Abstract
Introduction
Rasagiline is indicated for treating idiopathic Parkinson’s disease (PD) as monotherapy and adjunct therapy to levodopa in patients.
Objectives
To assess the post-marketing safety and tolerability of rasagiline in Chinese PD patients, as well as its effectiveness in improving motor symptoms.
Methods
This prospective, non-interventional, multicenter, cohort study included PD patients administered rasagiline monotherapy or adjunct therapy to levodopa. The primary outcome was the incidence of adverse drug reactions (ADRs) according to MedDRA® (version 22.0), and the secondary outcomes were the Parkinson’s Disease Unified Rating Scale (UPDRS) part III, Clinical Global Impression-Severity (CGI-S), and Clinical Global Impression-Global-Improvement (CGI-I), assessed at Weeks 4, 12, and 24.
Results
In total, 734 patients, 95 in the monotherapy subgroup and 639 in the adjunct therapy subgroup, were included in the safety population. The incidence rates of all ADRs were comparable between the monotherapy (15.8%) and adjunct therapy (13.6%) subgroups. The most common ADRs by system organ class were nervous system disorders (5.6%), gastrointestinal disorders (3.3%), psychiatric disorders (1.8%), vascular disorders (1.2%), and general disorders and administration site conditions (1.1%). Five (0.7%) participants experienced 5 serious ADRs. Improvements in UPDRS part III, CGI-S and CGI-I at Weeks 4, 12 and 24 from baseline were observed.
Conclusions
Safety data in this study indicated no extra safety concerns. Rasagiline is generally safe and well tolerated in Chinese PD patients. The safety profile and tolerability were in line with the established safety profile. Moreover, rasagiline reduced the severity of PD motor symptoms, confirming findings by previous clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first multicenter, large-scale, post-marketing study on the safety profile of rasagiline in Chinese PD patients in the real-world clinical practice. |
Rasagiline is generally safe and well tolerated as either monotherapy or adjunct therapy to levodopa, with no extra safety concerns. |
Rasagiline reduced the severity of PD motor symptoms, confirming findings by previous clinical trials. |
1 Introduction
Parkinson’s disease (PD) is a chronic progressive neurodegenerative disease that mainly manifests as motor and non-motor symptoms, such as tremor, rigidity, bradykinesia, dementia, and sleep disorders [1, 2]. A nationwide study conducted in 2015 reported that PD prevalence in China was 1.37% in individuals aged ≥ 60 years [3].
Parkinson’s disease is due to loss of dopaminergic neurons in the substantia nigra, as well as other dopaminergic and nondopaminergic areas in the brain [4]. Among all anti-PD drugs, monoamine oxidase B inhibitors (MAO-BIs) are recommended by PD guidelines as monotherapy or adjunct therapy to levodopa in PD patients [5,6,7,8]. Rasagiline, an irreversible MAO-B selective inhibitor, can cause an increase in extracellular levels of dopamine in the striatum, and the elevated dopamine level and subsequent increased dopaminergic activity improve PD symptoms [9,10,11]. Rasagiline mesylate was approved in China for the treatment of PD as monotherapy or as adjunct therapy to levodopa in June 2017. Two Phase 3 studies in China demonstrated that rasagiline monotherapy [12] and adjunct therapy to levodopa [13] were effective and safe in Chinese PD patients. Although the safety and tolerability of rasagiline have been demonstrated in a real-world setting in Germany [14], there are limited safety data on rasagiline in Chinese PD patients, especially in real-world clinical settings.
Therefore, this study aimed to assess the safety and tolerability of rasagiline as monotherapy or adjunct therapy to levodopa in Chinese PD patients, as well as its effectiveness in improving motor symptoms.
2 Methods
2.1 Study Design and Patients
This is a prospective, multicenter, non-interventional cohort study, conducted between January 2019 and August 2020 at the neurology clinics of 19 centers in China. Patients being diagnosed as idiopathic PD, and were prescribed with rasagiline tablets (Azilect®, Lundbeck, Denmark) as monotherapy (with no other anti-PD drugs) or adjunct therapy to levodopa (in cases with wearing-off), were eligible to be included in this study. The study protocol was approved by the ethics committee of the leading center (Beijing Hospital) for the study conduct in each of the participating centers (approval No. 2018BJYYEC-223-02). All patients or their legal representatives provided signed informed consent for participation in this study. Patients would be excluded if (1) they were unlikely, in the investigator’s opinion, to comply with the protocol; (2) they had a history of severe drug allergy or hypersensitivity or known hypersensitivity to rasagiline or its excipients as described in the Chinese packaging insert; (3) they had severe hepatic impairment; or (4) they had present or past use of contraindicated concomitant medications according to the Chinese packaging insert.
All patients received oral rasagiline 1 mg/day. Patients who did not receive any other anti-PD drugs when starting rasagiline were classified as the monotherapy subgroup, while those with wearing-off who used rasagiline as adjunct therapy to levodopa were classified as the adjunct therapy subgroup. Notably, during the treatment process, other anti-PD drugs could be added in both subgroups throughout the study period. The decision to treat the patient with rasagiline was based solely on the clinical judgment of the investigator and was clearly separated from the decision to include/exclude in the study. The administration of rasagiline was determined by physicians’ judgment, with patients’ condition and willingness in consideration, but not by the study protocol in advance. Any treatment for PD was prescribed in accordance with clinical practice.
2.2 Data Collection and Follow-Up
Data required by the protocol were entered via a secure on-line web-based Electronic Data Capture system, using an electronic case report form (eCRF) to capture the details. Patient characteristics (including demographic data and smoking history), history of PD, current and prior anti-PD treatments, comorbidities, and concomitant medications were collected at baseline (within 7 days of the first prescription of rasagiline). Changes in rasagiline dose and/or frequency were recorded during the study.
Safety was assessed by adverse events (AEs) and laboratory findings showing abnormal changes at Weeks 4 ± 2, 12 ± 4, and 24 ± 4. Adverse events were spontaneously reported by the patient or observed by the investigator using an AE/adverse drug reaction (ADR) report form according to MedDRA® version 22.0. The investigator assessed the relationship of the AE to the drug case-by-case and recorded findings on the AE/ADR report form. An ADR is an AE that is assessed as at least possibly related to the drug [14]. All ADRs, non-serious ADRs, serious ADRs (SADRs), SADRs leading to death, labeled ADRs, unlabeled ADRs, overdose ADRs, ADRs resulting from drug interaction(s), and ADRs leading to permanent discontinuation of rasagiline were reported. Details are shown in the Supplementary Information.
Parkinson’s disease severity at baseline was assessed using the Motor Subscale of the Parkinson’s Disease Unified Rating Scale (UPDRS) part III and the Clinical Global Impression-Severity (CGI-S). Patients with motor fluctuations underwent measurement of UPDRS in the ON state. The change in PD motor symptoms was assessed by changes in UPDRS part III and CGI-S from baseline, as well as the Clinical Global Impression-Global Improvement (CGI-I) at Weeks 4 ± 2, 12 ± 4, and 24 ± 4 (Supplementary Table S1).
2.3 Statistical Analysis
The sample size was calculated based on the rule-of-three [15]. With approximately 600 patients, the rule-of-three suggests that if none of the 600 patients shows the ADRs of interest, there is 95% confidence that ADR incidence is at most 3 in 600 patients (i.e., 0.5%). In addition, 750 patients were estimated to cover the anticipated 20% dropout rate. The eligible population (EP) included all patients who met the eligibility criteria and signed the Informed Consent Form. The safety population (SP) included all patients in the EP who were administered at least one dose of rasagiline and had at least one follow-up safety assessment. The effectiveness was analyzed in patients from the SP who had at least one baseline effectiveness assessment of interest (i.e., UPDRS part III or CGI-S) and at least one post-treatment effectiveness assessment (i.e., UPDRS part III , CGI-S, or CGI-I).
Only descriptive statistics were performed in this study. Quantitative variables were presented as mean ± standard deviation (SD) and qualitative (categorical) variables were described by number and percentage. The changes of the scores from baseline were calculated by the mixed model for repeated measures (MMRM) overall and by treatment modality. Baseline scores were used as covariates for adjustment, except for CGI-I scores (since there was no baseline CGI-I score, the baseline CGI-S score was used as a baseline covariate). SAS version 9.4 was used for statistical analysis.
3 Results
3.1 Characteristics of the Participants
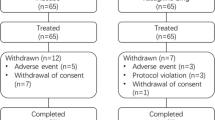
As shown in Fig. 1, there were 745 in the EP, 734 in the SP and ultimately 653 participants completed the 24-week follow-up. Thirty-eight patients in the SP had at least one baseline but no post-treatment effectiveness assessment. Effectiveness was analyzed in 696 participants. See Supplementary Information for details.
The participants were aged 64.0 ± 10.15 years at baseline, and 58.8 ± 10.21 years at diagnosis, including 55.0% of males. Details were recorded and shown in Table 1. All comorbidities of patients are summarized in Supplementary Table S2. In the SP, 197 (26.8%) and 77 (10.5%) patients had prior anti-PD and other medications, respectively (Supplementary Table S3). The total duration of rasagiline treatment was 148.7 ± 52.0 days.
3.2 Safety and Tolerability
Among the 734 participants in the SP, 102 (13.9%) reported a total number of 141 ADRs (Table 2). The incidence of all ADRs was similar between the monotherapy (15.8%) and adjunct therapy (13.6%) subgroups, while ADRs by system organ class (SOC) and preferred terms (PTs) were different between the two subgroups. The most common ADRs by SOC and PTs were nervous system disorders (5.6% overall), including dizziness (2.2%) and dyskinesia (1.1%). Other common ADRs (> 1%) were gastrointestinal disorders (3.3% overall) including dry mouth (1.2%), psychiatric disorders (1.8%), vascular disorders (1.2%), and general disorders and administration site conditions (1.1%).
Ninety-three unlabeled ADRs occurred in 68 (9.3%) patients, mostly in the adjunct therapy subgroup (78 ADRs in 55 [8.6%] patients). Besides the abovementioned dry mouth (1.2%) and dizziness (2.2%), other unlabeled ADRs included nausea (0.8% overall), constipation (0.4%), dreamy state (0.3%), head discomfort (0.1%), acne (0.1%), neutrophil count decrease (0.1%), white blood cell count decrease (0.1%), hypersensitivity (0.1%), and breast swelling (0.1%).
Fifty-one (6.9%) ADRs led to permanent discontinuation of rasagiline in the overall population (4 [4.2%] in the monotherapy subgroup and 47 [7.4%] in the adjunct therapy subgroup) (Supplementary Table S4). The most common ADRs leading to discontinuation were nervous system disorders (3.0%) including dizziness (1.5%), gastrointestinal disorders (1.5%), and psychiatric disorders (1.4%). Seven (1.0%) patients (all in the adjunct therapy subgroup) experienced 10 overdose ADRs, and 9 of the 10 ADRs occurred as a result of accidental overdose. All (100%) patients in the SP had concomitant anti-PD medications ongoing, or ended after initiation of rasagiline, 84.9% had concomitant anti-PD medications taken through the initiation of rasagiline, and 68.4% had concomitant medications other than anti-PD medications. No ADR resulting from drug interactions was reported. In total, 20 patients (3 and 17 in the monotherapy and adjunct therapy subgroups, respectively) had concomitant selective serotonin reuptake inhibitors, and none reported serotonin syndrome.
Non-serious ADRs were reported in 13.2% of the total cohort, with 13.9 and 13.1% in the monotherapy and adjunct therapy subgroups, respectively. Five participants (0.7%) experienced a total of five SADRs (one in the monotherapy subgroup and four in the adjunct therapy subgroup), including hypertension (n = 2), hypotension (n = 1), Parkinson’s disease (n = 1), and death (n = 1). The SADR leading to death occurred in the adjunct therapy subgroup. Advanced age and complex underlying chronic cardiovascular diseases were confounding factors for this SADR, and no evidence of a direct association with rasagiline treatment was found.
3.3 Effectiveness
The effectiveness analysis was performed in 696 participants who had at least one baseline effectiveness assessment of interest and at least one post-treatment effectiveness assessment. The overall mean (± SD) UPDRS part III score was 29.9 ± 14.44 at baseline. Improvements in the UPDRS part III score are shown in Fig. 2, with − 6.9 ± 0.35 in the monotherapy subgroup and − 4.8 ± 0.69 in the adjunct therapy subgroup at Week 24. Clinical Global Impression-Severity scores at baseline were 3.9 ± 1.18 and 2.4 ± 0.99 in the monotherapy subgroup and the adjunct therapy subgroup, respectively. Clinical Global Impression-Improvement scores at Weeks 4, 12 and 24 were 3.1 ± 0.85, 3.2 ± 0.91 and 3.2 ± 0.90, respectively. Improvements were similar between the two subgroups (Fig. 2).
4 Discussion
To our knowledge, this is the first multicenter, large-scale, post-marketing study on the safety profile of rasagiline in the real-world clinical practice in China. This study was conducted at the request of the Chinese Center for Drug Evaluation of National Medical Products Administration. Although this study was open-labeled, the data obtained are important for verifying the safety, tolerability, and effectiveness of rasagiline in a real-world clinical setting in terms of ADRs, PD motor symptoms, and disease severity in Chinese PD patients initiating rasagiline as monotherapy or as adjunct therapy to levodopa—an update of the Chinese packaging insert was also suggested. The major findings of this study confirm the good safety profile and remarkable effectiveness in line with the established evidence and clinical experience.
4.1 Safety and Tolerability
In this study, 734 PD patients received rasagiline monotherapy or adjunct therapy to levodopa for 24 weeks. The incidence of ADRs was considered to be low (15.8% in the monotherapy subgroup and 13.6% in the adjunct therapy subgroup), and the treatment was well tolerated. According to Phase 3 clinical trials in China [12, 13] and Japan [16, 17], in the monotherapy and adjunct therapy groups, the incidence of TEAEs and SAEs in rasagiline groups was similar to those of placebo. This is consistent with a recent meta-analysis of clinical trials, which showed no statistical difference in reported AEs between the 1 mg rasagiline and placebo groups, indicating satisfactory safety and tolerability of rasagiline, especially in an Asian subpopulation [18]. In this study, the real-world incidence rates of ADRs and SADRs were numerically lower than TEAEs and SAEs reported in randomized controlled clinical trials, providing supplementary evidence beyond clinical trials which set strict screening criteria for participants. The results confirmed the safety profile of rasagiline utilized in either monotherapy or adjunct therapy with levodopa. In a post-marketing study in Germany in 2010 [14], the incidence rates of AEs/ADRs were 2% (5/209) and 8% (46/545) in the rasagiline mono- and adjunct therapy subgroups, respectively, which were lower than observed in this study of Chinese PD patients (15.8% and 13.6% in the monotherapy and adjunct therapy subgroups, respectively). The differences might be related to the different ethnicities of the subject populations and the longer treatment period in this study (averaged 148.7 days in China vs 118.2 days in Germany). Future international multicenter real-world data may provide more comprehensive results.
The most common ADRs in this study were nervous system disorders (such as dizziness and dyskinesia), gastrointestinal disorders, and psychiatric disorders, corroborating the abovementioned clinical studies [12, 13]. In particular, the incidence rates of impulse-control disorder (ICD), hallucination, and somnolence were relatively low (0.1, 1.2, 0.8%, respectively) in this study, which were specially mentioned as AEs of dopaminergic therapy in the 2017 NICE guidance [19]. In addition, in the adjunct therapy subgroup but not monotherapy, dyskinesia was reported by only 8 patients (1.3%), probably due to stable extracellular dopamine level as a result of irreversible MAO-BI. Also, vascular ADRs, including hypertension, hypotension, blood pressure fluctuation and orthostatic hypotension, occurred in 9 patients (1.2%) in total, 8 of whom were from the adjunct therapy subgroup, which is consistent with previous findings that rasagiline does not exacerbate autonomic blood pressure dysregulation in early or mild PD [20].
Serious ADRs were observed in 1.1% patients in the monotherapy subgroup and 0.6% patients in the adjunct therapy subgroup. Adverse drug reactions leading to permanent discontinuation of rasagiline were reported in 4.2% and 7.4% patients in the monotherapy and adjunct therapy subgroups, respectively, indicating that ADRs were generally tolerable. In this study, one patient in the adjunct therapy subgroup died, which was a SADR. Notably, several confounding factors were found for this SADR, including advanced age (81 years), significant medical histories of idiopathic PD, and multiple comorbidities including high blood pressure, coronary heart disease, arrhythmia, arteriosclerosis, and lacunar infarction. There was no evidence of direct association with rasagiline treatment. In spite of several confounding factors, the principal investigator was unable to determine that the death was definitely unrelated to the study drug.
In addition, more than two-thirds (68.4%) of the patients in this study had concomitant medications other than anti-PD medications, and no serotonin syndrome or other ADRs associated with a PT drug interaction code were reported, indicating that rasagiline may be safe for patients with polypharmacy and possesses the potential to be utilized in PD co-morbidity conditions.
Finally, unlabeled ADRs were recorded in 15 SOCs, while dry mouth and dizziness were reported as the most common unlabeled ADRs in this study, with incidence rates of 3.2% and 2.1% in the monotherapy subgroup and 0.9% and 2.2% in the adjunct therapy subgroup, respectively. These incidence rates were low, indicating that rasagiline might be specific in some populations. Of the unlabeled ADRs, none were reported as being serious event, and most were confounded by the underlying diseases and concomitant medications that are often observed in PD patients. Therefore, the reported unlabeled ADRs in this study are consistent with the established safety profile and similar to the global cumulative post-marketing safety experience. In the future, data from large studies will be needed to determine the correlations between these ADRs and rasagiline. Unlabeled ADRs warrant further exploration to determine their safety profile. Importantly, the incidence of unlabeled ADRs in this study was low, and severity was moderate, which did not affect the quality of life and the use of rasagiline.
4.2 Effectiveness
In this study, improvements in motor symptoms as indicated by UPDRS part III, CGI-S, and CGI-I were observed from Week 4 after starting rasagiline treatment in both subgroups, indicating an early onset of action. In this study at Week 24 after initiating rasagiline treatment, mean changes in UPDRS part III from baseline was numerically beyond the changes reported in the Phase 3 trials of rasagiline monotherapy for early PD patients [12] and adjunct therapy for patients in later stages with motor fluctuations [13] in China. Therefore, rational use of rasagiline in real-world settings is thought to be achieving satisfactory therapeutic effects in China. Nevertheless, there might be other explanations, such as different study designs, inconsistencies in baseline scores, and different criteria for patient selection. Clinical Global Impression-Severity showed a slight reduction in the adjunct therapy subgroup but not in the monotherapy subgroup, probably because baseline scores were lower in the adjunct therapy subgroup. In addition, a slight improvement in CGI-I was found in both subgroups. In summary, in this real-world open-label study, UPDRS part III improvement in patients was more pronounced than that reported in the above Asian clinical trials, indicating that rasagiline could confer similar clinical benefits as reported in Caucasian populations. The conclusion drawn from our data should be taken with caution due to the open-label design and lack of placebo control in this study.
This study was associated with the strengths of the observational design including patients in a real-world clinical setting, favoring the external validity of results. The main limitation of this study was that assessment of treatment effectiveness was of relatively smaller power, since safety was set as the primary endpoint. In addition, a larger sample size and longer follow-up would facilitate the assessment of rare ADRs and the evaluation of the association between ADRs and rasagiline. Finally, possible bias could be due to potential subject selection for study participation and less recording of individual risks or disease factors. Nevertheless, these were adequately considered by the statistical analysis plan.
5 Conclusion
In conclusion, the findings of this real-world study confirmed the safety profile of rasagiline and demonstrated the effectiveness and tolerability of rasagiline administered according to the rasagiline Chinese package insert. Compared with previous studies, no additional prominent ADRs affecting patients’ quality of life and drug use were found in this study. In the real-world settings, rasagiline monotherapy or adjunct therapy to levodopa was generally safe and well tolerated, and could improve the overall severity of PD-associated motor symptoms after 4-week treatment. Therefore, this Phase 4 study provided complementary and additional support to the solid evidence on the effectiveness and safety of rasagiline obtained in previous clinical trials in the Chinese PD population.
References
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Todorova A, Jenner P, Ray CK. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol. 2014;14:310–22.
Qi S, Yin P, Wang L, Qu M, Kan GL, Zhang H, et al. Prevalence of Parkinson’s disease: a community-based study in China. Mov Disord. 2021;36:2940–4.
Benazzouz A, Mamad O, Abedi P, Bouali-Benazzouz R, Chetrit J. Involvement of dopamine loss in extrastriatal basal ganglia nuclei in the pathophysiology of Parkinson’s disease. Front Aging Neurosci. 2014;6:87.
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33:1248–66.
Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11–7.
Grosset DG, Macphee GJ, Nairn M, Guideline DG. Diagnosis and pharmacological management of Parkinson’s disease: summary of SIGN guidelines. BMJ. 2010;340: b5614.
Dezsi L, Vecsei L. Monoamine oxidase B inhibitors in Parkinson’s disease. CNS Neurol Disord Drug Targets. 2017;16:425–39.
McCormack PL. Rasagiline: a review of its use in the treatment of idiopathic Parkinson’s disease. CNS Drugs. 2014;28:1083–97.
Minguez-Minguez S, Solis-Garcia Del Pozo J, Jordan J. Rasagiline in Parkinson’s disease: a review based on meta-analysis of clinical data. Pharmacol Res. 2013;74:78–86.
Chang Y, Wang LB, Li D, Lei K, Liu SY. Efficacy of rasagiline for the treatment of Parkinson’s disease: an updated meta-analysis. Ann Med. 2017;49:421–34.
Zhang Z, Wang J, Chen S, Liu C, Zhang B, Peng R, et al. Efficacy and safety of rasagiline in Chinese patients with early Parkinson’s disease: a randomized, double-blind, parallel, placebo-controlled, fixed-dose study. Transl Neurodegener. 2018;7:32.
Zhang Z, Shao M, Chen S, Liu C, Peng R, Li Y, et al. Adjunct rasagiline to treat Parkinson’s disease with motor fluctuations: a randomized, double-blind study in China. Transl Neurodegener. 2018;7:14.
Reichmann H, Jost WH. Efficacy and tolerability of rasagiline in daily clinical use—a post-marketing observational study in patients with Parkinson’s disease. Eur J Neurol. 2010;17:1164–71.
Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ (Clinical Research ed). 1995;311:619–20.
Hattori N, Takeda A, Takeda S, Nishimura A, Kitagawa T, Mochizuki H, et al. Rasagiline monotherapy in early Parkinson's disease: a phase 3, randomized study in Japan. Parkinsonism Relat Disord. 2019;60:146–52. https://doi.org/10.1016/j.parkreldis.2018.08.024
Hattori N, Takeda A, Takeda S, Nishimura A, Kato M, Mochizuki H, et al. Efficacy and safety of adjunctive rasagiline in Japanese Parkinson's disease patients with wearing-off phenomena: A phase 2/3, randomized, double-blind, placebo-controlled, multicenter study. Parkinsonism Relat Disord. 2018;53:21–7. https://doi.org/10.1016/j.parkreldis.2018.04.025
Chang H-Y, Li Y-Y, Hong C-T, Kuan Y-C. Efficacy of rasagiline monotherapy for early Parkinson disease: a systematic review and meta-analysis of randomized controlled trials. J Psychopharmacol (Oxford, England). 2022;36:704–14.
Rogers G, Davies D, Pink J, Cooper P. Parkinson’s disease: summary of updated NICE guidance. BMJ (Clin Res ed). 2017;358: j1951.
Oka H, Sengoku R, Nakahara A, Yamazaki M. Rasagiline does not exacerbate autonomic blood pressure dysregulation in early or mild Parkinson’s disease. Clin Parkinsonism Relat Disord. 2022;6: 100124.
Acknowledgements
The authors thank the participating subjects and staff, and the investigators for their contributions to the study. The investigators not listed in the authors were Xinhua Wan (Peking Union Medical College Hospital), Zhengqi Lu (The Third Affiliated Hospital, Sun Yat-Sen University), Huifang Shang (West China Hospital, Sichuan University), Ping Jing (The Central Hospital of Wuhan), Baorong Zhang (The Second Affiliated Hospital, Zhejiang University School of Medicine), Yanping Wang (The Second Affiliated Hospital of Guangzhou Medical University), Weiguo Liu (The Affiliated Brain Hospital of Nanjing Medical University), Li Yang (Tianjin Medical University General Hospital), and Tao Feng (Beijing Tiantan Hospital, Capital Medical University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by H. Lundbeck A/S (Copenhagen, Denmark). Medical writing support was funded by H. Lundbeck A/S.
Conflict of interest
SC and ZS are employees of Lundbeck (Beijing) Pharmaceutical Consulting Co, Ltd. Other authors declare that they have no competing interests.
Availability of data and materials
The data set supporting the results of this article are included within the article and supplementary materials. Other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The study protocol was approved by the ethics committee of the leading center (Beijing Hospital) for the study conduct in each of the participating centers (approval No. 2018BJYYEC-223-02).
Consent to participate
All patients or their legal representatives provided signed informed consent for participation in this study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
WS and HC conceived and supervised the study; WS, ZHL, WM, MS, XH, YW, WW, ZGL, KZ, BT and HC performed the clinical trial; WS, ZHL, WM, SC and ZS wrote the manuscript; WS and HC made manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Su, W., Liang, Z., Mao, W. et al. Safety and Effectiveness of Rasagiline in Chinese Patients with Parkinson’s Disease: A Prospective, Multicenter, Non-interventional Post-marketing Study. Drug Saf 46, 637–646 (2023). https://doi.org/10.1007/s40264-023-01288-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01288-2