Abstract
Background and objective
Evidence highlights the allergenic potential of PEGylated drugs because of the production of anti-polyethylene glycol immunoglobulins. We investigated the risk of hypersensitivity reactions of PEGylated drugs using the Italian spontaneous adverse drug reaction reporting system database.
Methods
We selected adverse drug reaction reports attributed to medicinal products containing PEGylated active substances and/or PEGylated liposomes from the Italian Spontaneous Reporting System in the period between its inception and March 2021. As comparators, we extracted adverse drug reaction reports of medicinal products containing the same non-PEGylated active substances and/or non-PEGylated liposomes (or compounds belonging to the same mechanistic class). A descriptive analysis of reports of hypersensitivity reactions was performed. Reporting rates and time to onset of hypersensitivity reactions were also calculated in the period between January 2009 and March 2021. As a measure of disproportionality, we calculated the reporting odds ratio.
Results
Overall, 3865 adverse drug reaction reports were related to PEGylated medicinal products and 11,961 to their non-PEGylated comparators. Around two-thirds of patients were female and reports mostly concerned patients aged between 46 and 64 years. The frequency of hypersensitivity reactions reporting was higher among PEGylated versus non-PEGylated medicinal products (11.7% vs 9.4%, p < 0.0001). The hypersensitivity reaction reporting rates were higher for PEGylated medicinal products versus non-PEGylated medicinal products, with reporting rate ratios that ranged from 1.4 (95% confidence interval 0.8–2.5) for pegfilgrastim versus filgrastim to 20.0 (95% confidence interval 2.8–143.5) for peginterferon alpha-2a versus interferon alpha-2a. The median time to onset of hypersensitivity reactions was 10 days (interquartile range: 0–61) for PEGylated medicinal products, and 36 days (interquartile range: 3–216) for non-PEGylated comparators. Statistically significant reporting odds ratios were observed when comparing the reporting of hypersensitivity reactions for PEGylated versus non-PEGylated medicinal products (reporting odds ratio: 1.3; 95% confidence interval 1.1–1.4). However, when using all other drugs as comparators, the disproportionality analysis showed no association with hypersensitivity reactions for PEGylated nor non-PEGylated medicinal products, thus suggesting that many other triggers of drug-induced hypersensitivity reactions play a major role.
Conclusions
The findings of this analysis of the Italian spontaneous adverse drug reaction database suggest a potential involvement for PEGylation in triggering drug-related hypersensitivity reactions, especially clinically relevant reactions. However, when comparing both PEGylated and non-PEGylated drugs under study to all other drugs no disproportionate reporting of hypersensitivity reactions was observed, probably due to a masking effect owing to the presence in the same database of other medicinal products increasing the threshold required to highlight a safety signal when the entire database is used as a reference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Evidence highlights the allergenic potential of PEGylated drugs because of the production of anti-PEG immunoglobulins. |
The findings of this study suggest a potential involvement for PEGylation in triggering drug-related hypersensitivity reactions, especially clinically relevant ones. |
Anti-PEG antibodies screening is important to identify patients who may require a PEGylated drug with a reduced dosing strategy or the use of non-PEGylated drugs. |
1 Introduction
PEGylation is a widely used procedure consisting of the chemical conjugation of one or more molecules of polyethylene glycol (PEG) to a pharmacologically active compound with the main purpose of increasing its half-life [1, 2]. Polyethylene glycol moieties can be conjugated to poorly bioavailable compounds such as peptides, proteins, or nucleic acids as well as to the surface of nanocarriers (mainly lipid nanoparticles). The latter are increasingly being exploited to improve the pharmacokinetic and pharmacodynamic profiles of many drugs with poor biopharmaceutical properties allowing an improvement in the therapeutic index while providing organ and tissue targeting. Concerning nanotechnologies, PEG is used to prevent opsonization and to reduce the clearance of the nanocarriers by the reticuloendothelial system. In this case, PEG is linked to the nanocarrier surface as methoxy-PEG and only PEG2000 is used, as it was the most effective polymer based on experimental data [1].
As the number of PEGylated products on the market increases, safety concerns about the immunogenicity of PEG are rising in the scientific community, as recent evidence highlighted the allergenic potential of PEGylated drugs as a result of the production of anti-PEG immunoglobulins (Ig)G and IgE [3,4,5], triggering IgE- or complement-mediated hypersensitivity. In particular, the immunogenic potential of PEG may increase with increasing molecular weight and plasma concentrations [6, 7]. Sellaturay et al. reported a series of five cases of confirmed PEG-induced drug allergies (four cases of anaphylaxis and one case of a systemic allergic reaction) and concluded that the amount of PEG ingested as well as its molecular weight are important factors determining whether an allergic reaction occurs [7].
Anti-PEG antibodies have been detected also in patients who have never been exposed to PEGylated medicinal products, as PEG is present in many products such as toothpaste, shampoo, and detergent. Nevertheless, the titration of anti-PEG antibodies in the blood was not correlated to the activation of an immune response following the administration of PEGylated lipid nanoparticles [8].
The potential immunogenicity of PEGylated drugs is not clearly understood, and it is currently of particular interest as the first COVID-19 vaccines authorized for emergency use in the USA and in the European Union (Comirnaty and Spikevax) are based on PEGylated lipid nanoparticles. These are used to entrap and deliver a messenger RNA (mRNA) strand that, once introduced into the host cells, leads to the synthesis of the spike protein and to the activation of the immune response with the production of anti-spike antibodies. Lipid nanoparticles are vectors of choice for in vivo mRNA delivery [9]. Indeed, they protect the mRNA against degradation, can be easily synthesized in a scalable manner, can be targeted to the specific cell types, and facilitate endosomal escape.
As this technology has never been used for marketed vaccines in the pre-COVID-19 era, a safety concern about the potentially increased risk of allergic reactions to these vaccines has been raised. It has been hypothesized that some patients who have been vaccinated with mRNA vaccines, and already had anti-PEG antibodies, underwent anaphylactic reactions after the administration of those vaccines [10]. Post-marketing observational studies suggested that mRNA vaccines may be associated with an increased risk of severe allergic reactions as compared with conventional vaccines (11.1 vs 1.4 cases per million doses administered, respectively) [11]. In detail, the risk of an allergy after vaccination with an mRNA COVID-19 vaccine appeared to be increased in subjects with a history of allergies [12, 13]. Therefore, we analyzed the spontaneous reports of hypersensitivity adverse reactions (including anaphylactic shock) related to PEGylated drugs with the ultimate goal to explore the role of PEGylation on the drug-related allergy.
Specifically, the aims of this retrospective study were to assess the pattern, frequency, and characteristics (e.g., time to onset, severity, risk factors) of suspected hypersensitivity reactions associated with PEG-containing versus PEG-free active substances in the Italian Spontaneous Reporting System (SRS) and to evaluate the role of PEGylation in triggering drug-induced hypersensitivity reactions by carrying out a disproportionality analysis for both PEGylated and non-PEGylated medicinal products. In addition, we performed the same analyses for drugs delivered based on “Stealth®” nanotechnologies (e.g., liposomes), which are PEGylated or not.
2 Methods
2.1 Data Source
The Italian SRS database (Rete Nazionale di Farmacovigilanza) is a national pharmacovigilance database managed by the Italian Medicines Agency since 2001. Spontaneous and solicited reports of adverse drug reactions (ADRs) are transmitted by healthcare professionals and patients to local pharmacovigilance representatives in a hard copy form or through a web-based system and included into the Rete Nazionale di Farmacovigilanza after a consistency evaluation. Data extraction was carried out using VigiSegn (Version 3.0.0), a data warehousing system developed to analyze data from the SRS [14]. Drugs potentially implicated in ADRs are coded according to the Anatomical Therapeutic Chemical classification and suspected ADRs are categorized according to the Medical Dictionary for Regulatory Activities (Version 23.0) terminology [15]. Aggregated data concerning the exposure to the included medicinal products during the period between January 2009 (earliest available date for drug consumption data) and March 2021 were provided by the National Observatory on the Use of Medicines of the Italian Medicines Agency, which monitors the consumption and expenditure of medicines supplied by the National Health Service for both outpatient and inpatient assistance.
2.2 Study Drugs
We compiled a list of medicinal products currently marketed in Italy containing PEGylated (PEG+) active substances and/or PEGylated liposomes. To identify PEG+ medicinal products, the European public assessment reports of all the approved drugs in Europe were searched in the European Medicines Agency website as well as in the Italian Medicines Agency website. In addition, a thorough search of the scientific literature was also carried out.
As comparators, we selected medicinal products containing the same non-PEGylated (PEG−) active substances (if not available, compounds belonging to the same mechanistic class were identified) and, with regard to stealth nanoparticles, the same medicinal products containing non-PEGylated liposomal formulations. The PEG+/PEG− medicinal products for each active substance are shown in the Electronic Supplementary Material (ESM).
Spontaneous reports of hypersensitivity reactions related to PEG+/PEG− medicinal products received from the database inception to 31 March, 2021 (date of the last data drawn) were selected. Descriptive and frequency analyses were conducted to compare reports including hypersensitivity adverse reactions related to PEG+ medicinal products versus the corresponding non-PEGylated comparators.
2.3 Study Outcomes
Suspected events of interest were identified by using the Standardized MedDRA Query (SMQ) “Hypersensitivity”. Standardized MedDRA Queries include several preferred terms (PTs) referring to a range of clinical conditions, including well-defined diseases and symptoms.
As a subgroup analysis, we also evaluated the events identified by using the SMQ “Anaphylactic reaction”, whose PTs are also included in the SMQ “Hypersensitivity”. As SMQs are available as narrow or broad searches, we considered the specific narrow scope search [15].
We also specifically explored serious hypersensitivity reactions that were identified as those that: (i) led to death; or (ii) were life threatening; (iii) required hospitalization or prolongation of hospital stay; (iv) caused serious/permanent disability or (v) other clinically relevant conditions; or (vi) caused a congenital anomaly or birth defect [16].
2.4 Statistical Analysis
As a first step, to identify any temporal trend of adverse reactions of interest, the yearly frequency of reports of drug- and vaccine-related hypersensitivity and anaphylactic reactions collected in the Italian SRS database between 2001 and 2020 was assessed and represented with a line plot. Then, a descriptive analysis of patients’ age and sex, type of reporter, ADR seriousness and outcomes distribution for all the ADR reports, and specifically for hypersensitivity reactions of the PEG+/PEG− drugs under investigation was carried out. Data for continuous and categorical variables were respectively reported as median, along with interquartile range, and as absolute and relative frequency (percentages). Comparison of subjects’ characteristics between two defined groups (e.g., PEG+ vs PEG− medicinal products) was performed by the Mann–Whitney U test and by the Pearson Chi-square test for continuous and categorical variables, respectively.
The frequency of both serious and non-serious hypersensitivity reaction reports, comparing PEG+ with PEG− medicinal products, was assessed. As a subgroup analysis, we also evaluated the frequency of anaphylactic reactions, specifically.
Furthermore, to take into account the different uptake of the medicinal products included in the analyses, we estimated the reporting rates of hypersensitivity reactions, along with their 95% confidence intervals (CIs), in the period between January 2009 (earliest available date for drug consumption data) and March 2021. Reporting rates of hypersensitivity reactions for single PEG+/PEG− medicinal products were calculated by dividing the number of reports collected by drug consumption (expressed per 100,000 dispensed packages) in the same observation period. Reporting rate ratios, along with 95% CIs computed using the Poisson model, were calculated by comparing the reporting rates of PEG+ versus PEG− medicinal products.
To better evaluate the role of PEGylation in the context of a drug-induced hypersensitivity reaction, for both PEG+ and PEG− medicinal products, the crude reporting odds ratios (RORs), along with 95% CIs, were calculated as a measure of a signal of hypersensitivity reaction (i.e., cases) disproportionate reporting. This quantitative approach is based on the measurement of the frequency of drug-reaction pairs compared with distributions of all other ADRs (i.e., non-cases) from the whole database (excluding vaccines) reported in the same period. Reporting odds ratios were also calculated by comparing the frequency of hypersensitivity reactions reporting among each PEG+ (i.e., index drugs) versus PEG− (i.e., reference drugs) medicinal products. The statistical threshold to identify potential signals of disproportionate reporting was defined as the lower bound of the 95% CIs of the ROR of >1 in the presence of three or more reports [17].
For each medicinal product under investigation, the time to onset of hypersensitivity reports was also calculated as the number of days elapsed between the beginning of drug treatment and the onset of this adverse reaction. Furthermore, to assess the relationship between the proportion (i.e., frequency) of hypersensitivity reactions out of the total ADR reports for the study drugs injected subcutaneously and their PEG size, both bar plots and logistic regression models, which included the PEG size both as continuous and categorical covariates, were performed, separately. Frequencies were reported into the plot with error bars, which denoted a 95% CI computed following the Clopper–Pearson method for binomial CIs.
A p-value <0.05 was set up as the threshold for the statistical significance. All statistical analyses were performed using SAS Release 9.4 (SAS Institute, Cary, NC, USA). Plots were produced using R Foundation for Statistical Computing (R Development Core Team 2008, Vienna, Austria, Version 4.0.3, packages: ggplot2, ggpubr).
3 Results
The reporting trend of hypersensitivity and anaphylactic reactions in Italy showed that the yearly frequency of both drug- and vaccine-related anaphylactic reaction reporting in the Italian SRS database was constant from 2001 to 2020, while the trend of drug-related hypersensitivity reactions decreased from 35.8% in 2001 to 15.3% in 2020. The yearly frequency of vaccine-related hypersensitivity reactions remained almost constant during the study period, with a peak in 2015 (ESM).
Overall, 608,082 reports were collected in the Italian SRS from its inception to 31 March, 2021 and 172,066 (28.3%) of them concerned hypersensitivity reactions. The PEG+ medicinal products under investigation accounted for 3865 (0.6%) ADR reports, 451 (11.7%) of which were hypersensitivity reactions, while the PEG− medicinal products accounted for 11,961 (2.0%) ADR reports, 1129 (9.4%) of which reported hypersensitivity reactions.
Table 1 shows the main characteristics of all ADR reports, and of reports of hypersensitivity reactions specifically, related to PEG+/PEG− medicinal products. Concerning both overall ADRs and hypersensitivity reaction reports, around two-thirds of patients were female and they mostly concerned patients aged between 46 and 64 years (p < 0.0001 for each comparison), with similar frequencies between PEG+ and PEG− medicinal products. In both cases, the majority of ADRs were reported by physicians, especially for PEG+ versus PEG− medicinal products (p < 0.0001 for each comparison), and higher proportions of ADRs reported by patients or non-healthcare professionals (e.g., lawyers, pharmaceutical companies) were observed for PEG− versus PEG+ medicinal products (p < 0.0001 for each comparison). The proportion of serious ADRs was higher for PEG+ than for PEG− medicinal products (33.1% vs 23.6%; p < 0.0001) and, in particular, the proportion of serious hypersensitivity reaction reports was two times higher for PEG+ than for PEG− medicinal products (33.0% vs 16.3%, respectively; p < 0.0001). Regarding ADR outcomes, higher proportions of complete recovery were observed for PEG+ vs PEG− medicinal products, also when focusing on hypersensitivity reactions separately (p < 0.0001 for each comparison) (Table 1).
Overall, the frequency of anaphylactic reaction reports among the medicinal products under investigation was very low and they were mostly reported for PEG+ [N = 20 (0.5%); minimum–maximum range: 0.1–7.1%] vs PEG− medicinal products [N = 10 (0.1%); minimum–maximum range: 0.1–4.5%]. Among PEG+ medicinal products, anaphylactic reactions were mostly reported for liposomal doxorubicin (N = 8; 40%) and pegaspargase (N = 4; 20%) (Table 2).
Hypersensitivity reactions were more frequently reported for PEG+ versus PEG− medicinal products (11.7% vs 9.4%; p < 0.0001) but the frequency of hypersensitivity reactions reporting was significantly higher only for peginterferon alpha-2b, peginterferon beta-1a, certolizumab pegol, pegvisomant, pegaspargase, and PEGylated liposomal doxorubicin as compared with their respective PEG− comparators (p < 0.0001 for each comparison). Furthermore, a higher proportion of serious hypersensitivity reactions was observed for all PEG+ versus PEG− medicinal products, except for lipegfilgrastim versus filgrastim, peginterferon beta-1a versus recombinant interferon beta-1b, and methoxy-PEG epoetin beta versus darbepoetin alpha (p < 0.0001 for each comparison) (Table 2).
In the period between January 2009 and March 2021, the hypersensitivity reaction reporting rates were higher for PEG+ versus PEG− medicinal products, with reporting rate ratios that ranged from 1.4 (95% CI 0.8–2.5) for pegfilgrastim versus filgrastim to 20.0 (95% CI 2.8–143.5) for peginterferon alpha-2a versus interferon alpha-2a (Table 3).
Statistically significant RORs were observed when comparing the reporting of both serious [ROR: 2.5 (95% CI 2.0–3.2)] and non-serious [ROR: 1.3 (95% CI 1.1–1.4)] hypersensitivity reactions for PEG+ versus PEG− medicinal products. However, when using all other drugs as comparators, the disproportionality analysis showed no association with hypersensitivity reactions for PEG+ nor PEG− medicinal products (Table 4).
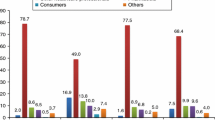
The median time to onset of hypersensitivity reactions was 10 (interquartile range: 0–61) days for PEGylated medicinal products, and 36 (interquartile range: 3–216) days for non-PEGylated comparators (Table 5). No statistically significant association between the frequency of hypersensitivity reaction reporting and increasing PEG molecular weight of medicinal products administered subcutaneously was found, as reported in the bar plots in Fig. 1.
Proportion of hypersensitivity reaction reports over the total number of adverse drug reactions (ADRs) with respect to ungrouped (A) and grouped (B) polyethylene glycol (PEG) size of PEGylated medicinal products that are injected subcutaneously. Error bars represent the 95% confidence interval around the computed percentages. N. number
4 Discussion
To our knowledge, this is the first study that explored the role of PEGylation in triggering hypersensitivity reactions using the Italian SRS database. In line with a recent Italian pharmacovigilance study, our analysis showed that drug-induced hypersensitivity reactions were mostly reported in female patients aged more than 40 years [18].
This study showed a disproportionate reporting of hypersensitivity reactions for most PEG+ medicinal products, i.e., peginterferon alpha-2b, peginterferon beta-1a, certolizumab pegol, pegvisomant, pegaspargase, and PEGylated liposomal doxorubicin, versus their respective PEG− comparators. However, when using all other drugs as comparators, no disproportionate reporting of hypersensitivity reactions for PEG+/PEG− medicinal product was observed. This may be due to a masking effect, owing to the presence of other medicinal products (e.g., antibiotics, other antineoplastic agents, and anti-inflammatory drugs) in the same database, which increase the threshold required for highlighting a signal [19].
A number of studies and clinical reports showed that PEG administration may be associated with moderate-to-severe hypersensitivity reactions, mainly due to complement system activation [4, 20] (complement activation-related pseudo allergy) or to the production of anti-PEG antibodies that may lead to an acceleration of the blood clearance (ABC phenomenon) of the PEGylated drug, resulting in efficacy loss and hypersensitivity reactions [21,22,23].
Many of the reported hypersensitivity reactions, mainly for PEG− medicinal products, had very long median times to onset. Indeed, the SMQ “hypersensitivity” does not only contain PTs pointing towards immediate hypersensitivity, but also PTs pertaining to delayed hypersensitivity (e.g., cutaneous adverse reactions). As a confirmation of this, most of the reports of hypersensitivity reactions for the medicinal products under investigation, especially those judged as serious, were cutaneous reactions.
Cutaneous hypersensitivity reactions have been frequently reported for interferons, and for peginterferon alpha-2b especially, and they have been extensively described in the literature [24,25,26,27,28,29]. A large body of evidence suggests that cutaneous hypersensitivity reactions are more frequent for peginterferon alpha as compared with its non-PEGylated form [28,29,30] and that switching from peginterferon alpha to conventional interferon alpha decreases the risk of severe allergic cutaneous reactions [30,31,32], suggesting that the likely culprit for the skin reaction may be the PEG component of peginterferon [30,31,32].
Additionally, pegaspargase was significantly associated with an increased reporting of hypersensitivity reactions. Although a large body of evidence proved that it causes less hypersensitivity as compared with asparaginase, its non-PEGylated form [33,34,35,36], such a difference could not be found in this study because asparaginase was not widely used in Italy during the study period (overall, only 632 packages were dispensed from 2018 to 2021) and no hypersensitivity reaction with this drug was identified in the Italian SRS.
Hypersensitivity reactions reported for certolizumab pegol mainly concerned cutaneous reactions such as hives and skin rashes. Cutaneous reactions, and psoriasiform skin eruptions in particular, are among the most common adverse reactions of tumor necrosis factor-α inhibitors and a considerable number of case reports describing adverse cutaneous reactions in patients treated with certolizumab have been published [37,38,39,40,41]. It has been proposed that increased levels of interferon-α due to tumor necrosis factor-α suppression and the activation of interleukin-23/T-helper-17 axis may play a crucial role in the development of such paradoxical reactions [42]. Therefore, cutaneous adverse reactions due to a tumor necrosis factor-α blockade seems to be a class effect.
It was postulated that the interaction between PEG and antibodies is stabilized and then enhanced when PEG is exposed on the surface of nanoparticles, suggesting that PEGylated nanocarriers might be even more immunogenic when compared with PEGylated drugs. This hypothesis seems to be confirmed looking at the data collected for liposomal doxorubicin, which is associated with important adverse events likely caused by complement activation, such as the hand-foot syndrome and infusion-related reactions [43]. As compared with the non-liposomal formulation, PEGylated liposomal doxorubicin is associated also with a wider range of cutaneous adverse reactions, reflecting its pharmacokinetics and tissue distribution [44].
Nevertheless, although a correlation between the presence of antibodies against methoxy-PEG and complement activation upon incubation with liposomal doxorubicin has not been found, the immunogenicity of this formulation is well known, and data drawn from liposomal doxorubicin experience cannot be transferred to other liposomal-based medicinal products. This is further demonstrated by the lack of a severe adverse effect registered in the case of PEGylated liposomes carrying irinotecan. In fact, PEG-induced complement activation-related pseudo allergy is dependent on several factors including liposome composition and physicochemical properties, along with the density of the PEG coating, the dose, and the frequency of administration. As an example, the immunogenicity of liposomal doxorubicin is attributed to both the negative charge and the oval shape of liposomes resulting from the crystallization of doxorubicin in the aqueous core. Equivalent formulations having a spherical shape did not lead to an increased complement activation [45]. In general, negatively or positively charged liposomes, with a cholesterol content higher than 45%, given at a high lipid dose through a slow infusion might be associated with a higher risk of the complement activation-related pseudo allergy phenomenon regardless of the presence of PEG moieties on the surface. As further proof of this, negatively charged non-PEGylated liposomes containing amphotericin B, used for the treatment of fungal infection, were found to increase levels of complement activation to a higher extent with respect to analogous non-charged liposomes [45].
In addition to these considerations, the results of our study are in line with those of a recently published pharmacovigilance study that explored the adverse events potentially associated with PEGylation by comparing ADR reports of PEG+ and PEG− drugs from the US Food and Drug Administration Adverse Event Reporting System database [46]. This study found that three immunogenicity-related PTs (i.e., rash, pruritus, and erythema) and the SMQs of hypersensitivity and anaphylactic reactions were slightly higher for PEGylated medicinal products, with a trend toward statistical significance, thus highlighting the importance of screening for anti-PEG antibodies to identify patients who may require a PEGylated drug with a reduced dosing strategy or the use of non-PEGylated drugs [46].
Furthermore, conflicting evidence exists on the role of PEG size in triggering hypersensitivity reactions [47]. Findings from this study suggest that PEG molecular weight is not statistically associated with an increased frequency of hypersensitivity reactions reporting; however, such statistical evidence does not necessarily translate into biological evidence, thus suggesting the need to conduct other studies investigating the role of PEG size in triggering hypersensitivity reactions.
The SRS is the cornerstone of pharmacovigilance for post-marketing surveillance of drugs and for the detection of new potential safety signals. In our study, we provided additional evidence concerning PEG-related hypersensitivity. To the best of our knowledge, this is the first pharmacovigilance study calculating the reporting rate of hypersensitivity reactions and to investigate the relationship between the frequency of hypersensitivity reaction reporting and PEG size for each of the medicinal products included in the analyses.
However, some limitations warrant caution. Many of the reports of hypersensitivity reactions for the medicinal products under investigation were long-term cutaneous reactions that were not necessarily due to hypersensitivity and/or allergic mechanisms, consequently accounting for a possible overestimation of the risk. The SRS has several known limitations that should be acknowledged, including under-reporting of suspected ADRs [48], selective over-reporting, missing demographic and clinical data, and a lack of a denominator (i.e., the total number of drug users), which precludes measuring the incidence of hypersensitivity reactions for both PEG+ and PEG− drugs. Furthermore, ROR estimates may also be affected by several biases ascribed to reporting trends in the SRS database. Finally, the analysis of SRS is mainly aimed at generating hypotheses, and causative relationships between the drugs and the studied ADRs can only be surmised. Indeed, drug-induced hypersensitivity reactions may also be due to specific excipients that are known to be involved in such adverse reactions, and delayed hypersensitivity specifically, rather than specific active principles [49]. Moreover, as it was not possible to stratify the analyses by route of administration, we compared drugs with more than one route of administration to drugs with only one route of administration (e.g., peginterferon beta-1a vs interferon beta-1a and pegaspargase vs asparaginase). However, it should be noted that there are no significant differences concerning the frequency of hypersensitivity reactions between the various parenteral routes of administration, but mostly between oral and parenteral routes of administration [50]. As we compared only drugs that are administered through parenteral routes of administration, we think this was unlikely to affect our analysis.
Finally, to really understand the role of PEG in the allergy phenomenon, more PEG+/PEG− liposomes comparators are needed, as lipid nanoparticles can activate the immune system by themselves and, as such, non-liposomal drugs cannot be used as controls in these studies. Therefore, findings from the analysis of the SRS need to be evaluated further through pharmacoepidemiological studies.
5 Conclusions
Most of the hypersensitivity reactions reported for both PEGylated and non-PEGylated medicinal products included in the analyses were cutaneous reactions due to delayed hypersensitivity mechanisms. The findings of this analysis of the Italian spontaneous ADR database suggest a potential involvement for PEGylation in triggering drug-related hypersensitivity reactions, especially clinically relevant reactions. However, when comparing both PEGylated and non-PEGylated drugs under study to all other drugs, there was no disproportionate reporting of hypersensitivity reactions, probably due to a masking effect, owing to the presence in the same database of other medicinal products increasing the threshold required to highlight a safety signal. Therefore, it is necessary to carry out more real-world studies to better investigate the potential involvement of PEGylation in triggering hypersensitivity reactions.
References
Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. 2016;105(2):460–75.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51.
Stone CA Jr, Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533-40.e8.
Chanan-Khan A, Szebeni J, Savay S, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14(9):1430–7.
Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216(2–3):106–21.
Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–22.
Sellaturay P, Nasser S, Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis). J Allergy Clin Immunol Pract. 2021;9(2):670–5.
Shi D, et al. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180: 114079.
Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–94.
de Vrieze J. Pfizer’s vaccine raises allergy concerns. Science. 2021;371(6524):10–1.
Luxi N, et al. Allergic reactions to COVID-19 vaccines: risk factors, frequency, mechanisms and management. BioDrugs. 2022;26(4):443–58.
Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–2.
Blumenthal KG, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–5.
Italian Medicines Agency (AIFA). Vigisegn. https://vigisegn.vigifarmaco.it. Accessed 29 Jan 2023.
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The medical dictionary for regulatory activities (MedDRA). Introductory guide for standardised MedDRA queries (SMQs) version 22.0. https://www.meddra.org. Accessed 29 Jan 2023.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) annex 1: definitions (rev 4). 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-annex-i-definitions-rev-4_en.pdf. Accessed 29 Jan 2023.
Eudvravigilance Expert Working Group. Guideline on the use of statistical signal detection methods in the Eudravigilance data analysis system. 2006. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/draft-guideline-use-statistical-signal-detection-methods-eudravigilance-data-analysis-system_en.pdf. Accessed 29 Jan 2023.
Pagani S, Lombardi N, Crescioli G, Vighi VG, Spada G, Andreetta P, et al., on behalf of the MEREAFaPS Study Group. Drug-related hypersensitivity reactions leading to emergency department: original data and systematic review. J Clin Med. 2022;11(10):2811.
Maignen F, Hauben M, Hung E, Van Holle L, Dogne JM. Assessing the extent and impact of the masking effect of disproportionality analyses on two spontaneous reporting systems databases. Pharmacoepidemiol Drug Saf. 2014;23(2):195–207.
Hamad I, Hunter AC, Szebeni J, Moghimi SM. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol Immunol. 2008;46(2):225–32.
Povsic TJ, et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol. 2016;138(6):1712–5.
Armstrong JK, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110(1):103–11.
Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, Sundy JS. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther. 2014;16(2):R63.
Hashimoto Y, Kanto H, Itoh M. Adverse skin reactions due to pegylated interferon alpha 2b plus ribavirin combination therapy in a patient with chronic hepatitis C virus. J Dermatol. 2007;34(8):577–82.
Meller S, et al. Allergic sensitization to pegylated interferon-α results in drug eruptions. Allergy. 2015;70(7):775–83.
Patrk I, Morović M, Markulin A, Patrk J. Cutaneous reactions in patients with chronic hepatitis C treated with peginterferon and ribavirin. Dermatology. 2014;228(1):42–6.
Hurst EA, Mauro T. Sarcoidosis associated with pegylated interferon alfa and ribavirin treatment for chronic hepatitis C: a case report and review of the literature. Arch Dermatol. 2005;141(7):865–8.
Gallelli L, Ferraro M, Mauro GF, De Sarro G. Generalised dermatitis induced by pegylated interferon-alpha-2b in a patient infected with genotype-1 hepatitis C virus: presentation of a case. Clin Drug Investig. 2005;25(4):281–4.
Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens induced by pegylated interferon alfa for chronic hepatitis C. Arch Dermatol. 2012;148(10):1213–4.
Veldt BJ, Schalm SW, Janssen HL. Severe allergic eczema due to pegylated alpha-interferon may abate after switching to daily conventional alpha-interferon. J Clin Gastroenterol. 2012;41(4):432.
Li Z, Ji F, Zheng Y, An J, Peng Z. Pegylated interferon, but not conventional interferon therapy induced severe skin lesions. Ann Hepatol. 2012;11(4):570–1.
Barut S, Yuksek J, Sezer E, Gunal O, Koseoglu D. Morbilliform drug eruption due to pegylated α-interferon can show complete regression after switching to non-pegylated interferon. J Dermatol. 2011;38(5):479–81.
Avramis VI, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99(6):1986–94.
Kim HJ, et al. Comparison of native Escherichia coli l-asparaginase versus pegylated asparaginase, in combination with ifosfamide, methotrexate, etoposide, and prednisolone, in extranodal NK/T-cell lymphoma, nasal type. Cancer Res Treat. 2018;50(3):670–80.
Kurtzberg J, Asselin B, Bernstein M, Buchanan GR, Pollock BH, Camitta BM. Polyethylene glycol-conjugated l-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children’s Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33(8):610–6.
Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma. 2011;52(12):2237–53.
Klein RQ, Spivack J, Choate KA. Psoriatic skin lesions induced by certolizumab pegol. Arch Dermatol. 2010;146(9):1055–6.
Babuna Kobaner G, Polat Ekinci A, Yilmaz Z, Copur S. Psoriasiform skin eruption in a patient receiving certolizumab-pegol for ankylosing spondylitis: report of a case and review of the literature. Dermatol Ther. 2018;31(5): e12693.
Kunadia A, Shulman K, Sami N. Certolizumab-induced lichenoid eruption in a patient with rheumatoid arthritis. BMJ Case Rep. 2021;14(12): e245875.
Sehgal R, Stratman EJ, Cutlan JE. Biologic agent-associated cutaneous adverse events: a single center experience. Clin Med Res. 2018;16(1–2):41–6.
Horai Y, et al. Development of hypocomplementemic urticarial vasculitis during certolizumab pegol treatment for rheumatoid arthritis: a case report. J Clin Pharm Ther. 2020;45(5):1179–82.
Brown G, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76(2):334–41.
Szebeni J, et al. Liposome-induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and Am Bisome. Nanomedicine. 2012;8(2):176–84.
Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136(12):1475–80.
Mostafa M, Elsadek NE, Emam SE, Abdelkader H, Farghaly Aly U, Sarhan HA. Complement activation-related pseudo allergy of PEGylated products: safety aspects, models, the role of anti-PEG antibodies, and ways to overcome. J Adv Biomed Pharm Sci. 2022;5:79–87.
Zhu Z, et al. PEGylated versus non-PEGylated drugs: a cross-sectional analysis of adverse events in the FDA Adverse Event Reporting System (FAERS) database. Int J Clin Pharmacol Ther. 2020;58(6):332–42.
Badiu I, Guida G, Heffler E, Rolla G. Multiple drug allergy due to hypersensitivity to polyethylene glycols of various molecular weights. J Investig Allergol Clin Immunol. 2015;25(5):368–9.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Caballero ML, Quirce S. Delayed hypersensitivity reactions caused by drug excipients: a literature review. J Investig Allergol Clin Immunol. 2020;30(6):400–8. https://doi.org/10.18176/jiaci.0562. (epub 2020 May 6).
Warrington R, Silviu-Dan F, Wong T. Drug allergy. Allergy asthma. Clin Immunol. 2018;14(Suppl. 2):60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Conflict of interest
Gianluca Trifirò has served in the last 3 years on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead, and Amgen; he was the scientific director of a Master program on pharmacovigilance, pharmacoepidemiology, and real-world evidence, which has received a non-conditional grant from various pharmaceutical companies; and he coordinated a pharmacoepidemiology team at the University of Messina until October 2020, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daichii Sankyo, PTC Pharmaceuticals). He is also a scientific coordinator of the academic spin-off “INSPIRE srl”, which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). None of these listed activities is related to the topic of the article. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article apart from those disclosed.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed equally to this work. All authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Crisafulli, S., Cutroneo, P.M., Luxi, N. et al. Is PEGylation of Drugs Associated with Hypersensitivity Reactions? An Analysis of the Italian National Spontaneous Adverse Drug Reaction Reporting System. Drug Saf 46, 343–355 (2023). https://doi.org/10.1007/s40264-023-01277-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01277-5