTS takotsubo syndrome
ICI-related myocarditis is frequently (in up to 30–40% of patients) associated with immune-related myositis and MG, a condition referred to as myocarditis/myositis/MG overlap syndrome (IM3OS), which is characterized by a hyper-acute onset (after a median of just one ICI infusion), a fulminant clinical course, and an ominous prognosis, with a poor response to immunomodulatory treatments and fatal outcome in up to two-thirds of patients [45] (Fig. 3).
From a clinical standpoint, immune-related MG manifests with fatigue and fluctuating muscle weakness, preferentially involving cranial, bulbar, and axial muscles. Cranial and bulbar involvement manifests with fluctuating ptosis, diplopia, dysarthria with slurred speech, and dysphagia, preferentially involving liquids. Respiratory muscle involvement might result in ventilatory dysfunction. Furthermore, the spectrum of immune-related myositis varies from asymptomatic elevation of creatine kinase (CK) levels to severe myopathies. In immune-related myositis, muscle weakness is often preceded by diffuse myalgias and presents with a limb-girdle pattern along with axial (mainly cervical) involvement. Therefore, patients complain about having difficulty rising from the toilet, getting up from chairs, or climbing stairs. Cervical muscle weakness, which is rarely observed in the idiopathic counterpart, manifests as dropped head. As skeletal muscle and neuromuscular junction are usually involved concomitantly, the resulting neurological picture is often complex and multidistrict, encompassing several of the aforementioned signs and symptoms.
Despite their well-known co-occurrence, the temporal relationship between cardiological and neuromuscular manifestations has never been elucidated. Interestingly, in a recent series of four patients with IM3OS, neurological symptoms were reported to precede the onset of myocarditis by a mean of 7.5 days [46]. Caution is recommended in every patient complaining of even mild symptoms potentially referable to a neuromuscular dysfunction, such as weakness or muscle aches, because they might predict a devastating overlapping syndrome.
6 Is There a Typical Patient Phenotype for the Overlap Syndrome?
Herein, we reported two representative cases of IM3OS. These cases are informative for several reasons. First, ICI-related myocarditis may range from asymptomatic to dramatic forms, manifesting with sudden arrhythmic death. Although combination regimen is a known risk factor, cases during ICI monotherapy may occur. Second, neurological symptoms often precede clinical or paraclinical evidence of myocardial inflammation. Third, in the two reported patients, CK and troponin levels seem to present opposite trends: while CK levels decrease rapidly and steadily after steroid introduction, troponin levels tend to increase and remain elevated after CK normalization. The same trend was reported in a recent systematic review including 14 patients with immune-related myositis and myocarditis: after the introduction of steroid treatment, CK decreased in all patients, while in 12 subjects (86%) troponin levels continued to increase [47]. CK and troponin have similar serum half-life (approximately 120 min) and their levels should rapidly decrease when the necrosis of myocytes or cardiomyocytes, respectively, stops. The fact that troponin, contrarily to CK, keeps rising at the administration of steroids in ICI-induced overlapping myositis/myocarditis may indicate that myocarditis is more resistant to immunosuppressive treatment than myositis. A recent retrospective study on 27 cases of myocarditis (probable or definite) found that CK peaked the fastest (median time to peak concentration 2 days prior to the diagnosis of myocarditis), with concentrations declining precipitously and returning to normal levels after a median of 17 days [48].
6.1 Case Vignette 1
A previously healthy 80-year-old man was diagnosed with cutaneous melanoma. After surgical excision, adjuvant anti-PD-1 was started, and 11 days after receiving the first dose, he developed diffuse muscle aches and proximal leg weakness, with difficulty to getting up from a chair or climbing the stairs. Within a few days, he developed bilateral ptosis, diplopia with ophthalmoplegia, dysarthria, and dysphagia for liquids; muscle weakness further deteriorated, compromising the ability to walk autonomously. The patient was admitted to the hospital 5 days after symptom onset. The ECG showed a previously unknown atrial fibrillation, and transthoracic echocardiogram (TTE) revealed a normal left ventricular ejection fraction (LVEF, 60%). Blood examination showed markedly increased levels of both CK (> 20,000 UI/L, normal value < 170 UI/L) and troponin-I (> 24,000 ng/L). Antibody testing revealed positivity to anti-acetylcoline receptor (AchR; titer: 0.85 nmol/L) and anti-titin antibodies. IM3OS was diagnosed and treated with 1 mg/kg/day intravenous methylprednisolone. Despite an initial improvement of neurological symptoms and CK-level reduction (> 6000 UI/L), the patient developed a fatal arrhythmia 5 days after admission.
6.2 Case Vignette 2
A 72-year-old man with a medical history of arterial hypertension, atrial fibrillation, and hypercholesterolemia received two cycles of anti-PD-1 for melanoma with nodal metastases. Two weeks after receiving the second infusion, he insidiously started to complain of difficulty to “keep his head straight,” fluctuating blurred vision, and mild voice lowering. Blood examination revealed raised CK levels (> 1700 UI/L), along with anti-AchR antibody and anti-titin antibody positivity. Immune-related-MG/myositis was diagnosed and immunosuppressive treatment with 1 mg/kg/day intravenous methylprednisolone was started. Neurological symptoms rapidly improved and CK levels returned to normal after 1 week. Despite having no cardiological symptoms and unremarkable ECGs and echocardiography, the patient was tested for troponin I, which was elevated (> 190 ng/L). He therefore underwent cardiac MRI, which showed signs of myocardial edema in the subendocardial posterior wall, indicating a concomitant myocarditis. Troponin levels continued to rise after immunosuppressant introduction (peaking at 390 ng/L), were still elevated at discharge (> 150 ng/L 2 weeks after steroid introduction), and normalized within 1 month. Steroid treatment was slowly tapered over 6 months without neurological relapses.
7 What Is the Role of ECG, Cardiovascular Imaging, and Cardiac Serum Biomarkers in Diagnosis?
At the onset of symptoms, the suspect of direct cardiotoxicity (e.g., myocarditis) needs to be dissected by combining cardiac biomarkers, ECG, and cardiovascular imaging [18]. The diagnosis is suspected on the basis of the occurrence of new cardiac symptoms, new ECG changes (bradycardia, tachyarrhythmias, atrio- or intraventricular conduction defects), and/or a new increase in troponin levels. Cardiovascular imaging is crucial in confirming cardiac dysfunction and excluding other possible causes (e.g., infectious myocarditis or acute coronary syndromes) [25].
Cardiovascular imaging has a definite role in both identifying patients with prior cardiovascular disease (CVD) and as a reference in recognizing any significant changes during therapy and follow-up. While TTE is the baseline imaging technique in patients undergoing any potentially cardiotoxic therapy (class I recommendation with LOE B in high-risk patients), cardiac magnetic resonance (CMR) represents the gold standard and is warranted when TTE is equivocal or identifies the need for further diagnostic work-up [18]. As it stands, the current definitions of cancer-therapy-related cardiac dysfunction are mainly based on LVEF/global longitudinal strain (GLS) reductions, ideally measured with the aid of 3D-echocardiography or even ultrasound-enhancing contrast agent. Baseline LVEF and GLS measures are recommended in all patients for whom TTE is indicated: it is known that baseline LVEF reduction or even borderline values (< 55%) are a risk factor for later development of cardiovascular toxicity, and these alterations help to stratify patients needing higher intensity surveillance [49].
In the case of suspected ICI-related myocarditis, both TTE and CMR are recommended in all patients; alternatively, if CMR is unavailable or not feasible/contraindicated (e.g., known or suspected metal foreign bodies, severe claustrophobia), cardiac positron emission tomography (PET) may be considered, although with significantly lower sensitivity. At this time, there are no specific CMR features of ICI-related myocarditis; standard-of-care modified Lake Louise criteria are used [50]. Additionally, endomyocardial biopsy remains the gold standard to characterize the histology pattern of myocarditis, and is to be considered when non-invasive tests cannot reliably establish it, or in clinically unstable patients for whom CMR cannot be executed in an urgent manner [51].
Cardiovascular imaging, associated with ECG and biomarkers, is also crucial in the identification of many other ICI-related cardiovascular toxicities, such as myocardial infarction, pericardial disease, and Takotsubo syndrome; these can be diagnosed according to the relative guidelines, as their diagnostic pathway is not significantly different in patients currently or previously having received ICI therapy [52, 53].
In addition to diagnostic purposes in case of suspect acute toxicity, 12-lead ECG is a simple and easily available test to screen for prior CVD. Routine ECG is a cost-effective surveillance measure for early identification of conduction abnormalities (e.g., atrioventricular block), QTc prolongation (possibly related to other anticancer drugs or concomitant agents), arrhythmias, or ischemia [54]. It might also be helpful in the preventive setting: even though no such correlation has yet been made in patients receiving ICIs, it has been shown that ECG changes such as P waves consistent with left atrial enlargement can be predictors of atrial fibrillation development during cancer chemotherapy [55], and that the presence of atrioventricular conduction defects is associated with atrial arrhythmias in patients undergoing hematopoietic stem cell transplantation [56]. In addition, QRS duration has been shown to be increased in ICI-related myocarditis, and even have a direct relation with increased cardiovascular risk [57].
More generally, any patient receiving ICIs that experiences palpitations or syncope should undergo a 12-lead ECG or even ambulatory ECG monitoring to rule out conduction delays, ventricular arrhythmias, and especially atrial fibrillation; additionally, it is crucial to keep in mind that a previous study has also shown how these conduction disorders were isolated in only 13% of patients, with the remaining majority occurring in conjunction with other ICI-related adverse events (mainly myocarditis, pericarditis, and thyroiditis) [26, 58].
The usefulness of cardiac biomarker assessment is less clearly proven in the setting of risk stratification prior to ICI administration, but it is still required to identify a change in laboratory values that is diagnostic for subclinical cardiac injury [18]. Furthermore, as discussed in the following section, cardiac biomarkers are crucial for predictive and preventive risk assessment.
In the case of ICI-related myocarditis, biomarkers such as troponin and brain natriuretic peptides (BNP and NT-proBNP) are sensitive indicators and can help guide diagnosis [59]. However, they are generic markers of cardiac damage and strain, and therefore can be elevated even in noninflammatory heart dysfunction; moreover, BNP and NT-proBNP may also be chronically elevated by tumor-related inflammation [60]. Notwithstanding the lack of specificity, several studies have shown that cardiac troponin (cTn) and BNP levels can be used for prognostic evaluations, since higher levels have been predictive of worse outcomes [25, 61].
8 Are Predictive, Prognostic, and Risk Stratification Biomarkers Available in Clinical Practice?
Despite the low incidence of cardiovascular toxicity, the high mortality rate strengthens the need for affordable prognostic and predictive biomarkers. Different observational studies have investigated clinical factors associated with an increased risk of immune-related myocarditis [62]. While ICI combination is regarded as the most important risk factor, female sex, age ≥ 75 years, diabetes mellitus, obesity, preexisting autoimmune diseases, and cardiovascular disease may enhance the susceptibility to cardiovascular events [25, 59]. Furthermore, patients affected by irAEs are more likely to experience more irAEs with scarce ability to predict type and severity [63]. In this regard, patients with a history of non-cardiovascular irAEs had a higher risk of ICI-related cardiotoxicity (Table 1). As anticipated, the ESC guidelines divided patients exposed to ICIs into two classes of risk, with slight differences in surveillance protocol (Fig. 4).
Proposed flowchart for the surveillance of cardiovascular toxicities with immune checkpoint inhibitors. The 2022 ESC guidelines defined high-risk patients as those receiving dual ICI and/or combination ICI-cardiotoxic therapy, with ICI-related non-cardiovascular events, prior cancer therapy-related cardiac dysfunction, or cardiovascular disease. Class of recommendations according to the 2022 ESC cardio-oncology guidelines [18]. AChR acetylcoline receptor antibodies, CK creatin kinase, ECG electrocardiogram, ICI immune checkpoint inhibitors, NP natriuretic peptides (B-type natriuretic peptide and NT-proBNP N-terminal pro-brain-natriuretic-peptide), TTE transthoracic echocardiography. Created with biorender.com
Serum biomarkers may be helpful in stratifying high-risk patients, formulating a diagnosis, and monitoring the treatment outcome. They should be promptly performed at baseline as a referral point and at symptom presentation/hospital admission. Among patients affected by ICI-induced myocarditis of a French cohort, the majority (26 out of 30 patients) presented elevated troponin T levels at hospital admission [26]. Conversely, only 14 patients underwent blood monitoring and had a BNP elevation. Mahmood et al. [25] found that the accuracy of troponin T at discharge was superior to admission and peak levels. Specifically, patients with final/discharge troponin T ≥ 1.5 ng/ml had the highest hazard of developing cardiovascular toxicity [hazard ratio (HR) 4.0; 95% confidence interval (CI) 1.5–10.9; p = 0.003], although the clinical relevance is uncertain.
The role of nonspecific blood biomarkers is still uncertain, although the absolute lymphocyte count (ALC) or the neutrophil-to-lymphocyte ratio (NLR) may reinforce the diagnostic hypothesis with a relevant prognostic value, as emerged for non-cardiac irAEs [64, 65]. Interestingly, variations of NLR were related to the entity of myocardial injury among patients affected by myocarditis [66]. Drobni et al. [67] found a statistically significant decrease in ALC from ICI-baseline to hospital admission for myocarditis presentation (p < 0.001). Parallelly, NLR was significantly increased from baseline to hospital admission (p < 0.001). A 100% increase in NLR was a slightly good predictor of cardiovascular toxicity, with an area under the curve of 0.74 (95% CI 0.57–0.90; p = 0.019).
The co-occurrence of MG and/or myositis is associated with a worse prognosis [33]. Patients diagnosed with myocarditis should therefore be carefully investigated for even mild and underreported signs or symptoms of neuromuscular dysfunction. CK and aldolase dosage are recommended in every patient with immune-related myocarditis. In IM3OS, average CK levels are reported to be 9645 UI/L, much higher than expected in isolated myocarditis or myositis. Since several cases of immune-related myositis with normal CK are reported, aldolase, whose concentration in higher in regenerating myocytes (preferentially damaged by muscle inflammation), should also be dosed [68].
If there is clinical or laboratory suspicious of concomitant neurological involvement, patients should be tested for AchR antibodies and anti-titin antibodies. Anti-AchR antibodies were reported to be positive in half of the cases of immune-related MG [69], but this percentage seems to be higher when MG occurs in the context of IM3OS [45]. Cases of immune-related MG with anti-muscle-specific receptor tyrosine kinase or anti-low-density lipoprotein-receptor-related protein 4 positivity are anecdotal; their search should therefore be performed only in anti-AchR-negative patients. Anti-striated muscle antibodies have been reported to be positive in about 75% of cases of IM3OS and might therefore represent a valuable biomarker of this overlapping condition [70].
9 Can Preventive Strategies Be Implemented?
While a preventive approach tailored to the specific ICI-related cardiovascular toxicity spectrum is mandatory, the literature regarding its design and application is quite limited, and mainly based on expert recommendations [71]. The 2022 ESC guidelines outlined a general strategy for risk assessment and management to be set up at the time of cancer diagnosis and before any cancer treatment. Such timing is crucial to enable the oncologist to take cardiovascular risk into account while identifying the optimal treatment plan, personalizing follow-up strategies and stratifying high-risk patients to be appropriately referred to the cardio-oncology specialist [18].
The choice of cardiac tests to be implemented (imaging, ECG, and/or biomarkers) should ultimately be tailored to the patient through consideration of prior individual risk and the range of possible complications specifically related to ICI therapy.
The baseline (pre-treatment) risk assessment should be conducted following a risk-scoring tool, such as the one developed by the Heart Failure Association–International Cardio-Oncology Society in patients treated with trastuzumab for early breast cancer [72]. In general, low-risk patients can be managed within the routine oncology follow-up, while patients at moderate risk may benefit from closer cardiac monitoring. By contrast, patients identified as having a baseline high or very high risk of later development of cardiovascular toxicity should then be referred to the specific cardio-oncology service to find and implement a personalized risk management and surveillance strategy [73], as outlined in the following section.
Although a baseline cardiovascular risk profile is mandatory, in the case of myocarditis, no specific risk factor nor diagnostic variable can reliably establish a definite risk profile before the initiation of ICI therapy, with the exception of patients with heart transplantation. In these patients, by counteracting immunosuppression, ICI significantly increases the occurrence of graft rejection, which in the case of heart transplantation is a form of myocarditis, in up to 40% of recipients experiencing the risk of graft loss during ICI therapy. Kidney and liver rejections are more frequently reported, although some preliminary experience from case reports/case series suggested that it is possible to uncouple immunosuppression and immune stimulation in these patients [74, 75].
In the ideal preventive strategy, the overall risk stratification should be balanced toward the expected benefit of ICI therapy in the individual patient: detection of a significant cardiovascular risk may not necessarily require withdrawal or contraindicate ICI therapy if the treatment is likely to cure the malignancy or meaningfully prolong its remission. A certain degree of asymptomatic or treatable cardiac dysfunction might be acceptable, provided that an intensified cardio-oncological follow-up plan and cardioactive therapy optimization are implemented. In this context, however, the lack of studies to guide patient-tailored management and/or preventive strategy of ICI discontinuation or reintroduction is a major unmet need.
10 Which Surveillance Protocols Exist?
As compared with ESMO, ASCO, SITC, and NCCN oncological guidelines, the ESC guidelines provided a surveillance protocol dependent on the underlying patient risk for the first time [18]. Serial ECGs and cardiac troponin measurements should be considered before ICI doses 2, 3, and 4, and, if normal, limited to a check every three doses until completion of therapy to detect subclinical ICI-related cardiovascular toxicity (class IIa recommendation; LOE B). Cardiovascular assessment (a) is recommended every 6–12 months in high-risk patients who require long-term (> 12 months) ICI treatment (class I recommendation; LOE C) and (b) may be considered every 6–12 months in all patients who require long-term (> 12 months) ICI treatment (class IIb recommendation; LOE C). Considering the high mortality of IM3OS and the promising role of biomarkers, CK, aldolase, and AchR antibodies could be considered at least during initial cycles to detect myocarditis early and start immunosuppressive treatment in a timely manner (Fig. 4).
11 What Is the Management Strategy, Including Refractory Cases?
The management of ICI-related cardiovascular toxicity consists of: (1) identifying and evaluating the type and severity of the cardiotoxicity; (2) deciding whether to withhold ICI therapy; (3) initiating steroid and immunomodulating therapy; (4) starting conventional cardiac treatment; and (5) restarting ICI therapy [76]. All the suspected cases of ICI-related myocarditis should promptly undergo a specific diagnostic algorithm, as previously described, and be classified as fulminant or non-fulminant myocarditis to adjust the management strategy.
The immunotherapy treatment must be withheld in all suspicious cases during the diagnostic pathway, regardless of clinical severity. The ESMO guidelines for managing immunotherapy toxicity recommended a four-sequential-step strategy consisting of diagnosis, grading, immunosuppression therapy for grade ≥ 2 events, and monitoring [15]. In case of isolated troponin elevation (grade 1), the ASCO guidelines recommend withdrawing ICIs and re-checking troponin level 6 h later; if troponin values normalize or the elevation seems to be unrelated to ICI therapy, on the basis of the physician’s differential diagnosis in the light of medical history, physical examination, and other test results, ICIs may be resumed [14].
The cornerstone of immunosuppression for irAEs is steroid treatment following the general rule that the minimal effective dose should be prescribed for the shortest duration, even if tapering and discontinuation should be monitored under oncologist advice. Notably, a possible negative prognostic impact on survival of steroid introduction under ICI raises concerns about their employment [77]. Nevertheless, steroid prescriptions for non-cancer-related syndromes and particularly for managing irAEs did not affect survival outcomes, although the channeling and immortal time bias should be considered for the increased probability of receiving concomitant treatments for ICI responders [78, 79].
A retrospective multicenter study registered the management of 126 patients affected by immune-related myocarditis [80]. Patient outcomes were evaluated according to initial steroid dose prescription as low dose (< 60 mg/day, 16.7%), intermediate dose (60–500 mg/day, 43.7%), and high dose (501–1000 mg/day, 39.6%). The high-dose group experienced a significantly lower risk of cardiovascular events after adjusting for age, sex, LVEF, and timing of steroid initiation (HR 0.27, 95% CI 0.09–0.84, p = 0.024). Moreover, the early administration of steroids (< 24 h from hospital admission) was associated with a significantly lower incidence of cardiovascular events regardless of age, sex, LVEF, and steroid dosage (HR 0.03, 95% CI 0.004–0.23, p = 0.001)
Thus, myocarditis management should be promptly started within 24 h from hospital admission, and high-dose steroid treatment, namely an intravenous bolus of 500–1000 mg of methylprednisolone once daily, is warmly recommended [15]. A clinical improvement should be monitored through patient semiology, cardiac serum biomarkers, ECG, and LVEF assessment. The subsequent deescalation of steroid dose is not strictly defined. However, a switch to oral prednisolone (1 mg/kg) is recommended, and a progressive weekly reduction should be carried out according to clinical and diagnostic cardiological findings [18].
A steroid-resistant ICI-myocarditis is diagnosed if a progressive recovery is not assessed within 3 days of high-dose steroid treatment [18]. A second-line strategy is not well established, and insufficient evidence emerged from case series, even with encouraging results. In particular, several immunomodulating agents have been administered, such as abatacept, ruxolitinib, alemtuzumab, tocilizumab, infliximab, mycophenolate, antithymocyte globulin, intravenous immunoglobulin, or plasmapheresis [81,82,83,84,85,86,87].
The immunotherapy rechallenge is debated and should be carefully discussed within a multidisciplinary team on an individual basis, considering the severity and recovery of the cardiac damage, the therapeutic alternatives, and the prognosis. According to the 2022 ESC guidelines, this multidisciplinary discussion is recommended in selected patients with previous uncomplicated ICI-related myocarditis (class of recommendation: I; LOE: C). This is in line with a retrospective analysis at Tel Aviv Sourasky Medical Center of three patients (out of seven diagnosed with myocarditis) who were selected to restart ICI treatment with concomitant low-dose steroids and weekly troponin monitoring. Only one patient with grade 3 myocarditis developed worsening cardiological symptoms after the first cycle and stopped ICI treatment permanently [88].
12 Knowledge Gaps and Future Perspectives
The 2022 ESC cardio-oncology guidelines aimed to first increase awareness of various clinicians, including general and emergency practitioners, on this emerging safety issue. However, knowledge gaps have been also identified to actually achieve a shared harmonized management, including strategic investments in cardio-oncology care networks and cardio-oncology services. The cardio-oncologist is becoming a key player and should be possibly included in the Molecular Tumor Board.
A key aspect of these ESC guidelines is represented by the lack of RCTs to guide decision-making. This is especially the case of screening/preventive strategies and pharmacological treatment of myocarditis, which is based only on limited case series. Notably, the ESMO, ASCO, SITC, and NCCN oncological guidelines provided recommendations “in isolation” without addressing multisystem toxicities, especially the overlap syndrome. Moreover, there is no consensus on recommending routine screening strategies. For instance, the ASCO guidelines stated that “there is no clear evidence regarding the efficacy or value of routine baseline or serial ECGs or troponin measurements in patients receiving checkpoint inhibitor therapy” [14], whereas the SITC guidelines stated that baseline ECG and troponin testing may be considered in patients deemed at higher risk of myocarditis (e.g., cardiac comorbidities, ICI combination regimen) [17]. Pharmacological management is more consistent across guidelines, although we support the design and conduction of RCTs to actually assess the efficacy of therapeutic strategies. Of note, the ongoing ATRIUM trial (ClinicalTrials.gov Identifier: NCT05335928; estimated completion date: April 2027) is comparing abatacept with placebo in terms of cardiovascular event reduction in hospitalized patients with ICI-related myocarditis.
Notwithstanding the vast battery of preclinical investigations, the underlying mechanisms are still far from being fully elucidated, and additional translational approaches are urgently needed to unravel the driver of myocarditis and relevant immunological basis. The characterization of drug- and patient-related risk factors is also a current need. The role of genetics is still in its infancy, and no specific genetic mutations have been identified as potential determinants of myocarditis, although germline variants and the composition of host microbiota have been associated with ICI toxicity [89]. The potentiation or synergic cardiotoxic effect of novel ICI combinations, including monoclonal antibody blocking lymphocyte-activation gene 3 (LAG-3) and vascular endothelial growth factor inhibitors deserve careful monitoring and assessment in the upcoming real-world use. In the RELATIVITY-047 trial, relatlimab (anti-LAG-3) in combination with nivolumab, showed a 0.6% incidence of grade 3 or 4 myocarditis (versus zero events in the nivolumab arm) [90], whereas the JAVELIN Renal 101 trial assessed avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma and found a 7.1% incidence of cardiovascular events in the combination arm (versus 3.9%), with myocarditis occurring in 1.4% of patients receiving ICI (versus 0.2%) [91]. Moreover, the role of concomitant drugs (e.g., lipid-lowering agents) with ICIs should be further investigated as a potential strategy in mitigating long-term cardiovascular events and optimizing patient outcome.
As a closing remark, we support creation, implementation, and sharing of real-world data, including large cardio-oncology registries, pharmacovigilance data, and prospective collection of patient-level “smart data” through “omics” approaches [92, 93]. This will advance our understanding of ICI-related cardiovascular toxicity, especially predictive biomarkers of preexisting latent autoimmunity, and eventually allow for the achievement of common screening and preventive, diagnostic, and management strategies, thus supporting safer prescribing.
References
Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2: e192535.
Michielin O, Lalani A-K, Robert C, Sharma P, Peters S. Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies. J Immunother Cancer. 2022;10: e003024.
Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–6.
Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8.
Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42:281–94.
Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–80.
Kfoury M, Najean M, Lappara A, Voisin A-L, Champiat S, Michot J-M, et al. Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias. Cancer Treat Rev. 2022;110: 102452.
Kanji S, Morin S, Agtarap K, Purkayastha D, Thabet P, Bosse D, et al. Adverse events associated with immune checkpoint inhibitors: overview of systematic reviews. Drugs. 2022;82:793–809.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–85.
Dearden H, Au L, Wang DY, Zimmer L, Eroglu Z, Smith JL, et al. Hyperacute toxicity with combination ipilimumab and anti-PD1 immunotherapy. Eur J Cancer. 2021;153:168–78.
Ghisoni E, Wicky A, Bouchaab H, Imbimbo M, Delyon J, Gautron Moura B, et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: an overlooked aspect in immunotherapy. Eur J Cancer. 2021;149:153–64.
Raschi E, Gatti M, Gelsomino F, Ardizzoni A, Poluzzi E, De Ponti F. Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: a clinical perspective from pharmacovigilance. Target Oncol. 2020;15:449–66.
Khalid AB, Calderon G, Jalal SI, Durm GA. Physician awareness of immune-related adverse events of immune checkpoint inhibitors. J Natl Compr Cancer Netw JNCCN. 2022;20:1316–20.
Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–126.
Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1217–38.
Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, version 1. 2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2022;20:387–405.
Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9: e002435.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–361.
Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–99.
Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58.
Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. 2022;43:4458–68.
Gelsomino F, Fiorentino M, Zompatori M, Poerio A, Melotti B, Sperandi F, et al. Programmed death-1 inhibition and atherosclerosis: can nivolumab vanish complicated atheromatous plaques? Ann Oncol. 2018;29:284–6.
Vuong JT, Stein-Merlob AF, Nayeri A, Sallam T, Neilan TG, Yang EH. Immune checkpoint therapies and atherosclerosis: mechanisms and clinical implications: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:577–93.
Nowatzke J, Guedeney P, Palaskas N, Lehmann L, Ederhy S, Zhu H, et al. Coronary artery disease and revascularization associated with immune checkpoint blocker myocarditis: report from an international registry. Eur J Cancer. 2022;177:197–205.
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–64.
Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–7.
Moslehi JJ, Salem J-E, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933.
Dolladille C, Ederhy S, Allouche S, Dupas Q, Gervais R, Madelaine J, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8: e000261.
Bonsu JM, Guha A, Charles L, Yildiz VO, Wei L, Baker B, et al. Reporting of cardiovascular events in clinical trials supporting FDA approval of contemporary cancer therapies. J Am Coll Cardiol. 2020;75:620–8.
Bonaca MP, Olenchock BA, Salem J-E, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–55.
Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–89.
Naqash AR, Moey MYY, Cherie Tan X-W, Laharwal M, Hill V, Moka N, et al. Major adverse cardiac events with immune checkpoint inhibitors: a pooled analysis of trials sponsored by the National Cancer Institute-Cancer Therapy evaluation program. J Clin Oncol. 2022;40:3439–52.
Dolladille C, Akroun J, Morice P-M, Dompmartin A, Ezine E, Sassier M, et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J. 2021;42:4964–77.
Rubio-Infante N, Ramírez-Flores YA, Castillo EC, Lozano O, García-Rivas G, Torre-Amione G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail. 2021;23:1739–47.
Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–311.
D’Souza M, Nielsen D, Svane IM, Iversen K, Rasmussen PV, Madelaire C, et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. 2021;42:1621–31.
Laenens D, Yu Y, Santens B, Jacobs J, Beuselinck B, Bechter O, et al. Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J Clin Oncol. 2022;40:3430–8.
Jiménez-Alejandre R, Ruiz-Fernández I, Martín P. Pathophysiology of immune checkpoint inhibitor-induced myocarditis. Cancers. 2022;14:4494.
Solimando AG, Crudele L, Leone P, Argentiero A, Guarascio M, Silvestris N, et al. Immune checkpoint inhibitor-related myositis: from biology to bedside. Int J Mol Sci. 2020;21:3054.
Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 2018;95:77–99.
Kwiecien I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, Dziedzic D, Domagala-Kulawik J. Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol Immunother. 2017;66:161–70.
Gergely TG, Kucsera D, Tóth VE, Kovács T, Sayour NV, Drobni ZD, et al. Molecular characterization of immune checkpoint inhibitor-induced cardiotoxicity reveals IL-17A as a driver of cardiac dysfunction after anti-PD-1 treatment. Br J Pharmacol. 2023;180:740–61.
Axelrod ML, Meijers WC, Screever EM, Qin J, Carroll MG, Sun X, et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. 2022;611:818–26.
Pathak R, Katel A, Massarelli E, Villaflor VM, Sun V, Salgia R. Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist. 2021;26:1052–61.
Rossi S, Cani I, Raschi E, Comito F, Rinaldi R, Ardizzoni A, et al. Neurological manifestations as a harbinger of myocarditis in patients treated with immune checkpoint inhibitors. J Clin Oncol. 2022;JCO2201602.
Nakagomi Y, Tajiri K, Shimada S, Li S, Inoue K, Murakata Y, et al. Immune checkpoint inhibitor-related myositis overlapping with myocarditis: an institutional case series and a systematic review of literature. Front Pharmacol. 2022;13: 884776.
Vasbinder A. Biomarker trends, incidence, and outcomes of immune checkpoint inhibitor-induced myocarditis. J Am Coll Cardiol CardioOnc. 2022;4:689–700.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–76.
Palaskas NL, Segura A, Lelenwa L, Siddiqui BA, Subudhi SK, Lopez-Mattei J, et al. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail. 2021;23:1725–35.
Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921–64.
Ederhy S, Cautela J, Ancedy Y, Escudier M, Thuny F, Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging. 2018;11:1187–90.
Viganego F, Singh R, Fradley MG. Arrhythmias and other electrophysiology issues in cancer patients receiving chemotherapy or radiation. Curr Cardiol Rep. 2016;18:52.
Lentz R, Feinglass J, Ma S, Akhter N. Risk factors for the development of atrial fibrillation on ibrutinib treatment. Leuk Lymphoma. 2019;60:1447–53.
Singla A, Hogan WJ, Ansell SM, Buadi FK, Dingli D, Dispenzieri A, et al. Incidence of supraventricular arrhythmias during autologous peripheral blood stem cell transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2013;19:1233–7.
Zlotoff DA, Hassan MZO, Zafar A, Alvi RM, Awadalla M, Mahmood SS, et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer. 2021;9: e002007.
Mir H, Alhussein M, Alrashidi S, Alzayer H, Alshatti A, Valettas N, et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol. 2018;34:1059–68.
Zamami Y, Niimura T, Okada N, Koyama T, Fukushima K, Izawa-Ishizawa Y, et al. Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol. 2019;5:1635–7.
Bando S, Soeki T, Matsuura T, Tobiume T, Ise T, Kusunose K, et al. Plasma brain natriuretic peptide levels are elevated in patients with cancer. PLoS ONE. 2017;12: e0178607.
Upadhrasta S, Elias H, Patel K, Zheng L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. 2019;5:6–14.
Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: CardioOncology primer. JACC CardioOncology. 2021;3:35–47.
Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6:1093–9.
Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist. 2019;24:1128–36.
Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. 2017;88:225–31.
Vinco G. P868. Neutrophil-to-lymphocyte ratio at the onset of acute myocarditis reflects the extent of myocardial necrosis. Eur Heart J Suppl. 2018;39:160.
Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, Lee C, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc. 2020;9: e018306.
Casciola-Rosen L, Hall J, Mammen A, Christopher-Stine L, Rosen A. Isolated elevation of aldolase in the serum of myositis patients: a potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol. 2012;30:548–53.
Marini A, Bernardini A, Gigli GL, Valente M, Muñiz-Castrillo S, Honnorat J, et al. Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology. 2021;96:754–66.
Seki M, Kitano S, Suzuki S. Neurological disorders associated with immune checkpoint inhibitors: an association with autoantibodies. Cancer Immunol Immunother. 2022;71:769–75.
Zamorano JL, Gottfridsson C, Asteggiano R, Atar D, Badimon L, Bax JJ, et al. The cancer patient and cardiology. Eur J Heart Fail. 2020;22:2290–309.
Battisti NML, Andres MS, Lee KA, Ramalingam S, Nash T, Mappouridou S, et al. Incidence of cardiotoxicity and validation of the Heart Failure Association-International Cardio-Oncology Society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res Treat. 2021;188:149–63.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–14.
Rünger A, Schadendorf D, Hauschild A, Gebhardt C. Immune checkpoint blockade for organ-transplant recipients with cancer: a review. Eur J Cancer. 2022;175:326–35.
Nguyen LS, Ortuno S, Lebrun-Vignes B, Johnson DB, Moslehi JJ, Hertig A, et al. Transplant rejections associated with immune checkpoint inhibitors: a pharmacovigilance study and systematic literature review. Eur J Cancer. 2021;148:36–47.
Zito C, Manganaro R, Ciappina G, Spagnolo CC, Racanelli V, Santarpia M, et al. Cardiotoxicity induced by immune checkpoint inhibitors: what a cardio-oncology team should know and do. Cancers. 2022;14:5403.
Aldea M, Orillard E, Mansi L, Marabelle A, Scotte F, Lambotte O, et al. How to manage patients with corticosteroids in oncology in the era of immunotherapy? Eur J Cancer. 2020;141:239–51.
De Giglio A, Aprile M, Di Federico A, Sperandi F, Melotti B, Gelsomino F, et al. Impact of baseline versus intercurrent steroids administration on upfront chemo-immunotherapy for advanced non-small cell lung cancer (NSCLC). Int J Mol Sci. 2022;23:10292.
De Giglio A, Mezquita L, Auclin E, Blanc-Durand F, Riudavets M, Caramella C, et al. Impact of intercurrent introduction of steroids on clinical outcomes in advanced non-small-cell lung cancer (NSCLC) patients under immune-checkpoint inhibitors (ICI). Cancers. 2020;12:2827.
Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031–4.
Salem J-E, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–9.
Nguyen LS, Bretagne M, Arrondeau J, Zahr N, Ederhy S, Abbar B, et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept. J Immunother Cancer. 2022;10: e004699.
Esfahani K, Buhlaiga N, Thébault P, Lapointe R, Johnson NA, Miller WH. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–6.
Tay RY, Blackley E, McLean C, Moore M, Bergin P, Gill S, et al. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer. 2017;117:921–4.
Jain V, Mohebtash M, Rodrigo ME, Ruiz G, Atkins MB, Barac A. Autoimmune myocarditis caused by immune checkpoint inhibitors treated with antithymocyte globulin. J Immunother. 2018;41:332–5.
Zhang RS, Padegimas A, Murphy KM, Evans PT, Peters CJ, Domenico CM, et al. Treatment of corticosteroid refractory immune checkpoint inhibitor myocarditis with infliximab: a case series. Cardio-Oncol Lond Engl. 2021;7:13.
Doms J, Prior JO, Peters S, Obeid M. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Ann Oncol. 2020;31:1273–5.
Peleg Hasson S, Salwen B, Sivan A, Shamai S, Geva R, Merimsky O, et al. Re-introducing immunotherapy in patients surviving immune checkpoint inhibitors-mediated myocarditis. Clin Res Cardiol. 2021;110:50–60.
Groha S, Alaiwi SA, Xu W, Naranbhai V, Nassar AH, Bakouny Z, et al. Germline variants associated with toxicity to immune checkpoint blockade. Nat Med. 2022;28:2584–91.
Raschi E, Comito F, Massari F, Gelsomino F. Relatlimab and nivolumab in untreated advanced melanoma: insight into RELATIVITY. Immunotherapy. 2023;15:85–91.
Rini BI, Moslehi JJ, Bonaca M, Schmidinger M, Albiges L, Choueiri TK, et al. Prospective cardiovascular surveillance of immune checkpoint inhibitor-based combination therapy in patients with advanced renal cell cancer: data from the phase III JAVELIN renal 101 trial. J Clin Oncol. 2022;40:1929–38.
Jing Y, Yang J, Johnson DB, Moslehi JJ, Han L. Harnessing big data to characterize immune-related adverse events. Nat Rev Clin Oncol. 2022;19:269–80.
Reynolds KL, Arora S, Elayavilli RK, Louv WC, Schaller TH, Khandelwal A, et al. Immune-related adverse events associated with immune checkpoint inhibitors: a call to action for collecting and sharing clinical trial and real-world data. J Immunother Cancer. 2021;9: e002896.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Conflicts of interest
The authors declare no potential conflict of interest relevant to the content of the manuscript.
Availability of data and material
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
ER conceptualized the manuscript. SR, ADG, and LP contributed in the design of the manuscript. MF contributed to visualization. ER wrote the original draft, with contribution of SR and ADG. All the authors contributed to the editing of the draft. All the authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Raschi, E., Rossi, S., De Giglio, A. et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: A Guide for Clinicians. Drug Saf 46, 819–833 (2023). https://doi.org/10.1007/s40264-023-01320-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01320-5