Abstract
Introduction
Drug-induced liver injury (DILI) is a rare but serious adverse event that can progress to acute liver failure (ALF). The evidence for treatment of DILI in children is scarce.
Objective
We aimed to comprehensively review the available literature on the therapies for both acetaminophen overdose (APAP) and idiosyncratic DILI in the paediatric population.
Methods
We included original articles conducted in a paediatric population (< 18 years) in which a therapeutic intervention was described to manage APAP or idiosyncratic DILI. Findings were summarized based on age groups (preterm newborn neonates, term and post-term neonates, infants, children and adolescents).
Results
Overall, 25 publications (fifteen case reports, six case series and four retrospective cohort studies) were included, including a total of 140 paediatric DILI cases, from preterm newborn neonates to adolescents. N-acetylcysteine was used to treat 19 APAP cases. N-acetylcysteine (n = 14), ursodeoxycholic acid (n = 3), corticosteroids (n = 31), carnitine (n = 16) and the combination of glycyrrhizin, reduced glutathione, polyene phosphatidylcholine and S-adenosylmethionine (n = 31) were the therapeutic options for treating idiosyncratic DILI. The molecular adsorbent recirculating system was used in the management of either APAP (n = 4) or idiosyncratic DILI (n = 2), while 20 paediatric ALF cases received continuous renal replacement therapy.
Conclusions
This systematic review identified DILI in the paediatric population who have received specific treatment. These interventions appear to be mainly extrapolated from low-quality evidence from the adult population. Thus, there is a need for high-quality studies to test the efficacy of known and novel therapies to treat DILI specifically addressed to the paediatric population.
PROSPERO registration number CRD42021214702.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Maturational processes during childhood hamper the assessment of therapeutic proposals. |
Specific interventions in paediatric DILI are extrapolated from evidence in adults. |
High-quality studies in therapeutic management of DILI in the paediatric population are needed. |
1 Introduction
Drug-induced liver injury (DILI) is an uncommon, complex and potentially severe adverse drug reaction to the use of medications, herbal products or dietary supplements. DILI has been typically classified into two types based on the mechanism of action. The intrinsic type is predictable, dose-related with a short latency period (hours to days) and most commonly associated with acetaminophen overdose (APAP). In contrast, idiosyncratic DILI is an unpredictable reaction to a drug or supplement used at a therapeutic dose and with a long latency period (days to months) [1].
Differences in pharmacokinetics and pharmacodynamics between children and adults rely on age-dependent developmental changes, which influence drug absorption, distribution and clearance. The reduction of total body water and increasing percentage of body fat, and the maturation of gastrointestinal physiology, renal function and of drug-metabolizing enzymes are some key processes during childhood that may explain these differences [2,3,4]. Indeed, the maturational processes during childhood may hamper the evaluation of therapeutic proposals.
DILI is generally considered to be rare in children and adolescents, but data on incidence are still sparse. Based on the World Health Organization (WHO) VigiBase data in the period 2000–2006, Ferrajolo et al. reported that hepatotoxicity was attributed to only 1.1% of the total adverse drug reactions in children and adolescents [5]. In nationwide prospective DILI registries, patients aged < 18 years ranged from 1% in the Spanish DILI Registry to 8% in the Indian Network for Drug-Induced Liver Injury [6, 7]. Data from the Pediatric Acute Liver Failure (PALF) study group showed that in the first 348 patients enrolled, DILI accounted for 18% of cases, mainly due to acetaminophen overdose [8].
Management of idiosyncratic DILI consists of the rapid discontinuation of the implicated agent in combination with supportive treatment if necessary. Clinical practice guidelines on idiosyncratic DILI have reviewed the use of therapeutical options in specific circumstances. Still, the evidence was limited due to the lack of high-quality studies [9]. On the other hand, N-acetylcysteine (NAC) remains the mainstay therapeutical option in the management of APAP [10, 11].
The existing differences in DILI between adults and children require a thorough assessment of the approaches used in the management of this complex condition in the paediatric population. We aimed to carry out an in-depth systematic review of the literature to summarize the therapeutic strategies for treating DILI and DILI-related acute liver failure (ALF) in the paediatric population.
2 Methods
2.1 Study Design and Search Strategy
This systematic review was conducted and reported following the PRISMA 2020 guidelines [12]. The protocol of the systematic review was registered in the international prospective register of systematic reviews (PROSPERO) with the registration number CRD42021214702.
A systematic literature search, up to June 30, 2022, was conducted in eligible literature published in PubMed, MEDLINE, EMBASE, Web of Science and SCOPUS, with no language or time restrictions. The following terms and Boolean operators were used in the search strategy: liver-related terms (‘liver impairment*’ or ‘liver injur*’ or ‘liver dysfunction*’ or ‘liver disease*’ or ‘liver disorder*’ or ‘liver toxicity’ or ‘liver damage’ or ‘liver problem’ or ‘hepatotoxicity’ or ‘liver failure’ or ‘hepatic*’ or ‘acetaminophen’ or ‘APAP’ or ‘overdose’ or ‘paracetamol’), combined with paediatric-related terms (‘infancy’ or ‘newborn*’ or ‘baby*’ or ‘babies’ or ‘neonat*’ or ‘preterm*’ or ‘prematur*’ or ‘schoolchild*’ or ‘kid*’ or ‘toddler*’ or ‘adolescent’ or ‘teen’ or ‘boy*’ or ‘girl*’ or ‘minor*’ or ‘pubert*’ or ‘pediatric*’ or ‘paediatric*’ or ‘infant*’) and therapeutical terms (‘medication*’ or ‘drug*’ or ‘manag*’ or ‘treat*’ or ‘therap*’). References of relevant studies, narrative/systematic reviews and meta-analyses were manually reviewed to identify additional studies eligible for inclusion. Relevant articles that could not be accessed were searched via inter-library loan, or the corresponding authors were contacted to request a copy. When required, authors were contacted to ask for further details about their studies. Retrieved literature was managed by Endnote 20 software and the Rayyan tool [13].
2.2 Inclusion and Exclusion Criteria
Published studies that fulfilled the following prespecified criteria were included: (i) original articles, including observational and experimental studies, case series and case reports; (ii) conducted in the paediatric population, i.e., aged < 18 years, who developed either idiosyncratic DILI or APAP due to pharmacological/herbal agents based on any threshold criteria; (iii) describe the use of therapeutical treatments, either pharmacological/herbal or extracorporeal therapeutical options, to manage idiosyncratic DILI or APAP and/or DILI-related ALF. Studies conducted in animal models or in vitro, in the adult population, or patients with acute liver injury due to other aetiologies (viral, autoimmune, metabolic, genetic, obstructive, neoplasm) were excluded. Editorials, letters, commentaries and other reports with no relevant data were also excluded.
2.3 Study Selection
The literature search was conducted by three independent researchers (HN, EA and IAA). The titles and abstracts were independently screened by pairs of authors. Full texts of relevant records identified were retrieved and reviewed, and their adequacy to be included was assessed. Differences in study selection were solved by discussion. In addition, original articles derived from conference abstracts which met the inclusion criteria were searched and reviewed. If the full-text article was not published, we considered the conference abstract eligible for inclusion if it contained sufficient relevant information.
2.4 Data Collection
Data were independently extracted by two researchers (HN and IAA), and discrepancies were solved by consulting a third author (EA). The following data were extracted from each of the included studies: surname of the first author, year of publication, study location, study design, number of patients, demographic data (age and sex), suspected culprit drug responsible for the DILI episode, DILI type (idiosyncratic or APAP), therapeutic intervention to manage DILI and outcome. Further, we collect data regarding DILI definition criteria, the severity of the episode and the pattern of DILI (hepatocellular, cholestatic, mixed). If this latter information was not available, we extracted liver biochemical parameters data when available and checked if cases fulfilled the DILI case definition, graded the severity of the episode, and computed the R value to ascertain the clinical pattern according to the current established criteria [14]. If a randomised clinical trial was considered for inclusion, the protocol was consulted to retrieve further information. If any data were unclear, corresponding authors were contacted to obtain further information.
2.5 Outcome Definition
The outcomes of interest were the efficacy of the therapeutic option(s) used to manage either APAP or idiosyncratic DILI and DILI-related ALF in the paediatric population, and the clinical outcomes of DILI (i.e., recovery, liver transplantation or death).
2.6 Quality Assessment
Quality assessment was undertaken based on the type of study. Thus, for case reports and case series, we used the quality appraisal tools developed by The Joanna Briggs Institute (JBI) [15], whilst for observational studies, the ROBINS-I tool was used [16]. Quality assessment was conducted independently by pairs of researchers (HN, EA and IAA), and disagreements were resolved by discussion.
2.7 Data Synthesis
We summarized the findings from the included studies in a narrative synthesis and classified them based on the therapeutic strategy used to manage DILI. Findings were presented based on age classification of the paediatric population [17]: preterm newborn neonates (from day of birth to the expected date of delivery plus 27 days), term and post-term neonates (from day of birth plus 27 days), infants or toddlers (from 1 month, i.e., 28 days, to 23 months), children (from 2 to 11 years), and adolescents (from 12 to < 18 years). We planned to perform a quantitative analysis to evaluate the efficacy of specific therapeutic options in the management of DILI in the paediatric population, but the different study designs (case reports, case series and retrospective cohort studies), along with non-comparable treatment regimens in terms of dosage and treatment duration, in different age groups, precluded statistical analysis.
3 Results
3.1 Literature Search and Study Characteristics
A total of 3,877 records were identified through the search strategy, of which 2,645 duplicates were excluded. After screening the title and abstract, 1,141 records failed to meet the inclusion criteria and were removed. A total of 91 full-text records were assessed for inclusion. Of them, 38 records were excluded as they were considered irrelevant for the study, 20 had no data on treatment, 16 were not original articles (reviews, letters or commentaries), and four were conducted in adult populations. One full-text article could not be retrieved. Therefore, 12 studies met the inclusion criteria. After reviewing the references of these studies, reviews, and meta-analyses identified in the literature search, 13 additional records were retrieved. Thus, 25 articles were finally included in this review (Fig.1).
Fifteen case reports, six case series, and four retrospective cohort studies, published between 1984 and 2022, with a total of 140 patients, from preterm newborn neonates to adolescents, were included (Table 1) [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Most of the studies were conducted in infants (n = 6) [20,21,22,23,24,25,26] and children (n = 13) [27,28,29,30,31,32,33,34,35,36,37,38,39]. In addition, one study reported findings from either preterm newborn neonates [18] or term or post-term neonates [19], and four studies included adolescents [36, 38, 40, 41] (of note, Novelli et al. [36] and Scheffner et al. [38] reported findings from both children and adolescents). In one study, the patient’s age was not reported [42]. Overall, 11 studies included patients with idiosyncratic DILI (n = 97) [25, 30,31,32,33,34,35, 37,38,39, 41], and 14 with APAP (n = 24) [18,19,20,21,22,23,24, 26,27,28,29, 36, 37, 40] (one study included both idiosyncratic and APAP cases [37]), whereas in one study, type of DILI could not be ascertained (n = 19) [42]. Therapeutical approaches used to manage DILI and DILI-related ALF were NAC (12 studies), ursodeoxycholic acid (UDCA) (three studies), corticosteroids (three studies), molecular adsorbent recirculating system (MARS) (three studies), continuous renal replacement therapy (CRRT, two studies), carnitine (one study), and the combination of glycyrrhizin, reduced glutathione (GSH), polyene phosphatidylcholine (PPC) and S-adenosylmethionine (one study). Ten studies provided heterogeneous DILI definitions [25, 27, 28, 34,35,36,37,38,39, 42]. Two studies [34, 35] adhered to the criteria set by the RegiSCAR group [43]. Karaarslan et al. [25] defined liver injury as an elevation of transaminases six times the upper limit of normal (ULN). Rumack [27] defined hepatotoxicity due to APAP as aspartate aminotransferase (AST) levels > 1,000 IU/L. Two studies defined idiosyncratic DILI as liver transaminases > 50 IU/L and bilirubin > 1.2 mg/dL, with abnormal coagulation parameters [38], or as a score > 5 in the Digestive Disease Week–Japan 2004 (DDW-J) scale [39]. For DILI-related ALF, two studies [28, 42] used the definition provided by the PALF study group [8], whilst another two studies [37, 41] used either the King’s College or the Clichy-Villejuif criteria [44, 45]. An additional study defined ALF cases as those who presented with bilirubin > 15 mg/dL, creatinine > 2 mg/dL, encephalopathy grade above II and International Normalized Ratio (INR) > 2.5, and elevations in alanine aminotransferase (ALT), AST and lactate [36]. The use of causality scales (Naranjo, DDW-J) was limited to only two studies [39, 41]. In most of the studies, pattern of liver injury was not described or could not be calculated. Most of the interventions did not show other related adverse effects. Severity of DILI episode was reported in 56 cases (40% of all), of whom nearly 60% presented with ALF or DILI progressed to ALF. Of the whole sample, 19 cases died (14%), four underwent liver transplantation (2.9%), and 117 recovered (84%). Therapeutic options for the management of DILI across the paediatric age groups are depicted in Fig. 2. Characteristics and outcome of DILI in the paediatric population are summarized in Table S1 (see the electronic supplementary material [ESM]).
Therapeutical options for the management of idiosyncratic drug-induced liver injury and acetaminophen hepatotoxicity in paediatric patients. ALF acute liver failure, CVVH continuous venovenous hemofiltration, GSH reduced glutathione, LTx liver transplantation, MARS molecular adsorbent recirculating system, NA not available, NAC N-acetylcysteine, PPC polyene phosphatidylcholine, SAM S-adenosylmethionine, TMP-SMZ sulfamethoxazole-trimethoprim, UDCA ursodeoxycholic acid
3.2 Preterm Newborn Neonates
3.2.1 N-acetylcysteine
One study described the case of a male preterm neonate who was started on intravenous paracetamol to promote ductal closure. After 5 days of therapeutic paracetamol dosing, the newborn developed acute transaminitis with coagulopathy (AST 994 µ/L, ALT 663 µ/L, alkaline phosphatase [ALP] 1,175 µ/L, INR of 4.2). Blood paracetamol levels were elevated, and NAC was started (200 mg/kg over 4 h, followed by 100 mg/kg over 16 h) along with supportive treatment, while paracetamol was withdrawn. Liver function tests improved, and the newborn recovered uneventfully [18].
3.3 Term and Post-term Neonates
3.3.1 N-acetylcysteine
One study reported the use of NAC in a 4-day-old boy with severe hepatocellular liver injury with coagulopathy (ALT 978 IU/L; AST 718 IU/L; total serum bilirubin 9 mg/dL and INR of 5.4), with clinical manifestations compatible with acetaminophen toxicity. The newborn received supportive treatment and intravenous NAC (150 mg/kg over 15 min, 50 mg/kg over 4 h and 100 mg/kg over 16 h), and recovered spontaneously without any adverse effects related to the treatment administered [19].
3.4 Infants or Toddlers
3.4.1 N-acetylcysteine
The use of NAC for the treatment of APAP was reported in 13 infants [20,21,22,23,24,25]. In six of them, parental or medical inadvertent overdosage of acetaminophen was the cause of DILI, while the remaining seven received NAC for idiosyncratic DILI. Infants presented with severe liver damage (n = 2) or ALF (n = 1). The vast majority (n = 12) recovered spontaneously after the administration of oral or intravenous NAC (initial doses ranging from 5 mg/kg/h to 1,000 mg) [20, 22,23,24,25]. The one infant who died was misdiagnosed with acute encephalopathy and received supportive treatment. However, his condition worsened to fulminant hepatic failure. Upon further inquiry, the mother reported that she had been giving increasing paracetamol doses to treat the infant’s fever. After serum paracetamol in blood was estimated, the treatment was changed to intravenous NAC (unreported dosage). However, the infant’s condition deteriorated and he died 24 h later [21].
3.4.2 Continuous Renal Replacement Therapy
One study reported a case of an 18-month male infant who presented with ALF after parental accidental overdosage of acetaminophen. He was started on supportive treatment and NAC (150 mg/kg over 1 h, followed by 50 mg/kg over 4 h and 100 mg/kg over 16 h). There was no improvement within the first 24 h, but his condition was aggravated. Decision to treat the infant with continuous venovenous hemofiltration (CVVH) as a rescue therapy was taken. A gradual clinical improvement was observed, and the infant recovered uneventfully after 10 days [26].
3.5 Children
3.5.1 N-acetylcysteine
The efficacy of NAC in treating APAP in children was assessed in three studies that included ten children aged between 2 and 5 years [27,28,29]. All of them recovered after administration of oral or intravenous NAC (initial doses ranged from 100 to 150 mg/kg).
Rumack [27] reported findings from a prospective nationwide study to evaluate APAP and its treatment. Of 417 children aged ≤ 5 years, with a history of known or suspected APAP, 55 had toxic plasma levels. Three out of these 55 patients showed acetaminophen hepatotoxic effects (AST > 1,000 IU/L) that coursed as a mild or moderate injury. The administration of oral NAC (loading dose 140 mg/kg, followed by 17 doses of 70 mg/kg every 4 h) resulted in full recovery.
In addition, in another two studies including seven children who developed ALF due to APAP, the use of intravenous NAC (doses ranged from 100 mg/kg/day to 150 mg/kg bolus followed by 12.5 mg/kg/h until recovery) led to a full recovery in these critically ill children [28, 29].
3.5.2 Ursodeoxycholic Acid
Three studies reported the use of UDCA in three children aged 3–7 years following idiosyncratic DILI due to trimethoprim-sulfamethoxazole (TMP-SMZ) and amoxicillin-clavulanate [30,31,32]. Two of them developed a cholestatic injury, and one presented with severe hepatocellular damage. Two children underwent a liver transplant and the remaining one recovered after treatment with UDCA (doses ranging from 16 to 30 mg/kg/day).
A 5-year-old girl developed a severe hepatocellular idiosyncratic DILI with jaundice following exposure to TMP-SMZ [30]. Supportive treatment was started, and UDCA (16 mg/kg/day) was administered due to the development of severe cholestasis. Despite transient improvement in liver biochemistry, she progressed to ALF and required liver transplantation.
Chawla et al. [31] described the case of a 3-year-old boy who had developed liver toxicity caused by amoxicillin-clavulanate. Two weeks after discontinuing the suspected culprit agent, the child was referred to hospital with abnormal liver enzymes (ALT 245 IU/L, ALP 630 IU/L, total bilirubin 3.5 mg/dL). UDCA (30 mg/kg/day) combined with conservative treatment were initiated. However, no clinical or biochemical improvements were noted after 4 months. Findings from a liver biopsy revealed canalicular cholestasis and signs of biliary obstruction. The microscopic pattern suggested a sclerosing cholangitis, and prednisone therapy was initiated. Due to clinical deterioration, liver transplantation was performed 8 months after DILI recognition.
Treatment with UDCA was also used in a child aged 7 years who had taken oral TMP-SMZ for 4 days [32]. He was hospitalized with jaundice and elevated liver enzymes (ALT 220 IU/L, ALP 1,028 IU/L, total bilirubin 8.4 mg/dL). UDCA administration (20 mg/kg/day) was started, but after 10 days, no improvement of cholestasis was observed. A liver biopsy was performed and vanishing bile duct syndrome diagnosis was confirmed. The UDCA dosage was increased to 30 mg/kg/day, and all liver parameters progressively improved until recovery.
3.5.3 Corticosteroids
Treatment with corticosteroids was detailed in three studies including 31 paediatric DILI patients with severe cutaneous reactions [33,34,35]. Patients recovered after the administration of doses ranging from 1 mg/kg/day to a median of 6.5 mg/kg of oral or intravenous corticosteroids.
Two children aged 2 and 8 years old had a drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS) due to TMP-SMZ [33, 35]. Both children presented with elevation in liver enzymes. Treatment with 1 mg/kg/day of prednisone and methylprednisolone, respectively, resulted in full and rapid recovery.
Ahluwalia and colleagues conducted a retrospective cohort study in which 29 paediatric DRESS cases (median age 11 years) were enrolled, four of them positive for human herpesvirus 6 (HHV6) [34]. Antibiotics (n = 20, TMP-SMZ accounted for 50% of cases) and antiepileptics (n = 9) were the causative agents. Among the 25 patients who were negative for HHV6, 20 patients were treated with systemic corticosteroids (median total dose 5 mg/kg, either oral or intravenously), while the remaining five patients were managed with supportive therapy alone. Patients who received steroids were more likely to have a definite diagnosis. Positive HHV6 patients received a median total dose of 6.5 mg/kg of systemic corticosteroids. When restricting analysis to negative HHV6 patients, a shorter period until cessation of disease progression, and a non-statistically significant trend toward shorter hospital length of stay and lower number of febrile days was observed between patients treated with corticosteroids and those who received standard supportive treatment. Although all patients recovered, adverse effects of systemic corticosteroid treatment were reported (recrudescence of DRESS due to a rapid taper of corticosteroids, steroid-induced acne exacerbation, atopic dermatitis flare secondary to tapering steroids, mild neutropenia and steroid-induced hyperglycaemia and hypertension). Notably, three patients who received supportive treatment also had secondary effects, including transaminitis needing hospitalization, abnormal thyroid function and xerosis in the lower bound extremities.
3.5.4 Molecular Adsorbent Recirculating System
Two studies described the use of MARS in children for the management of APAP and idiosyncratic DILI [36, 37]. Three children who had developed ALF recovered spontaneously, whereas the remaining one underwent a liver transplantation.
One girl aged 9 years old had ALF induced by APAP. She received supportive treatment and was treated with the MARS liver support device as a bridge for liver transplantation. Her clinical condition was resolved without liver transplantation after 35 h of MARS treatment, showing improvements in biochemical parameters and significant changes in the prognostic models Pediatric End-Liver Disease and Sequential Organ Failure Assessment [36].
Lexmond and colleagues performed a retrospective cohort study including 20 patients with ALF who were treated with MARS dialysis (one of them with APAP) and 32 ALF patients in a non-MARS treatment group (two of them due to APAP and valproic acid-induced liver injury, respectively) [37]. One child (with APAP) who was treated with MARS therapy (33 runs) recovered spontaneously with native liver. In contrast, of the two children who were not treated, one of them (with valproic acid DILI) required liver transplantation, whereas the second patient (with APAP) recovered spontaneously.
3.5.5 Carnitine
In a case series study, Scheffner et al. [38] analysed 14 cases (10 boys and 4 girls, median age 5 years) who developed valproic acid-induced ALF and died. Five of the children were given carnitine supplementation in doses ranging from 10 to 120 kg/mg. The remaining nine cases did not receive any treatment before death occurred.
3.5.6 Glycyrrhizin, Reduced Glutathione, Polyene Phosphatidylcholine, S-adenosylmethionine
In the retrospective study conducted by Wang et al. [39], 31 DILI cases (mean age 8.8 years, range from 0.3 to 14 years) were included (20 boys and 11 girls). The most common pattern of liver injury was mixed (48.4%), followed by hepatocellular (25.8%) and cholestatic (25.8%). Antimicrobials (41.9%), traditional Chinese medicines (29.0%) and antipyretics and analgesics (nimesulide, ibuprofen, paracetamol, metamizole; 19.4%) accounted for most of the hepatotoxic episodes. Patients were treated with supportive treatment with a combination of glycyrrhizin, GSH, PPC, and S-adenosylmethionine. In addition, those children with cholestasis were given a short-term treatment with corticosteroids. Except for one child who progressed to ALF and died (culprit drug not specified), all children recovered from liver injury.
3.6 Adolescents
3.6.1 N-acetylcysteine
One study described the case of a 17-year-old female adolescent who had ingested an overdose of acetaminophen. The Food and Drug Administration (FDA)-approved three-bag intravenous NAC protocol (loading dose 150 mg/kg, followed by 12.5 mg/kg over 4 h, and 6.25 mg/kg over 16 h) was started. After 10 h of treatment, no biochemical remission was evident, and near the end of the treatment, transaminases continued increasing (AST 990 IU/L, ALT 788 IU/L). She had an anaphylactoid reaction to NAC and treatment was stopped and replaced with corticosteroids and antihistaminic. After discussing the benefit–risk balance, NAC was restarted (12.5 mg/kg/h) along with scheduled steroids and antihistamines. There was no rechallenge, and the patient recovered after 6 days [40].
3.6.2 Molecular Adsorbent Recirculating System
In two studies [36, 41], MARS was used to treat one case of APAP and another of idiosyncratic DILI that presented with or progressed to ALF. One of the cases underwent liver transplantation, while the other one recovered.
Novelli et al. [36] reported the case of one 14-year-old boy who had ALF induced by APAP. In addition to supportive treatment, MARS was used as a bridge for liver transplantation. He successfully underwent a liver transplant after 49 h from the start of MARS.
A report by Ng et al. [41] described the case of a 17-year-old male adolescent who developed DRESS caused by TMP-SMZ. At hospital admission he presented with hepatocellular liver injury with jaundice. The suspected drug was withdrawn, and supportive treatment was started. However, he progressed to ALF, and fulfilled the King’s College criteria for liver transplantation [44]. He underwent two cycles of MARS liver dialysis with clinical improvement of both liver and skin injuries and achieved complete recovery 2 months later, without the need for liver transplantation.
3.6.3 Carnitine
One boy and one girl, median age 15 years, developed valproic acid-induced ALF. One of them was treated with carnitine 30 mg/kg but died, while the other one died before receiving any treatment [38].
3.7 Unspecified Age
3.7.1 Continuous Renal Replacement Therapy
The efficacy of CRRT in improving survival rates in a cohort of paediatric patients with ALF was evaluated in a retrospective cohort study [42]. Out of 136 cases with ALF, 45 (six with DILI-related ALF) received CRRT as part of their management prior to liver transplantation, while the other 91 (13 with DILI) were not treated with CRRT. Neither the type of liver injury nor the suspected agents were reported. All six patients who were treated with CRRT survived, while among cases with DILI who did not receive CRRT, the survival rate was 92%, with one patient who died.
3.8 Quality Assessment
Out of the 15 case reports included, only six reports clearly described all the domains in the case report checklist tool [18, 19, 24, 26, 32, 41], as per Fig. 3. Sufficient details on the clinical presentation of acute liver injury were reported in all included cases. In contrast, reporting the results of investigations was lacking in Hon and Leung [22] and Epperson et al. [40], and unclear in Chawla et al. [31]. There was lack of reporting details on treatment dose and duration in Ebenezer et al. [21], and clinical outcome and follow up in the reports from Hon and Leung and Simma et al. [22, 30].
Following assessment of the six case series, only one article [28] reported all the checklist’s domains as shown in Fig. 3. Two studies did not clearly define inclusion criteria of their cases [23, 38], and it was unclear whether consecutive cases were included in three series [23, 27, 38]. Only two studies reported complete inclusion of cases over a specific period [28, 36]. In contrast, Scheffner et al. [38] did not report all the liver failure cases identified following valproate therapy, and complete inclusion of cases was unclear in three studies [23, 27, 39]. It was unclear whether all clinical details of the included patients were reported in two studies [38, 39], whereas clinical information in Beringer et al. [23] was lacking at presentation and follow up. Four studies included statistical analysis that was deemed appropriate in two studies [28, 39], and unclear in the other two studies [27, 36].
The risk of bias from confounding was deemed serious across all four retrospective cohort studies [25, 34, 37, 42], as shown in Fig. 3. Moreover, we identified a significant risk of bias in selection of participants in two studies [34, 42]. The intervention in all studies was based on clinical decision; furthermore, details on duration of treatment were lacking. Therefore, all four studies were judged as moderate risk of bias in classification of interventions. Only one study excluded patients due to insufficient data and was deemed as moderate risk of bias for missing data [25]. Taking the retrospective nature of the studies into consideration, no deviation of the intervention or missing data were reported in all studies and they were deemed as low risk of bias in these domains. However, there was a moderate risk of bias in measuring outcomes and reporting results as assessors were aware of the intervention measured.
4 Discussion
Therapeutic management of DILI has gained relevance in recent years given the increasing awareness and research in the field, although the paediatric population remains a much more neglected area. This is the first systematic review to summarize the evidence on treatment of DILI in paediatric patients. Importantly, it highlights the lack of high-quality studies testing the efficacy of known and novel compounds specifically addressing this vulnerable population. Even though children have different pharmacokinetics and pharmacodynamics, treatments of DILI in general have been extrapolated from those used in the adult population.
Evidence about clinical characteristics of DILI in the paediatric population remains limited. Apart from APAP cases, cases of idiosyncratic DILI included in this systematic review were caused mainly by antibiotics and anticonvulsants. Hepatocellular injury was the most common pattern of liver injury among those cases in whom it could be ascertained. Despite the most frequent clinical presentation being ALF, most of the paediatric patients recovered, and in 2.9% of them a liver transplant was needed. These findings are consistent with prior evidence from the Drug-Induced Liver Injury Network (DILIN). DiPaola et al. characterized 57 idiosyncratic DILI cases in the paediatric population. Hepatocellular injury was the predominant pattern in this case series. Nearly 63% of children needed hospitalization, and 5% of them underwent liver transplantation. Antibiotics (mainly minocycline) and antiepileptics were the most common causes of hepatotoxicity [46]. Likewise, antibiotics (mainly amoxicillin-clavulanate, 31%) were the most common culprit drugs in a Spanish prospective multicentric study that characterized idiosyncratic DILI in 33 children. In addition, hepatocelular injury was the most common pattern (56%), and only one case died after undergoing liver transplantation [47]. In contrast, findings from a single center in India reported a much higher mortality rate, nearly 30%, in 39 paediatric DILI cases, mainly caused by anti-tuberculosis drugs [48], whilst in another single-center Chinese study including 69 paediatric DILI patients, mostly due to Chinese medicines (13%), the mortality rate was similar to western DILI cohorts (2.9%) [49] (Table S2, see ESM).
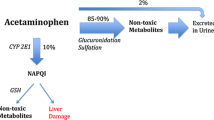
Paracetamol is the most extended analgesic and antipyretic in infants and has a demonstrated safety profile when administrated at therapeutic doses [50]. Thus, acetaminophen hepatotoxicity is intrinsically related with an unintentional administration of a higher dose over the recommended threshold [51]. Pharmacokinetics and metabolism of paracetamol differ in neonates and infants compared with older children and adults, firstly due to the immaturity of the cytochrome P-450 system. Levels of the cytochrome P-4502E1, responsible for the metabolism of acetaminophen, were lower in newborns and infants aged ≤ 1 year (10% and 30% of the value in adults, respectively) [52, 53]. Then, the glucuronidation pathway progressively increases its contribution in older ages, which leaves the sulphation metabolic pathway as the most significant route of paracetamol metabolism in the first months of life [54]. Lastly, GSH levels seem to remain more stable in newborns [55].
At therapeutic doses, acetaminophen is mostly converted to glucuronide and sulphate conjugates through glucuronidation and sulphation pathways. In addition, a minor fraction is oxidized to a reactive metabolite, NAPQI, responsible for APAP hepatotoxicity, which is detoxified through the glutathione pathway. When acetaminophen overdose occurs, glucuronidation and sulphation pathways get saturated, and the excess of NAPQI depletes glutathione and causes liver injury [56]. NAC is the only approved antidote for treating acetaminophen overdoses. It exerts its beneficial effect by replenishing hepatic glutathione stores, thereby scavenging the excess of NAPQI and enhancing the production of non-toxic metabolites. NAC treatment should be started within 8 h of acetaminophen ingestion as it has been proven most effective in this period window. Nonetheless, its beneficial effect extends throughout the first 24 h [57]. These findings are consistent with the results of this systematic review. All cases with APAP who were treated with NAC (either oral or intravenous) recovered without any reported adverse effects, except for one infant who died, possibly due to delayed administration of NAC [21].
Interestingly, in a randomized clinical trial with 184 non-acetaminophen ALF adult patients who received intravenous NAC or placebo, authors reported longer overall and transplant-free survival in patients with minimal hepatic encephalopathy (grades 0–I) [58]. In contrast, in another clinical trial involving the same number of paediatric patients with non-acetaminophen ALF, no benefits were found for the administration of intravenous NAC in the aforementioned endpoints in children with low grade of hepatic encephalopathy [59]. These differences were attributed to differences in the aetiology, underlying pathophysiology and age [59]. Hence, it is worth noting that the evidence described in studies limited to the adult population could not just merely be extrapolated to paediatric patients. Considering the developmental and maturational changes throughout the paediatric ages, and their influence on pharmacokinetics and pharmacodynamics [2,3,4], it is paramount to establish the most appropriate therapeutic strategies in this population.
Management of idiosyncratic DILI comprised a variety of therapeutical options, including the use of UDCA. This compound is commonly used to treat chronic cholestasis following DILI in clinical practice guidelines, albeit evidence is not substantiated in high-quality studies [9, 60]. The mechanisms described underlying the beneficial effects of UDCA in DILI comprise its immunomodulatory role, the protection of cholangiocytes against bile acids cytotoxic injury, the enhanced elimination of toxic compounds from the hepatocytes, antioxidant properties and antiapoptotic effects on liver cells [61,62,63]. Prior evidence on the use of UDCA for treating DILI in children is scarce. In a non-randomized uncontrolled pilot study in 22 patients aged between 4 months and 3 years with anticonvulsant-related hepatotoxicity, UDCA administration was associated with improvement in liver transaminases [64]. However, due to the limited sample size, methodological drawbacks and the piloting nature of the study, these findings should be read as preliminary conclusions, and stress the need for further high-quality studies to support the use of UDCA in children beyond empirical decisions.
DRESS is a complex disorder characterized by the onset of severe dermatological lesions along with hypersensitivity features, and the involvement of an internal organ, frequently the liver [65]. In a recent systematic review including 144 case reports and case series with 354 reported paediatric DRESS cases, nearly 60% of children were treated with systemic corticosteroids, while 13% received intravenous immunoglobulins (IVIg) [66]. In addition, in a case series of 49 well defined DRESS cases (84% with liver injury), corticosteroids (topical for mild cases, and systemic for more severe cases) were the main therapeutic option, whereas IVIg and cyclosporin were used only in a limited number of cases [67]. Overall, these findings are in line with the hypothesis that corticosteroids are the mainstay of treatment for DRESS, despite the lack of high-quality evidence to support its benefit. The use of corticosteroids relies on the role that the immune system plays in the pathophysiology of DILI [68]. Corticosteroids have anti-inflammatory and immunosuppressive properties that are exerted by the upregulation and downregulation of the transcription of anti-inflammatory genes and of genes related to the production of enzymes involved in the initiation or maintenance of the host inflammatory response, respectively, and by blocking the activation of T cells [69]. However, the use of immunomodulant agents such as IVIg for the management of paediatric DRESS is still debatable. While its efficacy as an add-on treatment complementary to corticosteroids has been described in a case series study with seven paediatric patients with severe DRESS [70], the limited sample size and the study design (case series of seven cases enrolled in a unique centre) prevent the drawing of firm conclusions.
The extracorporeal liver support system MARS is a blood detoxification system based on albumin dialysis able to remove albumin-bound and water-soluble substances selectively. It has been applied as a bridge to liver transplantation or recovery of liver function in patients with fulminant hepatic failure [71]. MARS has proven its efficacy in the adult population in the clinical trial setting [72], and prior experiences have suggested the effectiveness of MARS in the management of paediatric ALF by decreasing plasma concentrations of albumin-bound and water-soluble toxins, with a favourable safety profile [73]. Another extracorporeal support system used in the management of DILI in the paediatric population was continuous renal replacement therapy (CRRT). This therapy reduces ammonia levels and controls fluid overload in patients with liver failure [74]. Evidence of the use of CRRT in paediatric DILI patients is scant. Hence, only one study was identified in this review and supported its survival benefits in ALF patients [42]. In 2019, the Pediatric Continuous Renal Replacement Therapy expert panel raised recommendations about the use of renal replacement therapies in paediatric intoxications. For acetaminophen overdoses, the panel recommended the use of haemodialysis, and considered CRRT as an alternative treatment option in severe acetaminophen intoxications [75]. The use of these extracorporeal support systems has yielded some promising findings in the management of paediatric liver failure. However, the limited sample sizes and the heterogeneous study design, jointly with the absence of clinical trials in this specific paediatric population, underlines the need for further clinical trials to assess the efficacy, safety and cost effectiveness balance of these interventions.
Valproic acid is a broad-spectrum antiepileptic medication with well-known hepatotoxic potential [76]. Genetic variations in the polymerase γ gene have been found to predispose to valproic acid-induced hepatic failure [77]. Also, in paediatric patients, the metabolism of valproic acid mediated by CYP2C9 is more relevant than in adults, thus genetic variants might affect the susceptibility to valproic acid hepatotoxicity [78, 79]. One of the mechanisms by which valproic acid exerts its harmful toxic action is the depletion of carnitine stores and the interference with mitochondrial β-oxidation [80,81,82]. Thus, treatment with carnitine supplementation is founded on the deficiencies caused by valproic acid therapy. In the absence of carnitine, valproic acid is predominantly oxidized through the peroxisomal ω-oxidation pathway, which produces toxic metabolites that increase the risk of hepatotoxicity. However, carnitine supplementation promotes the proper metabolism of valproic acid and fatty acids through mitochondrial β-oxidation, which produces relatively non-toxic metabolites [81].
DILI due to valproic acid presents mainly in children under 3 years of age who receive other antiepileptics. In a consensus conference held in 1996, the panel of experts stated recommendations on carnitine dosage (100 mg/kg/day for oral administration). However, as panellists acknowledged, these recommendations were based on anecdotal reports and their own clinical experience, and they pointed out the need for well-designed controlled clinical trials [83]. Nevertheless, up-to-date evidence supporting the use of carnitine still relies on case reports and series [84, 85], and further high-quality evidence remains a compelling need.
Glycyrrhizin (or glycyrrhizic acid), in combination with other compounds (GSH, PPC, and S-adenosylmethionine), was described as the therapeutic intervention for managing idiosyncratic DILI in a Chinese study [39]. Glycyrrhizin is a derivative from a traditional Chinese medicine named liquorice, widely prescribed for the treatment of acute and chronic liver diseases in China and Japan. Based on the evidence of in vitro and in vivo investigations, it has been proposed that glycyrrhizin exerts an anti-oxidative stress activity and poses anti-inflammatory and immunomodulatory properties [86]. The use of liquorice compounds in the setting of clinical trials to test their efficacy in the management of DILI is limited to few studies in the adult population [87, 88]. However, due to methodological flaws found in these trials [89], these preliminary findings should be interpreted with caution. In addition, the evidence supporting the efficacy of the remaining antioxidant compounds in treating DILI is still sparse [90,91,92]. Further studies assessing the synergistic effect of a therapeutic intervention with antioxidant compounds in DILI is warranted.
To date, there is no specific therapy approved for the management of idiosyncratic DILI in adults, while NAC remains as the antidote for APAP [9, 60, 93]. In this systematic review, we highlight that evidence in the paediatric population is even more scarce, and mostly limited to low-quality studies. Indeed, there was heterogeneity in both the DILI definition and treatment regimes. The relatively low incidence of DILI in the paediatric population and the difficulties of conducting high-quality studies calls for the setting up of an international multicentric interdisciplinary network of centres with the aim of establishing a prospective registry to enrol paediatric patients with DILI using a harmonized case definition [94]. This registry would contribute to the performance of high-quality studies based on standardised diagnostic criteria to improve the quality and consistency of research outputs in this specific population.
All included studies have significant limitations in methodological design and risk of bias, most of them were case reports and series, with ‘very low-quality evidence’ using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group [95]. This highlights the main limitation of the systematic review, which is the poor-quality evidence derived from the included studies. Nonetheless, the strengths of this review come from performing an extensive search and following high methodological standards. Therefore, our review demonstrates an urgent case of need for high-quality studies in this field.
5 Conclusions
This systematic review of the therapeutic management of DILI in the paediatric population identified a limited number of DILI in children. Paediatric DILI is usually caused by accidental acetaminophen overdoses, or by antibiotics and anticonvulsants. The most common clinical presentation was a hepatocellular severe liver injury or ALF. Recovery rate after management was high, and only a minor proportion of patients needed a liver transplant or died. Specific interventions in the paediatric population appear to be merely extrapolated from those used in the adult population, albeit they are sustained by low-quality evidence. Therefore, as management of DILI in the paediatric population mainly relies on empirical clinical decisions, future high-quality studies focused on testing the efficacy and safety of known and novel compounds in this specific population are needed.
References
Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5(1):58. https://doi.org/10.1038/s41572-019-0105-0.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. https://doi.org/10.1056/NEJMra035092.
Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395–404. https://doi.org/10.1111/bcp.12267.
van den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2018;58(Suppl 10):S10–25. https://doi.org/10.1002/jcph.1284.
Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, et al. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70(5):721–8. https://doi.org/10.1111/j.1365-2125.2010.03754.x.
Stephens C, Robles-Diaz M, Medina-Caliz I, Garcia-Cortes M, Ortega-Alonso A, Sanabria-Cabrera J, et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J Hepatol. 2021;75(1):86–97. https://doi.org/10.1016/j.jhep.2021.01.029.
Devarbhavi H, Joseph T, Sunil Kumar N, Rathi C, Thomas V, Prasad Singh S, et al. The Indian Network of drug-induced liver injury: etiology, clinical features, outcome and prognostic markers in 1288 patients. J Clin Exp Hepatol. 2021;11(3):288–98. https://doi.org/10.1016/j.jceh.2020.11.002.
Squires RH, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652–8. https://doi.org/10.1016/j.jpeds.2005.12.051.
European Association for the Study of the Liver. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70(6):1222–61. https://doi.org/10.1016/j.jhep.2019.02.014.
Squires JE, McKiernan P, Squires RH. Acute liver failure: an update. Clin Liver Dis. 2018;22(4):773–805. https://doi.org/10.1016/j.cld.2018.06.009.
Chiew AL, Reith D, Pomerleau A, Wong A, Isoardi KZ, Soderstrom J, et al. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust. 2020;212(4):175–83. https://doi.org/10.5694/mja2.50428.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–15. https://doi.org/10.1038/clpt.2011.58.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. JBI manual for evidence synthesis. 2020. https://synthesismanual.jbi.global. Accessed 9 July 2022.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919. https://doi.org/10.1136/bmj.i4919.
European Medicines Agency and Heads of Medicines Agencies. Guideline on good pharmacovigilance practices (GVP). Amsterdam: European Medicines Agency; 2018.
Raghu K, Berry MJ. Acute liver failure secondary to therapeutic paracetamol dosing in an extremely preterm neonate. BMJ Case Rep. 2022;15(5): e245406. https://doi.org/10.1136/bcr-2021-245406.
Walls L, Baker CF, Sarkar S. Acetaminophen-induced hepatic failure with encephalopathy in a newborn. J Perinatol. 2007;27(2):133–5. https://doi.org/10.1038/sj.jp.7211641.
Muñiz AE, Rose SR 2nd, Liner SR, Foster RL. Unsuspected acetaminophen toxicity in a 58-day-old infant. Pediatr Emerg Care. 2004;20(12):824–8. https://doi.org/10.1097/01.pec.0000148032.06964.b0.
Ebenezer K, Agarwal I, Fleming D. Acute hepatic failure in an infant caused by acetaminophen (paracetamol) toxicity. Ann Trop Paediatr. 2008;28(4):301–3. https://doi.org/10.1179/146532808X375495.
Hon KL, Leung AK. Be careful, mom and doc: hepatotoxicity associated with prescribed medications in young infants. Int J Pediatr. 2009;2009: 673269. https://doi.org/10.1155/2009/673269.
Beringer RM, Thompson JP, Parry S, Stoddart PA. Intravenous paracetamol overdose: two case reports and a change to national treatment guidelines. Arch Dis Child. 2011;96(3):307–8. https://doi.org/10.1136/adc.2010.192005.
Savino F, Lupica MM, Tarasco V, Locatelli E, Garazzino S, Tovo PA. Fulminant hepatitis after 10 days of acetaminophen treatment at recommended dosage in an infant. Pediatrics. 2011;127(2):e494–7. https://doi.org/10.1542/peds.2010-1965.
Karaarslan U, Colak M, Topal S, Atakul G, Soydan E, Caglar A, et al. The association between N-acetylcysteine treatment and hepatic healing in patients with non-acetaminophen-induced liver injury in pediatric intensive care: a single-center retrospective study. Arch Pediatr. 2022;29(2):140–4. https://doi.org/10.1016/j.arcped.2021.11.006.
Awasthi P, Jindal A, Sharma Y, Williams V, Ravikumar N, Nallasamy K, et al. Continuous venovenous hemofiltration as a rescue therapy for severe acetaminophen toxicity in a toddler. J Pediatr Intensive Care. 2021;10(2):159–61. https://doi.org/10.1055/s-0040-1712158.
Rumack BH. Acetaminophen overdose in young children. Treatment and effects of alcohol and other additional ingestants in 417 cases. Am J Dis Child. 1984;138(5):428–33. https://doi.org/10.1001/archpedi.1984.02140430006003.
Di Giorgio A, Sonzogni A, Picciche A, Alessio G, Bonanomi E, Colledan M, et al. Successful management of acute liver failure in Italian children: a 16-year experience at a referral centre for paediatric liver transplantation. Dig Liver Dis. 2017;49(10):1139–45. https://doi.org/10.1016/j.dld.2017.05.026.
Brenner M, Zosel A, Stanton M, Gummin D. An unusual case of severe pediatric acetaminophen overdose treated with increased dose N-acetylcysteine. Clin Toxicol. 2019;57(10):997.
Simma B, Meister B, Deutsch J, Sperl W, Fend F, Ofner D, et al. Fulminant hepatic failure in a child as a potential adverse effect of trimethoprim-sulphamethoxazole. Eur J Pediatr. 1995;154(7):530–3. https://doi.org/10.1007/BF02074828.
Chawla A, Kahn E, Yunis EJ, Daum F. Rapidly progressive cholestasis: an unusual reaction to amoxicillin/clavulanate acid therapy in a child. J Pediatr. 2000;136:121–3. https://doi.org/10.1016/s0022-3476(00)90064-7.
Cho HJ, Jwa HJ, Kim KS, Gang DY, Kim JY. Urosodeoxycholic acid therapy in a child with trimethoprim-sulfamethoxazole-induced vanishing bile duct syndrome. Pediatr Gastroenterol Hepatol Nutr. 2013;16(4):273–8. https://doi.org/10.5223/pghn.2013.16.4.273.
Hubiche T, Milpied B, Cazeau C, Taieb A, Leaute-Labreze C. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222(2):140–1. https://doi.org/10.1159/000324506.
Ahluwalia J, Abuabara K, Perman MJ, Yan AC. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172(4):1090–5. https://doi.org/10.1111/bjd.13512.
Maarouf M, Wickenheiser M, Krase JM, Wolter S, Shi VY. Trimethoprim-sulfamethoxazole-induced drug reaction with eosinophilia and systemic symptoms in a child with congenital renal disease. Pediatr Dermatol. 2018;35(6):e391–2. https://doi.org/10.1111/pde.13607.
Novelli G, Rossi M, Morabito V, Pugliese F, Ruberto F, Perrella SM, et al. Pediatric acute liver failure with molecular adsorbent recirculating system treatment. Transpl Proc. 2008;40(6):1921–4. https://doi.org/10.1016/j.transproceed.2008.05.075.
Lexmond WS, Van Dael CM, Scheenstra R, Goorhuis JF, Sieders E, Verkade HJ, et al. Experience with molecular adsorbent recirculating system treatment in 20 children listed for high-urgency liver transplantation. Liver Transpl. 2015;21(3):369–80. https://doi.org/10.1002/lt.24037.
Scheffner D, Konig S, Rauterberg-Ruland I, Kochen W, Hofmann WJ, Unkelbach S. Fatal liver failure in 16 children with valproate therapy. Epilepsia. 1988;29(5):530–42. https://doi.org/10.1111/j.1528-1157.1988.tb03757.x.
Wang SZ, Gao S, Liu YM, Huang YL, Chen YS, Wang XX, et al. Clinical characteristics of drug-induced liver injury in 31 pediatric cases. Chin J Hepatol. 2012;20(3):193–5. https://doi.org/10.3760/cma.j.issn.1007-3418.2012.03.011.
Epperson LC, Weiss ST, Cao DJ. A case report of a severe, unusually delayed anaphylactoid reaction to intravenous N-acetylcysteine during treatment of acute acetaminophen toxicity in an adolescent. J Med Toxicol. 2021;17(1):75–9. https://doi.org/10.1007/s13181-020-00804-5.
Ng CT, Tan CK, Oh CC, Chang JP. Successful extracorporeal liver dialysis for the treatment of trimethoprim-sulfamethoxazole-induced fulminant hepatic failure. Singap Med J. 2013;54(5):e113–6. https://doi.org/10.11622/smedj.2013067.
Deep A, Stewart CE, Dhawan A, Douiri A. Effect of continuous renal replacement therapy on outcome in pediatric acute liver failure. Crit Care Med. 2016;44(10):1910–9. https://doi.org/10.1097/CCM.0000000000001826.
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156(3):609–11. https://doi.org/10.1111/j.1365-2133.2006.07704.x.
O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–45. https://doi.org/10.1016/0016-5085(89)90081-4.
Bernuau J, Goudeau A, Poynard T, Dubois F, Lesage G, Yvonnet B, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986;6(4):648–51. https://doi.org/10.1002/hep.1840060417.
DiPaola F, Molleston JP, Gu J, Cirulli ET, Chalasani N, Barnhart H, et al. Antimicrobials and antiepileptics are the leading causes of idiosyncratic drug-induced liver injury in American children. J Pediatr Gastroenterol Nutr. 2019;69(2):152–9. https://doi.org/10.1097/MPG.0000000000002383.
Ocete Hita E, Martin Garcia JA, Gimenez Sanchez F, Flores Gonzalez JC, Abril Molina A, Salmeron Escobar J, et al. Hepatotoxicity due to drugs or natural products in children. An Pediatr (Barc). 2013;78(4):248–59. https://doi.org/10.1016/j.anpedi.2012.06.012.
Devarbhavi H, Karanth D, Prasanna KS, Adarsh CK, Patil M. Drug-Induced liver injury with hypersensitivity features has a better outcome: a single-center experience of 39 children and adolescents. Hepatology. 2011;54(4):1344–50. https://doi.org/10.1002/hep.24527.
Zhu Y, Li YG, Wang JB, Liu SH, Wang LF, Zhao YL, et al. Causes, features, and outcomes of drug-induced liver injury in 69 children from China. Gut Liver. 2015;9(4):525–33. https://doi.org/10.5009/gnl14184.
Cuzzolin L, Antonucci R, Fanos V. Paracetamol (acetaminophen) efficacy and safety in the newborn. Curr Drug Metab. 2013;14(2):178–85. https://doi.org/10.2174/1389200211314020005.
Caravati EM. Unintentional acetaminophen ingestion in children and the potential for hepatotoxicity. J Toxicol Clin Toxicol. 2000;38(3):291–6. https://doi.org/10.1081/clt-100100934.
Bjorkman S. Prediction of cytochrome p450-mediated hepatic drug clearance in neonates, infants and children: how accurate are available scaling methods? Clin Pharmacokinet. 2006;45(1):1–11. https://doi.org/10.2165/00003088-200645010-00001.
Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–67. https://doi.org/10.1016/j.pharmthera.2008.02.005.
Pacifici GM, Allegaert K. Clinical pharmacology of paracetamol in neonates: a review. Curr Ther Res Clin Exp. 2015;77:24–30. https://doi.org/10.1016/j.curtheres.2014.12.001.
van Ganzewinkel C, Derijks L, Anand KJ, van Lingen RA, Neef C, Kramer BW, et al. Multiple intravenous doses of paracetamol result in a predictable pharmacokinetic profile in very preterm infants. Acta Paediatr. 2014;103(6):612–7. https://doi.org/10.1111/apa.12638.
Mazaleuskaya LL, Sangkuhl K, Thorn CF, FitzGerald GA, Altman RB, Klein TE. PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genom. 2015;25(8):416–26. https://doi.org/10.1097/FPC.0000000000000150.
Jaeschke H, Akakpo JY, Umbaugh DS, Ramachandran A. Novel therapeutic approaches against acetaminophen-induced liver injury and acute liver failure. Toxicol Sci. 2020;174(2):159–67. https://doi.org/10.1093/toxsci/kfaa002.
Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856–64, 64 e1. https://doi.org/10.1053/j.gastro.2009.06.006.
Squires RH, Dhawan A, Alonso E, Narkewicz MR, Shneider BL, Rodriguez-Baez N, et al. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a placebo-controlled clinical trial. Hepatology. 2013;57(4):1542–9. https://doi.org/10.1002/hep.26001.
Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR, Practice parameters committee of the American College of Gastroenterology. ACG clinical guideline: diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2021;116(5):878–98. https://doi.org/10.14309/ajg.0000000000001259.
Yoshikawa M, Tsujii T, Matsumura K, Yamao J, Matsumura Y, Kubo R, et al. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16(2):358–64. https://doi.org/10.1002/hep.1840160213.
Lapenna D, Ciofani G, Festi D, Neri M, Pierdomenico SD, Giamberardino MA, et al. Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol. 2002;64(11):1661–7. https://doi.org/10.1016/S0006-2952(02)01391-6.
Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36(3):525–31. https://doi.org/10.1053/jhep.2002.36088.
Asgarshirazi M, Shariat M, Dalili H, Keihanidoost Z. Ursodeoxycholic acid can improve liver transaminase quantities in children with anticonvulsant drugs hepatotoxicity: a pilot study. Acta Med Iran. 2015;53:351–5.
Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996;15(4):250–7. https://doi.org/10.1016/s1085-5629(96)80038-1.
Afiouni R, Zeinaty P, Kechichian E, Zoghaib S, Matar S, Helou-Mallat J, et al. Pediatric drug reaction with eosinophilia and systemic symptoms: a systematic review of the literature, with a focus on relapsing cases. Pediatr Dermatol. 2021;38(1):125–31. https://doi.org/10.1111/pde.14446.
Bedouelle E, Ben Said B, Tetart F, Milpied B, Welfringer-Morin A, Maruani A, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): series of 49 French pediatric cases. J Allergy Clin Immunol Pract. 2022;10(1):267–74 e5. https://doi.org/10.1016/j.jaip.2021.07.025.
Cueto-Sanchez A, Niu H, Del Campo-Herrera E, Robles-Diaz M, Sanabria-Cabrera J, Ortega-Alonso A, et al. Lymphocyte profile and immune checkpoint expression in drug-induced liver injury: an immunophenotyping study. Clin Pharmacol Ther. 2021;110(6):1604–12. https://doi.org/10.1002/cpt.2423.
Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. https://doi.org/10.1186/1710-1492-9-30.
Marcus N, Smuel K, Almog M, Prais D, Straussberg R, Landau D, et al. Successful intravenous immunoglobulin treatment in pediatric severe DRESS syndrome. J Allergy Clin Immunol Pract. 2018;6(4):1238–42. https://doi.org/10.1016/j.jaip.2017.10.016.
Boyle M, Kurtovic J, Bihari D, Riordan S, Steiner C. Equipment review: the molecular adsorbents recirculating system (MARS). Crit Care. 2004;8(4):280–6. https://doi.org/10.1186/cc2895.
He GL, Feng L, Duan CY, Hu X, Zhou CJ, Cheng Y, et al. Meta-analysis of survival with the molecular adsorbent recirculating system for liver failure. Int J Clin Exp Med. 2015;8(10):17046–54.
Quintero Bernabeu J, Ortega Lopez J, Juamperez Goni J, Julio Tatis E, Mercadal-Hally M, Bilbao Aguirre I, et al. The role of molecular adsorbent recirculating system in pediatric acute liver failure. Liver Transpl. 2018;24(2):308–10. https://doi.org/10.1002/lt.24966.
Zoica BS, Deep A. Extracorporeal renal and liver support in pediatric acute liver failure. Pediatr Nephrol. 2021;36(5):1119–28. https://doi.org/10.1007/s00467-020-04613-4.
Raina R, Grewal MK, Blackford M, Symons JM, Somers MJG, Licht C, et al. Renal replacement therapy in the management of intoxications in children: recommendations from the Pediatric Continuous Renal Replacement Therapy (PCRRT) workgroup. Pediatr Nephrol. 2019;34(11):2427–48. https://doi.org/10.1007/s00467-019-04319-2.
Valproate. LiverTox: clinical and research information on drug-induced liver injury. 2020. https://www.ncbi.nlm.nih.gov/books/NBK548284/. Accessed 16 July 2022.
Stewart JD, Horvath R, Baruffini E, Ferrero I, Bulst S, Watkins PB, et al. Polymerase gamma gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52(5):1791–6. https://doi.org/10.1002/hep.23891.
Budi T, Toth K, Nagy A, Szever Z, Kiss A, Temesvari M, et al. Clinical significance of CYP2C9-status guided valproic acid therapy in children. Epilepsia. 2015;56(6):849–55. https://doi.org/10.1111/epi.13011.
Zhu MM, Li HL, Shi LH, Chen XP, Luo J, Zhang ZL. The pharmacogenomics of valproic acid. J Hum Genet. 2017;62(12):1009–14. https://doi.org/10.1038/jhg.2017.91.
Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opin Pediatr. 2007;19(2):206–10. https://doi.org/10.1097/MOP.0b013e32805e879a.
Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101–11. https://doi.org/10.1080/15563650902752376.
Price KE, Pearce RE, Garg UC, Heese BA, Smith LD, Sullivan JE, et al. Effects of valproic acid on organic acid metabolism in children: a metabolic profiling study. Clin Pharmacol Ther. 2011;89(6):867–74. https://doi.org/10.1038/clpt.2011.47.
De Vivo DC, Bohan TP, Coulter DL, Dreifuss FE, Greenwood RS, Nordli DR, et al. L-carnitine supplementation in childhood epilepsy: current perspectives. Epilepsia. 1998;39(11):1216–25. https://doi.org/10.1111/j.1528-1157.1998.tb01315.x.
Chan YC, Tse ML, Lau FL. Two cases of valproic acid poisoning treated with L-carnitine. Hum Exp Toxicol. 2007;26(12):967–9. https://doi.org/10.1177/0960327107087799.
Glatstein M, Bonifacio Rino P, de Pinho S, Scolnik D, Pivko-Levi D, Hoyte C. Levocarnitine for the treatment of valproic acid-induced hyperammonemic encephalopathy in children: the experience of a large, tertiary care pediatric hospital and a poison center. Am J Ther. 2019;26(3):e344–9. https://doi.org/10.1097/MJT.0000000000000706.
Li X, Sun R, Liu R. Natural products in licorice for the therapy of liver diseases: progress and future opportunities. Pharmacol Res. 2019;144:210–26. https://doi.org/10.1016/j.phrs.2019.04.025.
Tang LN, Lin F, Sen Z, Sun YY, Yao Y. Magnesium isoglycyrrhizinate used in the treatment of chemotherapeutic drugs-induced acute liver dysfunction: a phase III clinical trial. TUMOR. 2012;738–43.
Wang Y, Wang Z, Gao M, Zhong H, Chen C, Yao Y, et al. Efficacy and safety of magnesium isoglycyrrhizinate injection in patients with acute drug-induced liver injury: a phase II trial. Liver Int. 2019;39(11):2102–11. https://doi.org/10.1111/liv.14204.
Niu H, Sanabria-Cabrera J, Alvarez-Alvarez I, Robles-Diaz M, Stankeviciute S, Aithal GP, et al. Prevention and management of idiosyncratic drug-induced liver injury: systematic review and meta-analysis of randomised clinical trials. Pharmacol Res. 2021;164: 105404. https://doi.org/10.1016/j.phrs.2020.105404.
Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol. 2013;60:38–44. https://doi.org/10.1016/j.fct.2013.07.008.
Lei X, Zhang J, Xu Q, Li J, Qian Y, Zhang J, et al. Exploring the efficacy and safety of polyene phosphatidylcholine for treatment of drug-induced liver injury using the Roussel Uclaf causality assessment method: a propensity score matching comparison. J Int Med Res. 2021;49(8):3000605211039810. https://doi.org/10.1177/03000605211039810.
Zhu SS, Dong Y, Gan Y, Tang HM, Xu ZQ, Chen DW, et al. Efficacy and safety of ademetionine for treatment of drug-induced liver disease in children. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2010;24(2):136–8.
Licata A, Minissale MG, Stankevičiūtė S, Sanabria-Cabrera J, Lucena MI, Andrade RJ, et al. N-Acetylcysteine for preventing acetaminophen-induced liver injury: a comprehensive review. Front Pharmacol. 2022;13: 828565. https://doi.org/10.3389/fphar.2022.828565.
Peire MA, Lucena MI, Ruiz-Extremera A, Jara P, Romero-Gonzalez J, Andrade RJ. Drug-induced hepatotoxicity in children. Where we are and where we are going. An Esp Pediatr. 2002;56(5):434–42.
Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 12 July 2022.
Acknowledgements
We acknowledge the authors contacted for their generous help by providing information for this study. This article is based upon work from COST Action "CA17112 - Prospective European Drug-Induced Liver Injury Network", supported by COST (European Cooperation in Science and Technology). www.cost.eu
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The present study has been supported by grants from Instituto de Salud Carlos III (ISCIII) cofounded by Fondo Europeo de Desarrollo Regional-FEDER (contract numbers: PI21/01248; PT20/000127), Consejería de Salud de Junta de Andalucía (contract number: PI-0310-2018), and Agencia Española del Medicamento. CIBERehd and Plataforma ISCIII Ensayos Clínicos are funded by ISCIII. HN holds a postdoctoral research contract funded by Junta de Andalucia (POSTDOC_21_00780). I.A.-A. holds a Sara Borrell contract funded by ISCIII (CD20/00083). This article/publication is based upon work from COST Action "CA17112 - Prospective European Drug-Induced Liver Injury Network", supported by COST (European Cooperation in Science and Technology) www.cost.eu. The funding sources have no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable. No ethical approval was needed because data were retrieved from previous published studies in which informed consent was obtained by the primary investigators.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material (data transparency)
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Author’s contribution
HN (conceptualization, data curation, writing—original draft); EA (conceptualization, data curation, writing—original draft); IA-A (conceptualization, data curation, writing—original draft); IM-C (writing—review and editing); GPA (writing—review and editing); CA (writing—review and editing), RJA (conceptualization, writing—review and editing); MIL (conceptualization, writing—review and editing). All authors have read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Niu, H., Atallah, E., Alvarez-Alvarez, I. et al. Therapeutic Management of Idiosyncratic Drug-Induced Liver Injury and Acetaminophen Hepatotoxicity in the Paediatric Population: A Systematic Review. Drug Saf 45, 1329–1348 (2022). https://doi.org/10.1007/s40264-022-01224-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01224-w