Abstract
Background
Aged care residents are vulnerable to the harmful effects of medicines; however, data on the prevalence and preventability of adverse medicine events in aged care residents are scarce.
Aim
To determine the prevalence and preventability of adverse medicine events in Australian aged care residents.
Methods
A secondary analysis of data from the Reducing Medicine-Induced Deterioration and Adverse Reactions (ReMInDAR) trial was conducted. Potential adverse medicine events were identified and independently screened by two research pharmacists to produce a short-list of potential adverse medicine events. An expert clinical panel reviewed each potential adverse medicine to determine the likelihood that the event was medicine related (based on the Naranjo Probability Scale criteria). The clinical panel assessed preventability of medicine-related events using Schumock-Thornton criteria.
Results
There were 583 adverse events due to medicines, involving 154 residents (62% of the 248 study participants). There was a median of three medication-related adverse events (interquartile range [IQR] 1–5) per resident over the 12-month follow-up period. The most common medication-related adverse events were falls (56%), bleeding (18%) and bruising (9%). There were 482 (83%) medication-related adverse events that were preventable, most commonly falls (66% of preventable adverse medicine events), bleeding (12%) and dizziness (8%). Of the 248 residents, 133 (54% of the cohort) had at least one preventable adverse medicine event, with a median of 2 (IQR 1–4) preventable adverse medicine events per resident.
Conclusion
In total, 62% of aged care residents in our study had an adverse medicine event and 54% had a preventable adverse medicine event in a 12-month period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In our study involving aged care residents, preventable adverse medicine events were common. |
Our study showed that almost two-thirds of aged care residents experience an adverse medicine event in a 12-month period. |
In our study, 50% of aged care residents experienced a preventable adverse medicine event in a 12-month period. |
1 Introduction
Harm from medicines is common and residents of aged-care facilities are particularly vulnerable to the harmful effects of medicines [1]. Reasons are that aged-care residents have a median of five chronic conditions [2] and use multiple medicines to manage their chronic conditions (between 9 and 11 medicines on average [3]). Almost all aged-care residents (95%) have at least one medication-related problem identified during a pharmacist-led medicines review [3], further increasing the risk of medication-related harm. Harm associated with medicines use is frequently described as an adverse event (AE). The World Health Organization defines AEs due to medicines as “any untoward medical occurrence that may present during treatment with a medicine but which does not necessarily have a causal relationship with this treatment” [4]. Adverse medicine events include unintended, harmful effects of medicines as well as harm that occurs due to medication errors or medication-related harm associated with system-related factors, e.g., medication-related harm due to poor continuity of care between different care settings or providers or disruption to medication supply [5]. An adverse drug reaction (ADR) is a sub-type of adverse medicine event that is defined as “a response to a medicinal product which is noxious and unintended and which occurs at doses normally used for the prophylaxis, diagnosis or therapy of disease”[4]. Commonly identified AEs due to medicines in aged-care residents include falls, delirium and hospital admission [6].
International evidence suggests that up to one in ten residents in aged-care facilities experience an AE due to their medicines every month [7,8,9]. The majority of these AEs are serious, life-threatening or fatal [10, 11]. Over half of this harm is preventable [10, 11]. Evidence on the extent of adverse medicine events in Australian aged-care facilities is limited. A systematic review of the outcomes from medication management reviews in Australian aged-care residents found that in a pooled sample of 1374 residents who received medication reviews, there were 609 cases of toxicity or ADRs identified [12]. This figure is likely to underestimate the true prevalence of adverse medicine events in Australian aged care, because ADRs are only one kind of adverse medicine event and residents who didn’t receive a medication review may still have experienced an ADR but were not counted in this figure. In 2020, a published scoping review of studies addressing any aspect of AEs associated with use of medicines in Australian aged-care residents identified only 7 relevant studies [13]. The studies included in the review tended to address AEs associated with specific classes or types of medicines, and the overall prevalence of AEs due to medicines was not reported [13]. A study assessing AEs in 72 aged-care facilities in New South Wales and the Australian Capital Territory between 2013 and 2016 found 60,268 AEs documented over the 3-year period [14]. In this study, AEs were categorised as falls, behavioural incidents, medication incidents (including medication errors or missed doses) and impact or injuries (AEs not falling into the other three categories). Falls (37% of AEs) and behavioural incidents (33%) were most common, with medicine incidents accounting for 9% of AEs [14]. Although this study provides some insight into AEs due to medicines in the aged-care setting, it is likely to have underestimated their extent. For example, falls are commonly precipitated due to medicines, but this study did not distinguish between medication-related falls and falls for other reasons.
Data on the preventability of adverse medicine events in aged-care residents are also scarce. A study conducted in 1997 in 18 nursing homes in the USA found that among 410 residents who experienced at least one adverse medicine event, 226 residents (55%) had at least one adverse event that was preventable [15]. The most common types of adverse medicine events were neuropsychiatric events (29% of residents with a preventable AE), falls (20%) and haemorrhage (15%) [15]. A 2011 systematic review found that the median prevalence of adverse medicine events in older people in ambulatory-care settings was 23% (interquartile range [IQR] 19–31%) and that across all age groups the median proportion of patients with preventable AEs was 17% (IQR 12–24%) [16]; however, the studies in this review were not restricted to the aged -care setting and the prevalence of adverse medicine events and preventable adverse medicine events in the vulnerable aged-care population may differ.
We located no studies which assessed the extent of preventable adverse medicine events in the Australian aged-care setting. It is unclear whether international estimates of the prevalence of AEs are applicable to the Australian setting due to differences in both the aged-care population and the way care is provided to residents. In addition, the preventability of adverse medicine events in Australian aged-care residents is unknown. The aim of this study was to determine the prevalence and preventability of adverse medicine events in Australian aged-care residents.
2 Methods
This study used data collected as part of the Reducing Medicine Induced Deterioration and Adverse Reactions (ReMInDAR) randomised controlled trial, which occurred between August 2018 and June 2020. The ReMInDAR trial has been described in detail previously [17, 18]. Briefly, the ReMInDAR trial aimed to reduce medicine-induced deterioration by trialling a sessional visiting pharmacist service compared to usual care. Participants were eligible for inclusion in the trial if they were taking four or more regular medicines or at least one anticholinergic or sedative medicine. Aged-care residents with moderate or severe dementia or pre-existing frailty at baseline were not eligible to participate in the study [18]. The trial recruited 248 participants living in 39 residential aged-care facilities in South Australia and Tasmania over a period of 12 months. Participants had a median age of 87 years and 32% (n = 80) were male. One hundred and twenty participants were randomised to receive the pharmacist-led intervention and 128 were randomised to receive usual care [18].
For all trial participants, adverse medicine events were identified by key word search of the aged-care facility care record by trained research assistants. Adverse events were extracted from the care record and included falls (non-injurious and injurious including fractures), bleeding and bruising, delirium, confusion, dizziness and faecal impaction. We conducted an in-depth analysis of this AE data for the present study.
2.1 Screening for Potential Adverse Medicine Events
A research pharmacist screened all AEs collected during the ReMInDAR trial and created a suspected adverse medicine event case report detailing patient characteristics, a description of the AE, medicines used (including date started and medicines suspected to be associated with the AE), and any other relevant details, such as new-onset illness. A second research pharmacist independently reviewed all events screened by the first research pharmacist to produce a short-list of potential adverse medicine events. Both research pharmacists had expertise in clinical pharmacy, medication safety and quality use of medicines in older people. Adverse events were independently coded by the two research pharmacists using the Naranjo Probability Scale criteria to assess adverse medicine event causality [19]. Events considered by both research pharmacists to be ‘doubtful’ on the Naranjo scale were excluded from further review. Discrepancies in the research pharmacists’ assessment of doubtful AEs were resolved by a third pharmacist reviewer if required. That is, if one research pharmacist classified an event as ‘doubtful’ and the other classified it as potential or higher likelihood, a third reviewer resolved the discrepancy. Events that were considered by both pharmacists to be either potential, possible, probable, or definite adverse medicine events were then subject to clinical panel review.
2.2 Panel Review to Determine Causality and Preventability of Adverse Medicine Events
An expert clinical panel, comprising two clinical pharmacists and a medical practitioner, reviewed each potential adverse medicine event identified in the screening phase to determine the likelihood that the AE was medicine related, as well as the preventability of the AE. The clinical panel rated adverse medicine events as being definite, probable, possible or doubtful based on the Naranjo scale [19].
Where the likelihood of causality that the AE was classified as medicine induced was determined to be ‘possible’ or greater, the clinical panel then assessed each medicine-induced AE for preventability using the modified Schumock-Thornton criteria [20, 21].
Severity was not assessed due to insufficient outcome information for assessment. For all ratings made by the panel, consensus was reached by discussion.
Based on the ratings made by the expert clinical panel, we determined the extent of medication-related harm in residential aged-care facilities and the preventability of that harm. The rate of adverse medicine events was measured as a composite outcome of adverse medicine events judged by the clinical panel to be possibly, probably or definitely medicine induced. We report descriptive statistics of the total number of AEs identified in the study cohort and the number of AEs that the clinical panel determined to be medication related and preventable. The types of AEs most frequently occurring in each category are also reported. The characteristics (age and gender) of aged-care residents who experienced AEs and the number of AEs per resident are described. Results are reported as frequencies, percentages and medians.
Ethics approval to conduct the study was received from the University of South Australia Human Research Ethics committee (ID: 0000036440) and the Tasmania Health and Medical Human Research Ethics Committee, University of Tasmania (ID: H0017022).
3 Results
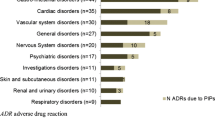
A total of 1978 AEs were identified during the study period, involving 225 of the 248 residents who participated in the study (91% of participants). The most prevalent types of AE were falls (21% of the 1978), gastroenteritis (15%), bleeding (11%) and cough (11%) (Table 1). The clinical panel determined that 583 AEs (29% of those identified) were medication related (Fig. 1). The most prevalent medication-related AEs were falls (56%), bleeding (18%) and bruising (9%) (Table 1). The panel determined that 482 (83%) of the 583 medication-related AEs were preventable (Fig. 1). The most prevalent types of preventable adverse medicine events were falls (66%), bleeding (12%) and dizziness (8%) (Table 1).
Characteristics of residents who had an AE are shown in Table 2; median age of 87 years, approximately two-thirds were female and they used a median of 14 medicines (Table 2). Medicines commonly used by people in the study population included paracetamol, laxatives, antithrombotic agents, proton-pump inhibitors and opioid analgesics (Table 2). One hundred and fifty-four residents (68% of the 225 residents with an AE and 62% of the 248 participants in the study) had a medication-related AE, with a median of 3 medication-related AEs (IQR 1–5) per resident over the 12-month follow-up period (Table 2). At least one medication-related AE was judged by the panel to be preventable for 133 of the 154 residents with a medication-related AE (86%). Residents had a median of 2 (IQR 1–4) preventable adverse medicine events (Table 2).
4 Discussion
Overall, 62% of our study population (154 of 248 residents) had an adverse medicine event, and 54% of our study population (133 of 248 residents) had a preventable adverse medicine event during the 1-year study period. Our study has, for the first time, quantified the prevalence and preventability of adverse medicine events in a cohort of Australian aged-care residents. Our study showed that in a 1-year period there were 583 adverse medicine events in our study population of 248 older adults living in aged care. Five in six (83%) of the adverse medicine events were preventable. One hundred and fifty-four residents experienced at least one AE due to their medicines and most residents experienced three or more AEs due to medicines. Australia-wide, there are approximately 190,000 aged-care residents [22]. Extrapolating our results to the entire Australian aged-care population of 190,000 people suggests that, each year, there could be over 117,000 people in aged care in Australia who experience an AE due to their medicines and for 102,000 people the medication-related AE is likely to be preventable.
Our estimate of the prevalence and preventability of adverse medicine events in aged-care residents is comparable to prior research conducted internationally. In the present study, we found that 62% of aged-care residents had an AE due to medicines over a 12-month period. The incident rate of AEs in the trial was 20 adverse medicine events per 100 resident months, with no difference in the rate of AEs for intervention or control group patients [18]. A 2022 study from Japan found that over a 1-year study period, 73% of 459 aged-care residents in the study experienced an adverse medicine event (equivalent to an incidence of 36.4 AEs per 100 resident months of follow-up) [23]. One-third of adverse medicine events were preventable (equivalent to 13.2 preventable adverse medicine events per 100 residents months of follow-up) [23]. In the Japanese study, falls (70% of preventable adverse medicine events) and neuropsychiatric events (65% of preventable adverse medicine events) were the most common types of preventable adverse medicine events. In our study, 318 of 407 falls (78%) were preventable medication-related AEs. This finding is similar to the results of a 2022 study from Japan, which identified the incidence of medication-related falls in aged care. In this study, 645 falls occurred over a 1-year period with 480 (74%) determined to be adverse medicine events [24].
Unlike our study where bleeding was the second most common preventable adverse medicine event (11%), in the study from Japan there was only one preventable bleed related to medicines (0.2% of all preventable adverse medicine events) [23]. Similarly, in prior studies, gastrointestinal (GI) adverse medicine events were relatively common in aged-care residents. For example, in the 2022 study from Japan, 26% of adverse medicine events and 3% of preventable adverse medicine events were GI events [23] and in a study from the USA, published in 2000, 12% of all adverse medicine events were GI related, and 11% of preventable adverse medicine events were GI related [10]. Another US study conducted in the early 2000s found that 17% of all adverse medicine events, and 16% of preventable adverse medicine events, were GI related [25]. In our study, only 1% of adverse medicine events and 0.4% of preventable adverse medicine events were GI events, a prevalence much lower than that seen in prior research. It is worth noting that 15% (n = 293) of all AEs in our study were GI AEs but only 5 were deemed by the panel to be adverse medicine events. A number of factors are likely to have influenced the identification of different types of adverse medicine events in the different studies, including the methods used to collect data relating to adverse medicine events, the methods used to assess AEs, or the types of medicines used by study participants. Differences in the type of information recorded by aged-care facility staff in the different study settings may also have contributed to the different prevalence of GI adverse medicine events across the different studies.
In our study, medicines commonly used by participants with AEs and adverse medicine events included antithrombotic agents, proton-pump inhibitors, opioid analgesics antidepressants and cardiovascular medicines. Our study, which did not include a comparison group of people with no AEs, was not designed to identify associations between the medicines used by study participants and the prevalence of AEs. However, it is worth noting that falls and bleeding were the two most common medication-related AEs and preventable medication-related events in our study (related AEs of dizziness and bruising were also common) and use of medicines that can cause falls or bleeding was also common. Prior studies have shown that medication-related falls are common in aged-care residents. A prospective cohort study involving 1655 residents from 84 aged-care facilities in the UK found that 519 residents (31%) had a fall over an 18-month period and that the risk of falling was higher in people using multiple medicines (OR 1.041, 95% CI 1.011–1.071, p = 0.006) and people using psychotropic medicines like antidepressants and benzodiazepines (OR 1.392, 95% CI 1.1–1.76, p = 0.006) [26]. A root cause analysis of fall-related hospital admissions from aged-care residents in South Australia found that, in a population of 383 aged-care residents, there were 47 hospital admissions for falls in a 5-month period [27]. Almost three-quarters of hospital admissions for falls occurred in people using multiple medicines, and in all cases the resident had recently used a medicine which increases the risk of falls [27]. We located only one study, which found that use of warfarin by aged-care residents was associated with increased risk of hospital admission for any type of bleeding event (OR 1.26, 95%CI 1.11–1.43), and increased risk of specific bleeding events including CNS bleeding (OR 1.64, 95% CI 1.19–2.26) and GI bleeding (OR 1.18, 95% CI 1.03–1.36), and that use of aspirin was associated with increased risk of hospital admission for CNS bleeding (OR 1.36, 95% CI (1.05–1.78) [28]. The findings of our study support and add to these findings of prior research. A previous analysis of the ReMInDAR trial data investigated factors predictive of AEs in this aged-care population. The results of this analysis showed that increasing age, higher body weight, use of sedative or hypnotic medicines and a prior history of falls were independent risk factors for having an adverse medicine event, while better cognition (as measured by higher scores on the Montreal Cognitive Assessment) was associated with reduced risk of having an adverse medicine event [29].
Our study provides insight into which AEs in aged-care residents are most likely to be medicine related. These insights can be used to improve existing triage tools. For example, one-fifth of all AEs in our study population were falls and more than half of medication-related AEs and preventable medication-related AEs were falls. In contrast, gastroenteritis was also a common AE in our aged-care population (15% of all AEs) but was rarely a medication-related AE or a preventable medication-related AE. Identifying the types of AEs in aged-care residents that are medication related and preventable allows us to target interventions to reduce medication-related harm in these areas.
Our study has several limitations. First, the Schumock-Thornton criteria were used to assess preventability of adverse medicine events; however, data were not collected relating to each item within the criteria meaning that a breakdown of the reasons for preventability of AEs cannot be reported. This information would be useful in designing interventions to reduce preventable adverse medicine events in aged-care residents and should be the focus of future research. Second, severity of adverse medicine events was not reported in our study. Unfortunately, the level of detail required to accurately, and consistently report AE severity for study participants was not available in the aged-care records and so a decision was made not to include an assessment of severity in our study. A US study conducted in the early 2000s found that, of 338 preventable adverse medicine events in aged care, 32% were serious events and 7% were life-threatening events [25]. These results indicate that examining the severity of AEs is an important component in interpreting adverse medicine events in aged-care residents, and this should be the focus of future research. Our analysis of AEs included residents in both the intervention and control arms of the ReMInDAR trial. These groups differed in their level of interaction with health care professionals, with the intervention group receiving 8-weekly visits and review by study pharmacists to identify AEs early, meaning that there may have been the potential for differences in both the rate of detection and resolution of adverse medicine events between groups. However, the primary analysis of the ReMInDAR trial data showed that there was no difference in the rate of AEs and no difference in the rate of preventable AEs between intervention and control group participants [18], so this is unlikely to have influenced the results of this medication-related AE analysis. Our study included 248 aged-care residents from 39 aged-care facilities across South Australia and Tasmania and our study participants, who were not frail and did not have moderate or severe dementia at baseline, were not representative of people living in aged care in Australia [18]. Replication of our study in a large sample from more Australian states, and in a population more representative of the Australian aged-care resident population would strengthen our findings.
5 Conclusion
Our study has shown that 62% of aged-care residents have an adverse medicine event and 54% of aged-care residents have a preventable adverse medicine event in a 12-month period, with a median of two preventable adverse medicine events per resident. Falls were identified as the most common preventable adverse medicine event in our study.
References
Lim R, et al. The extent of medication-related hospital admissions in australia: a review from 1988 to 2021. Drug Saf. 2022;45(3):249–57.
Inacio M, et al. Health status and healthcare trends of individuals accessing Australian aged care programmes over a decade: the Registry of Senior Australians historical cohort. Intern Med J. 2021;51:712–24.
Pharmaceutical Society of Australia, Medicine safety: aged care. 2020, PSA: Canberra. 2020.
World Health Organization, Safety of Medicines. A guide to detecting and reporting adverse drug reactions. 2002. WHO: Geneva.
Australian Government Department of Health. Guiding principles for medication management in residential aged care facilities. 2012. https://www.health.gov.au/internet/main/publishing.nsf/Content/guide-med-mgmt-aged-care.
Clegg A, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–62.
Al-Jumaili A, Doucette W. A systems approach to identify factors influencing adverse drug events in nursing homes. J Am Geriatr Soc. 2018;66(7):1420–7.
Tenhunen M, Smithers B, Tucker B. Identifying medication-related adverse drug events in nursing facilities in East Texas. Consult Pharm. 2016;31(8):436–9.
Handler S, et al. Epidemiology of medication-related adverse events in nursing homes. Am J Geriatr Pharmacother. 2006;4(3):264–72.
Gurwitz J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109(2):87–94.
Kapoor A, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254–61.
Chen E, et al. Process, impact and outcomes of medication review in Australian residential aged care facilities: a systematic review. Austral J Ageing. 2019;38(Suppl. 2):9–25.
Qasim H, et al. A comprehensive evaluation of studies in the adverse effects of medications in Australian aged care facilities: a scoping review. Pharmacy. 2020;8:56.
St Clair B, Jorgensen M, Georgiou A. Incidence of adverse events in residential aged care. Austral Health Rev. 2021;46:405–13.
Field T, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629–34.
Tache S, Sonnichsen A, Ashcroft D. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45:977–89.
Lim R, et al. Reducing medicine-induced deterioration and adverse reactions (ReMInDAR) trial: study protocol for a randomised controlled trial in residential aged-care facilities assessing frailty as the primary outcome. BMJ Open. 2020;10: e032851.
Roughead E, et al. Effect of an ongoing pharmacist service to reduce medicine-induced deterioration and adverse reactions in aged-care facilities (nursinghomes): a multicentre, randomised controlled trial (the ReMInDAR trial). Age Ageing. 2022;51:1–9.
Naranjo C, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Schumock G, Thornton J. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6):538.
Iftikhar S, et al. Causality and preventability assessment of adverse drug reactions and adverse drug events of antibiotics among hospitalized patients: a multicenter, cross-sectional study in Lahore, Pakistan. PLoS ONE. 2018;13(6): e0199456.
Australian Institute of Health and Welfare, People using aged care. 2021, AIHW. https://www.gen-agedcaredata.gov.au/Topics/People-using-aged-care. Accessed 21 Jan 2022.
Ayani N, et al. Epidemiology of adverse drug events and medication errors in four nursing homes in Japan: the Japan Adverse Drug Events (JADE) Study. BMJ Qual Saf. 2022;31:878–81.
Oya N, et al. Over half of falls were associated with psychotropic medication use in four nursing homes in Japan: a retrospective cohort study. Int J Environ Res Public Health. 2022;19(5):3123.
Gurwitz J, et al. The incidence of adverse drug events in two large academic long-term care facilities. Am J Med. 2005;118:251–8.
Izza M, et al. Polypharmacy, benzodiazepines, and antidepressants, but not antipsychotics, are associated with increased falls risk in UK care home residents: a prospective multi-centre study. Eur Geriatr Med. 2020;11:1043–50.
Sluggett J, et al. Root cause analysis of fall-related hospitalisations among residents of aged care services. Aging Clin Exp Res. 2020;32:1947–57.
Quilliam B, et al. Effect of antiplatelet and anticoagulant agents on risk of hospitalization for bleeding among a population of elderly nursing home stroke survivors. Stroke. 2001;32:2299–304.
Dorj G, et al. Risk factors predictive of adverse drug events and drug-related falls in aged care residents: secondary analysis from the ReMInDAR trial. Drugs Aging. 2023;40:49–58.
Acknowledgements
We thank the trial participants, pharmacists, general practitioners and the staff of residential aged-care facilities and research assistants for their support and participation. We also thank members of the stakeholder advisory group and the consumer advisory committee for their valuable advice and support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Australian Government Department of Health Pharmacy Trial Program.
Conflicts of Interest
RB was employed as the ReMInDAR partnership engagement and trial manager to oversee the operations management for the trial. The authors have no other competing interests
Ethics Approval
Ethics approval to conduct the study was received from the University of South Australia Human Research Ethics committee (ID: 0000036440) and the Tasmania Health and Medical Human Research Ethics Committee, University of Tasmania (ID: H0017022).
Consent to Participate
The trial adopted an “opt out approach”, as requested by the residential aged-care facilities and consumer advisors and approved by the ethics committees. In compliance with the National Statement of Ethical Conduct in Human Research section 2.3.6, flyers were displayed at all participating residential aged-care facilities at least one month prior to recruitment to inform the residents, family members and staff of the introduction of the pharmacist service. In addition, flyers were provided to all potential residents. The flyer explained the pharmacist service, information on what to do if the residents did not wish to participate and informed the residents that they could opt out at any time throughout the study period. The trial excluded persons with moderate or severe dementia, as determined by the last facility recorded Psychogeriatric Assessment scale (PAS) or through administration of the Montreal Cognitive assessment (MoCA) tool during eligibility screening, meaning that the eligible residents had the capacity to decline participation if they wished to do so. Participants who enrolled in the trial could withdraw from the trial for any reason or without having to give reasons.
Consent for Publication
Not applicable as all data available to the study team were anonymous.
Availability of Data and Materials
The datasets generated during and/or analysed during the current study are available from the data custodian by request.
Code Availability
Not applicable
Author Contributions
LKE: study design, data analysis and interpretation, writing of the manuscript. GD, AAQ, RLB, DR, JW: study design, data analysis and interpretation, review of the manuscript for important intellectual content. RL, NLP, TLK, NNP, LB, EER: study design, review of the manuscript for important intellectual content. All authors read and approved the final version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kalisch Ellett, L.M., Dorj, G., Andrade, A.Q. et al. Prevalence and Preventability of Adverse Medicine Events in a Sample of Australian Aged-Care Residents: A Secondary Analysis of Data from the ReMInDAR Trial. Drug Saf 46, 493–500 (2023). https://doi.org/10.1007/s40264-023-01299-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01299-z