Abstract
Introduction
Concerns over serious respiratory depression in children led to two European Union (EU) referral procedures (in 2013 and 2015) to review the benefit–risk balance of codeine in this population when used for pain relief, cough or cold. Consequently, codeine should no longer be used in children aged < 12 years and restrictions were introduced for treatment in children ≥ 12 years.
Objective
This multinational collaborative study aimed to assess the effectiveness of these risk minimisation measures by evaluating changes in prescribing of codeine and alternative treatments.
Method
Children under 12 and 12–18 years old were followed between 2010 and 2017 to analyse quarterly trends in prescribing of codeine and alternative treatments in electronic health records from France, Germany, Norway, Spain and the United Kingdom using interrupted time series analysis.
Results
Overall prescribing of codeine in children decreased in all five countries, reaching near zero prevalence in children under 12 years of age. This was accompanied by an increase in use of other opioid analgesics in France (from 0.15 to 0.56 prevalence per 100 person-years immediately after the first referral), Norway (from 0.0006 to 0.0013 at the end of the study), the United Kingdom (from 0.018 to 0.05 at the end of the study), and an increase in non-opioid analgesics in Norway (from 0.045 to 0.075 at the end of the study) after the referral on pain relief indication. The referral on cough/cold indication led to a decrease in use of opioid and non-opioid antitussives in children aged < 12 years in France (from 10 to 7 and 20 to 16, respectively) and had no impact in other countries. Overall prescribing trends for codeine and alternatives were similar across both age groups within each country.
Conclusion
The decrease in use of codeine shows that healthcare professionals followed the adopted measures and switched prescribing practices for pain management in children aged < 18 years towards opioid or non-opioid analgesics depending on national clinical and reimbursement settings. Whist the magnitude of the first referral on pain differed between countries, the second referral on cough/cold had only a minimal impact on the use of codeine and antitussives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reports of serious and sometimes fatal respiratory depression in children associated with codeine when used for pain relief, cough or cold led to the imposition of risk minimisation measures in the European Economic Area through two European Union referrals in 2013 and 2015, including contraindications and restrictions of use of this substance in particular in children under 12 years of age. |
This study aimed at analysing prescribing patterns of codeine and alternative medicines in children in France, Germany, Norway, Spain and the United Kingdom before and after the referrals using interrupted time series analysis. |
Whilst the use of codeine in children dropped in the five countries until reaching near zero prevalence by the end of the study period, an increase in prescribing of certain alternative analgesics for pain management could be observed in some participating countries, revealing a heterogenous impact of the risk minimisation measures linked to different national clinical and reimbursement practices. |
1 Introduction
Codeine-containing products have been authorised nationally in Europe for decades (the European Union reference date for codeine is September 1954 [1]) for the management of pain in adults and children, and in some countries for cough, either on prescription or over the counter. The substance is commonly combined with other analgesics such as non-steroidal anti-inflammatory drugs and non-opioid analgesics with the aim of increasing the analgesic effect due to the different modes of action of the individual drugs. The pharmacological effect of codeine on pain is due to its conversion into morphine by an enzyme called CYP2D6 [2].
In November 2007, the Medicines and Healthcare products Regulatory Agency (MHRA) issued a Drug Safety Update on the very rare risk of side effects in breastfed babies from maternal ingestion of codeine, following a report of respiratory depression resulting in death in a breastfed newborn whose mother was a CYP2D6 ultra-rapid metaboliser [3]. In August 2012 based on further reports associated with the substance, the United States (US) Food and Drug Administration (FDA) published a Drug Safety Communication warning stating that codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare but life-threatening adverse events or death [4,5,6]. The concerned children had evidence of being 'CYP2D6 ultra-rapid metabolisers', meaning that codeine is converted into morphine in their body at a faster rate than normal, resulting in high levels of morphine in the blood that can cause toxic effects such as breathing difficulties. As a result, the US FDA imposed a boxed warning on the packaging and a contraindication of US products against the use of codeine for the post-operative pain management in children after tonsillectomy or adenoidectomy, regardless of the metabolic status.
In light of the above, the Pharmacovigilance Risk Assessment Committee (PRAC) [7] decided to thoroughly evaluate the issue of serious opioid toxicity in order to determine whether any risk minimisation measures (RMM) should be introduced in the European Economic Area (EEA) to ensure safe use of codeine consistently across its countries. In October 2012, a referral under Article 31 of Directive 2001/83/EC [8] was initiated to review the benefit–risk balance of codeine-containing medicinal products (including combination products) indicated in the management of pain in children. The PRAC issued its conclusions in June 2013 based on all available evidence including pharmacokinetic data, clinical studies, post-marketing data and published literature, which were agreed upon by the Coordination Group for Mutual Recognition and Decentralised procedures Human (CMDh) [9]. Although morphine-induced side effects may occur at all ages, the current evidence suggested that children aged < 12 years were at special risk of life-threatening respiratory depression with codeine due to their reduced ability to metabolise codeine. Moreover, the efficacy of codeine for pain relief in children was not superior to that of other analgesics, such as non-steroidal anti-inflammatory drugs and non-opioid analgesics, although it was considered that codeine still had a place in the treatment of acute pain in the paediatric population. To address their concerns over the risks, the PRAC and CMDh recommended the following legally binding RMM to be implemented at the national level in all EEA countries: (i) contraindications in all paediatric patients (0–18 years of age) who undergo tonsillectomy or adenoidectomy for obstructive sleep apnoea syndrome, in children whom respiratory function might be compromised, and those of any age who are known to be CYP2D6 ultra-rapid metabolisers, (ii) restrictions of use for the treatment of acute moderate pain only in patients >12 years of age who cannot be relieved by other analgesics such as paracetamol or ibuprofen (alone) and (iii) codeine should be used at the lowest effective dose for the shortest period of time. A Public Health Communication in lay language was published on the EMA website together with the PRAC assessment report, PRAC recommendation, CMDh decision and the relevant annexes including the details of the required amendments to the Summary of Product Characteristics (SmPC) of codeine-containing products, list of products impacted and the timetable for the implementation of the agreed amendments at the national level [10]. The Public Health Communication was translated in all EEA languages and subsequently published on the websites of the National Competent Authorities in line with the Guideline on Good Pharmacovigilance Practices (GVP) Module XV on Safety communication [11] to ensure consistent messages are addressed to the public in a timely manner and in the official languages of the countries where the medicinal products are placed on the market.
As similar risks could apply to the use of codeine for cough and cold in children, a second EEA-wide review was launched in April 2014 [12]. In total, 14 cases of codeine intoxication in children (aged from 17 days to 6 years) related to the treatment of cough and respiratory infection were identified in the published literature, four of which had a fatal outcome. As a consequence, in April 2015, codeine for cough and cold was contraindicated by the PRAC and CMDh in children aged < 12 years, and not recommended in children aged between 12 and 18 years with compromised respiratory function and/or considered CYP2D6 ultra-rapid metabolisers. These restrictions were largely in line with the previous recommendations for codeine when used for pain relief and a similar public health communication was issued. The changes applied to the SmPC of codeine-containing products following both referrals can be found in Table A of the electronic supplementary material (ESM).
To assess the effectiveness of these RMM, a collaborative study was performed between the European Medicines Agency (EMA) and three National Competent Authorities (NCAs) (Agence Nationale de securité du médicament et des produits de santé [ANSM] in France, Statens Legemiddelverket [NOMA] in Norway and Agencia Española de Medicamentos y Productos Sanitarios [AEMPS] in Spain). The objectives were to (i) describe the use of codeine, alternative analgesics and antitussives between 2010 and 2017 (included) in patients below 18 years of age in the five participating countries and (ii) assess whether the two referrals were associated with any changes in the use of alternative treatments in this patient population.
2 Methodology
2.1 Population
This collaborative drug utilisation study aimed to describe quarterly prescription trends in France, Germany, Norway, Spain and the United Kingdom (UK) based on electronic health records from the Système National des Données de Santé (SNDS) [13], IMS® Disease Analyzer Germany (GP practices and paediatric practices) [14, 15], Norwegian prescription database (NorPD) [16], Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP) [17] and IQVIA Medical Research Data (IMRD-UK) [18], respectively. These databases are further described in Table B of the ESM.
The study period covered January 2010 to December 2017. The population consisted of children under the age of 18 years, stratified between those aged < 12 years and those 12–18 years of age exposed to codeine, alternative analgesics or antitussives as recorded in the databases.
An overview of the different treatments’ indications in the participating countries is provided in Table C of the ESM.
2.2 Exposure
The following exposure groups were defined in each database: (i) codeine-containing products; (ii) opioid antitussives other than codeine; (iii) opioid analgesics other than codeine; (iv) non-opioid analgesics and (v) non-opioid antitussives. Table D of the ESM provides further details on the composition of the exposure groups and related WHO ATC classification [19].
2.3 Data Management and Analysis
A common protocol, data extraction plan and table shells were developed and agreed by the participating analysts with knowledge of the respective databases to ensure a consistent approach in the data extraction and calculation of exposure measures [20].
The aggregate table shells were completed by the participating analysts from ANSM for SNDS, NOMA for NorPD, AEMPS for BIFAP and EMA for IMS® Disease Analyzer Germany and IMRD. After validation, the information was sent to EMA for analysis at a central level.
The quarterly prevalence was calculated as the number of children with a prescription for a drug of interest (patient level) in a specific calendar quarter per 100 person-years. Children contributed to the denominator if they were eligible to receive a prescription at any time between the first and last day of the period, therefore observable at any time in the database. The same analyses were performed at prescription level. However, since the results were in line with the analyses at the patient level, only the latter are described in this paper.
An interrupted time series (ITS) regression analysis [21, 22] was performed to assess whether the regulatory actions taken as a result of the codeine referrals in June 2013 (on pain relief indication, referred to as ‘first referral’) and in April 2015 (cough or cold indication, referred to as ‘second referral’) were associated with statistically significant changes in the use of these treatments. Models were adjusted for autocorrelation (i.e. seasonality) [23] if identified with the Durban-Watson statistic. Model selection was assessed through a visual inspection of the white noise and Inverse Autocorrelation Function (IACF) plots.
Norwegian data were omitted from the analysis on changes to antitussives prescribing after the second referral as no codeine-containing products were authorised for cough or cold in Norway.
2.4 Outcomes
The following coefficients were estimated from the ITS models: (i) coefficient β1 indicating the pre-referral slope and interpreted as the change in outcome associated with a time unit increase, (ii) the change in level from pre- to post-referral (β2) and (iii) the change in slope from pre- to post-referral (β3). The post-interruption slope can be determined by summing coefficients β1 and β3 with statistical significance obtained using post-estimation procedures [24]. Level of significance was set at p < 0.05. All analyses were performed using SAS.
The results presented in section 3 highlight the statistically significant changes in the use of codeine and its alternative medicines during the study period (i.e. p value < 0.05 in Tables 1, 2, 3, 4, 56 and Tables 1–6 of the ESM). All prevalence data are expressed per 100 person-years.
2.5 Ethical Approval
Upon agreement of the common protocol, the analysts requested ethical approval when applicable. The use of BIFAP data for this project was approved by the Scientific Committee of BIFAP (protocol reference: 10/2019) and an Ethics Committee (CEIM regional de la Comunidad de Madrid: aprobación 08-06-2020, acta CEIm 06/20). The use of IMRD-UK was approved by the Scientific Review Committee under the reference number 19THIN080.
3 Results
3.1 Overall Trends in Codeine Prescribing in Children Below 12 Years of Age
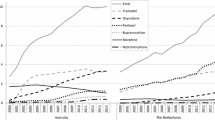
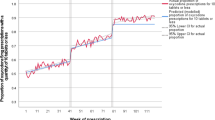
A decreasing trend in the prevalence rate in children was visible in Spain and the UK prior to the first referral (from 4.63 to close to 0 and 0.26 to 0.15, respectively). Despite a slight increase pre-referral in France (from 2.84 to 3.82), the prevalence rate dropped immediately afterwards in this country (− 3.15) and in Norway (− 0.017) (Fig. 1, Table 1). A decrease in post-referral trend was then continuously observed, except in Spain and the UK, reaching near zero use by the time of the second referral in the five countries (Fig. 2, Table 2). A slight increase in trend could be seen in the UK post-second referral.
3.2 Overall Trends in Alternatives to Codeine Prescribing in Children Below 12 Years of Age
For other opioid analgesics, there was an immediate increase in use after the first referral in France (from 0.15 to 0.56), and a trend increase in Norway (from 0.0006 to 0.0013) and the UK (from 0.018 to 0.05). For non-opioid analgesics, an immediate decrease in use was visible in Germany, whereas a trend decrease appeared in France and the UK, and a trend increase in Norway (from 0.045 to 0.075) (Figs. 3a, b and 4, Tables 3, 4).
Quarterly (n = 32) trends for alternative opioid medicines for treatment of pain in children < 12 years of age in France (a), Norway (b), Germany (c), Spain (d) and the United Kingdom (e) between 1 January 2010 and 31 December 2017, in relation to the first referral on pain relief indication (June 2013)
Quarterly (n = 32) trends for alternative non-opioid medicines for treatment of pain in children < 12 years of age in France (a), Norway (b), Germany (c), Spain (d) and the United Kingdom (e) between 1 January 2010 and 31 December 2017, in relation to the first referral on pain relief indication (June 2013)
As shown in Table E of the ESM, tramadol (including in combination with non-opioids products) was the most prescribed alternative opioid analgesic across all countries, while prescribed ibuprofen (Norway, Germany and Spain) and paracetamol (France and the UK) appeared to stand out as non-opioid analgesics.
Following the second referral, an immediate increase in use of opioid and non-opioid antitussives in France changed into a decrease in trends over time (from 10 to 7 and from 20 to 16, respectively). No changes were visible in the three other countries immediately nor later post-second referral (only an increasing trend of opioid antitussives in Germany which remained extremely close zero use) (Figs. 5, 6, Tables 5, 6).
Quarterly (n = 32) trends for alternative opioid medicines for treatment of cough or cold in children < 12 years of age in France (a), Germany (b), Spain (c) and the United Kingdom (d) between 1 January 2010 and 31 December 2017, in relation to the second referral on cough or cold indication (April 2015)
Quarterly (n = 32) trends for alternative non-opioid medicines for treatment of cough or cold in children < 12 years of age in France (a), Germany (b), Spain (c) and the United Kingdom (d) between 1 January 2010 and 31 December 2017, in relation to the second referral on cough or cold indication (April 2015)
3.3 Overall Trends in Children Aged 12 Years and Above
Codeine prescribing trends in children aged ≥ 12 years in relation to the first referral were rather similar to those for children aged < 12 years, with a decrease in use in France, Norway and the UK (Fig. 1, Table 1 of the ESM). A decreasing trend was visible just before the second referral in Germany, Spain and the UK, but only perdured after the second referral in France and the UK (Fig. 4, Table 4 of the ESM).
The overall prescribing trends for both opioid and non-opioid alternatives were similar across both age groups within each participating country over time (Figs. 2, 3, 5, 6 and Tables 2, 3, 5, 6 of the ESM).
4 Discussion
This study was conducted to assess changes in prescribing of codeine and alternative medicines authorised for pain relief and cough or cold indications in children following restrictions on use of codeine imposed at the EU level. The results are in line with another European multicentre drug utilisation study on the impact of the first referral on prescribing of codeine for pain in children [25]. The present study, however, goes further as it looks at a longer period including the second referral on cough or cold indication and extends the drug utilisation analysis to alternative analgesics and antitussives.
4.1 Observations on Prevalence of Use and Prescribing Trends Across Age Groups and Countries
Whilst the use of codeine decreased throughout the study period in the five participating countries until reaching extremely low (or no) use in children aged < 12 years, the decrease could be observed prior to the first referral in Spain and the UK. At the end of the study period, an extremely small increase could be seen in the UK. However this is qualified as minimal considering the overly low prevalence.
The use remained higher in children aged 12 years and above, and was highly variable between countries, hinting at different national clinical practices. For example, in December 2017 the prevalence rate was 0.11 per 100 person-years in Norway compared with 6.14 in Spain. In line with the use of codeine, the use of alternative opioid analgesics remained higher in older children, while the use of non-opioid analgesics was higher in children aged < 12 years throughout the study period in all countries. The use of non-opioid alternatives remained overall higher than opioid alternatives in both age groups in most countries (apart for antitussives in Spain and in children aged ≥ 12 years in France and the UK).
Important differences in patterns of use of alternative treatments could be observed between the two age groups and between countries. In France, for example, the prevalence rate for non-opioid analgesics at the end of the study was 140 in children aged < 12 years and 80.75 in children aged ≥ 12 years, while in Norway, the rate was < 1 in both age groups. Similar observations hold for the other alternative medicines analysed in this study.
4.2 Switching to Alternative Treatments
Changes to prescribing trends of alternative medicines following the referrals led to assumptions on switching patterns towards the use of opioid analgesics in France, Norway and the UK, and towards the use of non-opioid analgesics in Norway for pain management in children under 12 years of age. As the study did not aim to assess the prescribing trends at an individual substance level, it is difficult to point out which of the substances were prescribed instead of codeine. However, as shown in Table E of the ESM, tramadol (including in combination with non-opioids products) was the most prescribed alternative opioid analgesic used across all countries. As for non-opioid analgesics, ibuprofen was the most prescribed in Norway, Germany and Spain, while paracetamol was the most prescribed in France and the UK. Considering that the overall use of alternative opioid antitussives decreased or flattened over time after the second referral in line with the use of codeine, it can be assumed that no switching towards these substances occurred, however this was not tested.
4.3 Contextualisation of the Results
The variability in prescribing trends of alternative medicines across countries and across the two age groups might have been influenced by national reimbursement status and/or clinical guidelines or recommendations. In France, marketing of the only plain codeine-containing product authorised for pain relief in children (Codenfan®) was ceased by the Marketing Authorisation Holder (MAH) in 2013, justifying the immediate drop in use of codeine-containing products after the first referral. This was associated with an increase in use of other opioid analgesics such as tramadol (from 3 years of age) or even morphine according to certain conditions as recommended by French guidelines for the management of intense pain in children [26, 27].
In Spain, a change in national reimbursement status became effective on September 1, 2012 [28] for certain medicinal products indicated in a wide range of minor symptoms like cough or cold. Most of the products excluded from reimbursement concerned those categorised in groups 1 (codeine-containing products), 2 (opioid antitussives) and 5 (non-opioid antitussives) (Table D of the ESM). Consequently, prescriptions in these groups dropped by 64%, 93% and 89%, respectively.
In the UK, codeine and antitussives use started to decrease before 2013 while a steep increase was visible for opioid analgesics in children aged < 12 years after the pain referral. It can be assumed that patients were switched from codeine to these medications. This behaviour might have been triggered by the earlier warning of the harm of codeine-containing products in 2009 [3]. In addition, back in 2010, the UK Commission on Human Medicines advised that over-the-counter (OTC) liquid medicines containing codeine should not be used for cough suppression in people aged < 18 years [29], which might have also contributed to the early decrease in use of these products.
5 Opportunities and Limitations
5.1 Opportunities
Three NCAs participated with EMA in the study. The whole process worked well thanks to very good interactions between the parties and strong expertise regarding the characteristics of the national datasets. The common data extraction plan was consistently used by the participants, allowing the data to be retrieved in a structured format, and simplifying data pooling and analysis centrally by EMA.
5.2 Limitations
The impact of the two referrals is difficult to compare across countries due to different baseline prescriptions prevalence.
As the study was performed in prescription and dispensation databases, the results do not take account of OTC medications intake, when these can be sold directly to a consumer without a prescription from a healthcare professional, which can be substantial for these indications. The OTC status is regulated at a national level, and can therefore vary across countries [30]. The use of non-opioid medicinal products is as a consequence most probably underestimated, and the decrease in the use of codeine could have led to an increase in use of OTC non-opioid medicines that cannot be measured in this study.
The specific indications for which codeine was prescribed were not considered in the analysis, nor was the prevalence of use of individual alternative substances. It is therefore difficult to ascertain which substances were precisely used instead of codeine.
Whilst Annex IV of each of the referrals’ documents [10, 12] provide a timetable for the language translations of the regulatory agreements and for the subsequent submission of the appropriate variations by the MAHs to NCAs, the time to implementation of the regulatory actions may vary between countries. This depends on various aspects, including the type of variation submitted for each codeine-containing product based on the changes to the SmPC required [31], on the time needed for the relevant authorities to integrate the measures into local prescribing policies, and on varying levels of awareness and communication of the underlying clinical issue prior to and after the referrals. These could all act to confuse any trends in the time series.
Most data sources have some form of blinding of dates of birth, typically rounding off to the nearest month, 6 months or year. This will lead to the misclassification of age for some subjects at some time points and in turn will lead to some 11-year-old children being classified as aged 12–18 years and vice-versa. If prescribing varies with age, such misclassification could result in spurious seasonality, but would not change yearly prescribing patterns.
6 Conclusion
This study shows that the RMM introduced following the first referral procedure (2013) on the pain indication in children had different impacts across the participating countries on the use of codeine-containing products and alternative treatments. While a significant drop in codeine use is visible after the referral in France and Norway, prescriptions started to decrease before the regulatory intervention in Spain and the UK. Mindful of the safety risk potentially caused by codeine, healthcare professionals switched prescribing patterns for pain management in children towards alternative opioid analgesics like tramadol in France, Norway and the UK, and towards the use of non-opioid analgesics like prescribed ibuprofen and paracetamol in Norway. The RMM recommended in the frame of the second codeine referral (2015) did not impact the trends in the use of opioid and non-opioid antitussives, apart from France where the use decreased for both types of products in children under 12 years.
References
List of European Union reference dates and frequency of submission of periodic safety update reports (PSURs). https://www.ema.europa.eu/documents/other/list-european-union-reference-dates-frequency-submission-periodic-safety-update-reports-psurs_en.xls. Accessed 16 June 2022.
Dean L, Kane M. Codeine therapy and CYP2D6 genotype. 2012 Sep 20 [updated 2021 Mar 30]. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kane MS, Kattman BL, Malheiro AJ, editors. Medical genetics summaries [Internet]. Bethesda: National Center for Biotechnology Information (US); 2012 (PMID: 28520350).
Codeine: very rare risk of side-effects in breastfed babies. Medicines and Healthcare products Regulatory Agency (MHRA) website. https://www.gov.uk/drug-safety-update/codeine-very-rare-risk-of-side-effects-in-breastfed-babies. Accessed 16 June 2022.
Safety review update of codeine use in children; new Boxed Warning and Contraindication on use after tonsillectomy and/or adenoidectomy. U.S. Food and Drug Administration. https://www.fda.gov/media/85072/download#:~:text=In%20August%202012%2C%20FDA%20announced,ultra%2Drapid%20metabolizers%20of%20codeine. Accessed 16 June 2022.
Ciszkowski C, et al. Codeine, ultrarapid-metabolism genotype, and postoperative death. N Engl J Med. 2009. https://doi.org/10.1056/NEJMc0904266.
Kelly LE, Rieder M, van den Anker J, Malkin B, Ross C, Neely MN, Carleton B, Hayden MR, Madadi P, Koren G. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129(5):e1343–7. https://doi.org/10.1542/peds.2011-2538.
Pharmacovigilance Risk Assessment Committee (PRAC). https://www.ema.europa.eu/en/committees/pharmacovigilance-risk-assessment-committee-prac. Accessed 16 June 2022.
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32001L0083. Accessed 16 June 2022.
Coordination Group for Mutual Recognition and Decentralised Procedures - Human (CMDh). https://www.ema.europa.eu/en/committees/working-parties-other-groups/coordination-group-mutual-recognition-decentralised-procedures-human-cmdh. Accessed 16 June 2022.
Codeine-containing medicines for the treatment of pain relief in paediatric patients. https://www.ema.europa.eu/en/medicines/human/referrals/codeine-containing-medicines. Accessed 16 June 2022.
Guideline on good pharmacovigilance practices (GVP) Module XV—Safety communication (Rev 1). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-xv-safety-communication-rev-1_en.pdf. Accessed 16 June 2022.
Codeine-containing medicinal products for the treatment of cough or cold in paediatric patients. https://www.ema.europa.eu/en/medicines/human/referrals/codeine-containing-medicinal-products-treatment-cough-cold-paediatric-patients. Accessed 16 June 2022.
Système National des Données de Santé (SNDS). https://www.snds.gouv.fr/SNDS/Accueil. Accessed 16 June 2022.
Becher H, Kostev K, Schroder-Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47(10):617–26. https://doi.org/10.5414/cpp47617.
Rathmann W, et al. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. 2018. https://doi.org/10.5414/CP203320.
Norwegian prescription database (NorPD). http://www.norpd.no/. Accessed 16 June 2022.
Maciá-Martínez MA, Gil M, Huerta C, et al. Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP): a data resource for pharmacoepidemiology in Spain. Pharmacoepidemiol Drug Saf. 2020;29(10):1236–45. https://doi.org/10.1002/pds.5006.
IQVIA Medical Research Data (IMRD-UK) (database version used September 2018). https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/the-health-improvement-network-thin-database/. Accessed 16 June 2022.
ATC/DDD Index 2022. https://www.whocc.no/atc_ddd_index/. Accessed 16 June 2022.
Gini, R. et al. Different strategies to execute multi-database studies for medicines surveillance in real-world setting: a reflection on the European model. Clin Pharmacol Ther. https://doi.org/10.1002/cpt.1833
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002. https://doi.org/10.1046/j.1365-2710.2002.00430.x.
Section Interrupted time series analyses of the ENCePP Guide on Methodological Standards in Pharmacoepidemiology. https://www.encepp.eu/standards_and_guidances/methodologicalGuide.shtml. Accessed 16 June 2022.
Schaffer AL, Dobbins TA, Pearson S-A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMS Med Res Methodol. 2021. https://doi.org/10.1186/s12874-021-01235-8.
Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Sage J. 2015. https://doi.org/10.1177/1536867X1501500208.
Hedenmalm K, et al. A European multicentre drug utilisation study of the impact of regulatory measures on prescribing of codeine for pain in children. Pharmacoepidemiol Drug Saf. 2019. https://doi.org/10.1002/pds.4836.
Haute Autorite de Santé (HAS). https://www.has-sante.fr/jcms/c_2010340/en/prise-en-charge-medicamenteuse-de-la-douleur-chez-l-enfant-alternatives-a-la-codeine. Accessed 16 June 2022.
HAS Fiche Mémo. Prise en charge médicamenteuse de la douleur chez l’enfant : alternatives à la codéine. Janvier 2016. https://www.sfpediatrie.com/sites/www.sfpediatrie.com/files/medias/documents/prise_en_charge_medicamenteuse_de_la_douleur_chez_lenfant_alternatives_a_la_codeine_-_fiche_memo.pdf. Accessed 16 June 2022.
Confer to the Resolution of the Spanish Ministry of Health (Resolución de 2 de agosto de 2012 de la Dirección General de Cartera Básica de Servicios del Sistema Nacional de Salud y Farmacia, por la que se procede a la actualización de la lista de medicamentos que quedan excluidos de la prestación farmacéutica en el Sistema Nacional de Salud. https://www.boe.es/eli/es/res/2012/08/02/(2). Accessed 16 June 2022.
UK Commission on Human Medicines. Codeine-containing liquid over-the-counter medicines. https://www.gov.uk/drug-safety-update/codeine-containing-liquid-over-the-counter-medicines. Accessed 16 June 2022.
Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003. https://doi.org/10.2165/00002018-200326150-00001.
Variation procedures for nationally authorised medicinal products. CMDh website. https://www.hma.eu/human-medicines/cmdh/procedural-guidance/variation.html. Accessed 16 June 2022.
Acknowledgements
This study used five databases including the Système National des Données de Santé (French National Health Data System–SNDS), IMS® Disease Analyzer Germany, Norwegian prescription database (NorPD), Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP) and IQVIA Medical Research Data (IMRD-UK). EPI-PHARE has permanent regulatory access to data of the French National Health Data System (French decree No. 2016-1871, French law articles Art. R. 1461-13/14, French data protection authority decision CNIL-2016-316). Because data are anonymised, no informed consent or specific approval by an ethics committee was required for this study. IQVIA Medical Research Data (IMRD) incorporates data from THIN, a Cegedim Database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
The authors declared no competing interests for this work. The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agency/agencies or organisations with which the author(s) is/are employed/affiliated. BIFAP is a public program for independent research financed by the Spanish Agency for Medicines and Medical Devices (AEMPS). The results, discussion, and conclusions of this work are only of the authors and do not represent in any way the position of the AEMPS on this subject. The excellent collaboration of the primary care physicians (general practitioners/paediatricians) as well as the support from the regional health administrations providing BIFAP data is acknowledged.
Ethics approval
The use of BIFAP data for this project was approved by the Ethics Committee (CEIM regional de la Comunidad de Madrid: aprobación 08-06-2020, acta CEIm 06/20).
Consent to participate
The use of BIFAP data for this project was approved by the Scientific Committee of BIFAP (protocol reference: 10/2019). The use of IMRD-UK was approved by the Scientific Review Committee under the reference number 19THIN080.
Consent for publication
Not applicable.
Availability of data and material (data transparency)
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Author contributions
Robert Flynn developed the study protocol, data extraction plan and table shells. All authors were consulted and agreed on these documents. Robert Flynn, Karin Hedenmalm, Aikaterini-Christina Deli, Miguel-Angel Maciá-Martinez, Patricia García-Poza, David Olsen and Pierre Nguyen extracted the data from the different databases. Robert Flynn ran the analyses centrally. Kelly Plueschke, Chantal Quinten and Robert Flynn drafted the manuscript and Karin Hedenmalm, Miguel-Angel Maciá-Martinez, Patricia García-Poza, David Olsen and Pierre Nguyen contributed by providing comments. All authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Plueschke, K., Flynn, R., Hedenmalm, K. et al. Prescribing Patterns of Codeine and Alternative Medicines in Children in Europe. Drug Saf 45, 1069–1081 (2022). https://doi.org/10.1007/s40264-022-01214-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01214-y